Necrotizing Mucormycosis of Wounds Following Combat Injuries, Natural Disasters, Burns, and Other Trauma
Abstract
1. Introduction
2. Microbiology
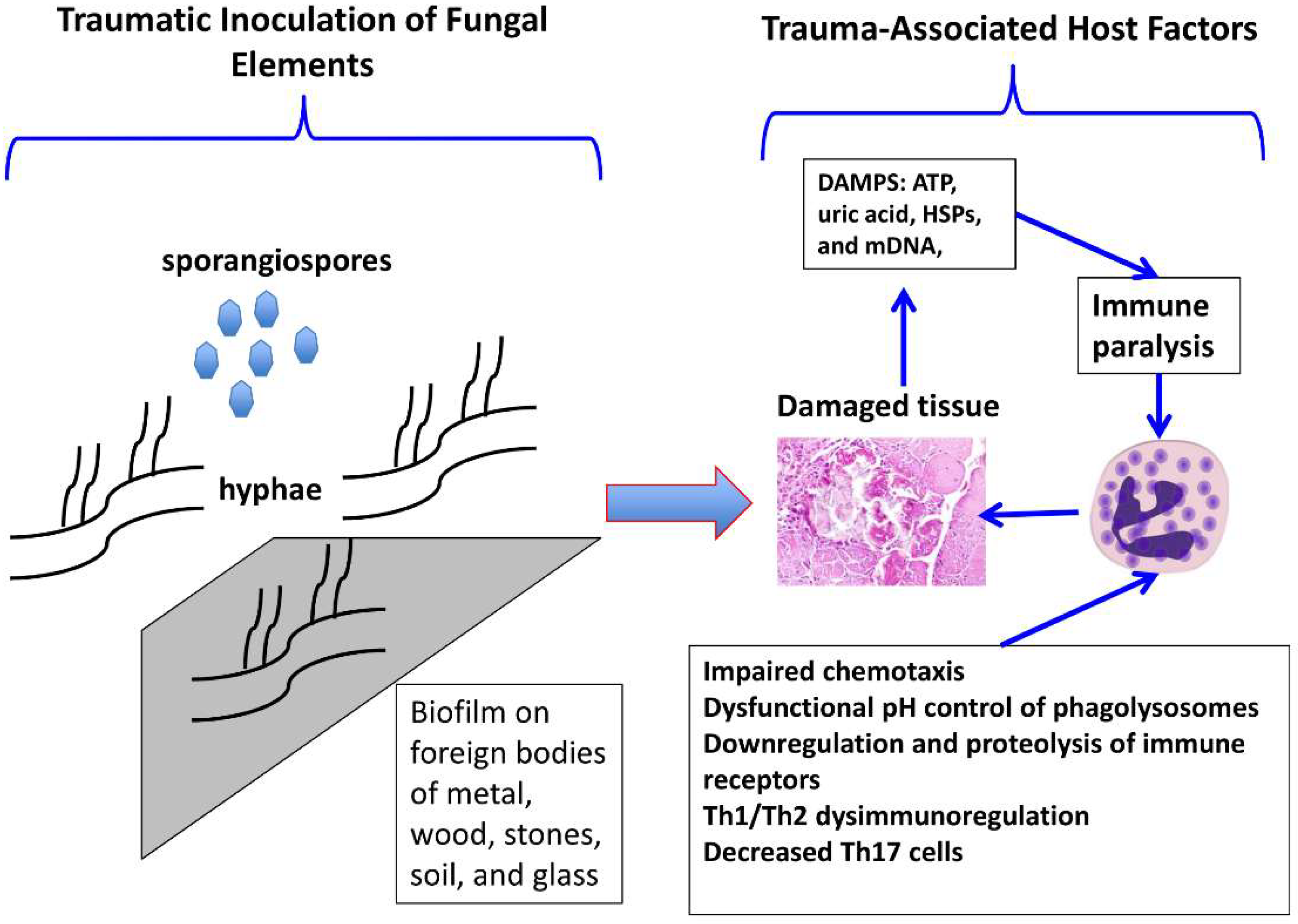
3. Pathogenesis
4. Epidemiology
4.1. Mucormycosis Following Combat-Related Injury
4.2. Mucormycosis Following Natural Disasters
4.3. Mucormycosis Following Burn Injuries
4.4. Mucormycosis Following Civilian Industrial, Agricultural, and Automotive Injuries
4.5. Trauma-Related Mucormycosis in Children
4.6. Trauma-Related Osteoarticular Mucormycosis
5. Principles of Management
5.1. Microbiological Diagnosis
5.2. Surgical Management and Antifungal Therapy
6. Future Directions
Acknowledgments
Conflicts of Interest
References
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Lortholary, O.; Walsh, T.J.; Skiada, A.; Roilides, E.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl. 1), S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.R.; Rodriguez, C.J. Combat-Related Invasive Fungal Wound Infections. Curr. Fungal Infect. Rep. 2014, 8, 277–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kronen, R.; Liang, S.Y.; Bochicchio, G.; Bochicchio, K.; Powderly, W.G.; Spec, A. Invasive Fungal Infections Secondary to Traumatic Injury. Int. J. Infect. Dis. 2017, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Devauchelle, P.; Jeanne, M.; Frealle, E. Mucormycosis in Burns Patients. J. Fungi 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Park, B.J. Invasive fungal infections after natural disasters. Emerg. Infect. Dis. 2014, 20, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lelievre, L.; Garcia-Hermoso, D.; Abdoul, H.; Hivelin, M.; Chouaki, T.; Toubas, D.; Mamez, A.C.; Lantieri, L.; Lortholary, O.; Lanternier, F.; et al. Posttraumatic mucormycosis: A nationwide study in France and review of the literature. Medicine (Baltimore) 2014, 93, 395–404. [Google Scholar] [CrossRef]
- Warkentien, T.E.; Shaikh, F.; Weintrob, A.C.; Rodriguez, C.J.; Murray, C.K.; Lloyd, B.A.; Ganesan, A.; Aggarwal, D.; Carson, M.L.; Tribble, D.R.; et al. Impact of Mucorales and Other Invasive Molds on Clinical Outcomes of Polymicrobial Traumatic Wound Infections. J. Clin. Microbiol. 2015, 53, 2262–2270. [Google Scholar] [CrossRef]
- Warkentien, T.; Rodriguez, C.; Lloyd, B.; Wells, J.; Weintrob, A.; Dunne, J.R.; Ganesan, A.; Li, P.; Bradley, W.; Gaskins, L.J.; et al. Invasive mold infections following combat-related injuries. Clin. Infect. Dis. 2012, 55, 1441–1449. [Google Scholar] [CrossRef]
- Neblett Fanfair, R.; Benedict, K.; Bos, J.; Bennett, S.D.; Lo, Y.C.; Adebanjo, T.; Etienne, K.; Deak, E.; Derado, G.; Shieh, W.J.; et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012, 367, 2214–2225. [Google Scholar] [CrossRef]
- Maegele, M.; Gregor, S.; Yuecel, N.; Simanski, C.; Paffrath, T.; Rixen, D.; Heiss, M.M.; Rudroff, C.; Saad, S.; Perbix, W.; et al. One year ago not business as usual: Wound management, infection and psychoemotional control during tertiary medical care following the 2004 Tsunami disaster in southeast Asia. Crit. Care 2006, 10, R50. [Google Scholar] [CrossRef] [PubMed]
- Snell, B.J.; Tavakoli, K. Necrotizing fasciitis caused by Apophysomyces elegans complicating soft-tissue and pelvic injuries in a tsunami survivor from Thailand. Plast. Reconstr. Surg. 2007, 119, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Paolino, K.M.; Henry, J.A.; Hospenthal, D.R.; Wortmann, G.W.; Hartzell, J.D. Invasive fungal infections following combat-related injury. Mil. Med. 2012, 177, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Etienne, K.A.; Gillece, J.; Hilsabeck, R.; Schupp, J.M.; Colman, R.; Lockhart, S.R.; Gade, L.; Thompson, E.H.; Sutton, D.A.; Neblett-Fanfair, R.; et al. Whole genome sequence typing to investigate the Apophysomyces outbreak following a tornado in Joplin, Missouri, 2011. PLoS ONE 2012, 7, e49989. [Google Scholar] [CrossRef] [PubMed]
- Patiño, J.F.; Castro, D. Necrotizing lesions of soft tissues: A review. World J. Surg. 1991, 15, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Kyriopoulos, E.J.; Kyriakopoulos, A.; Karonidis, A.; Gravvanis, A.; Gamatsi, I.; Tsironis, C.; Tsoutsos, D. Burn injuries and soft tissue traumas complicated by mucormycosis infection: A report of six cases and review of the literature. Ann. Burns Fire Disasters 2015, 28, 280–287. [Google Scholar] [PubMed]
- Schaal, J.-V.; Leclerc, T.; Pasquier, P.; Bargues, L. Epidemiology of fungal infection in burns: Therapeutic implications. Burns 2012, 38, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Kordy, F.N.; Al-Mohsen, I.Z.; Hashem, F.; Almodovar, E.; Al Hajjar, S.; Walsh, T.J. Successful treatment of a child with post-traumatic narcotizing fasciitis caused by Apophysomyces elegans: Case report and review of literature. Pediatr. Infect. Dis. J. 2004, 23, 877–879. [Google Scholar] [CrossRef]
- Leliefeld, P.H.; Wessels, C.M.; Leenen, L.P.; Koenderman, L.; Pillay, J. The role of neutrophils in immune dysfunction during severe inflammation. Crit. Care 2016, 20, 73. [Google Scholar] [CrossRef]
- Gupta, D.L.; Bhoi, S.; Mohan, T.; Galwnkar, S.; Rao, D.N. Th1Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine 2016, 88, 214–221. [Google Scholar] [CrossRef]
- Weintrob, A.C.; Weisbrod, A.B.; Dunne, J.R.; Rodriguez, C.J.; Malone, D.; Lloyd, B.A.; Warkentien, T.E.; Wells, J.; Murray, C.K.; Bradley, W.; et al. Combat trauma-associated invasive fungal wound infections: epidemiology and clinical classification. Epidemiol. Infect. 2015, 143, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.R.; Rodriguez, C.J.; Weintrob, A.C.; Shaikh, F.; Aggarwal, D.; Carson, M.L.; Murray, C.K.; Masuoka, P.; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group. Environmental Factors Related to Fungal Wound Contamination after Combat Trauma in Afghanistan, 2009–2011. Emerg. Infect. Dis. 2015, 21, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.J.; Weintrob, A.C.; Shah, J.; Malone, D.; Dunne, J.R.; Weisbrod, A.B.; Lloyd, B.A.; Warkentien, T.E.; Murray, C.K.; Wilkins, K.; et al. Risk factors associated with invasive fungal infections in combat trauma. Surg. Infect. (Larchmt) 2014, 15, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, L.R.; Weintrob, A.C.; Tribble, D.R.; Rodriguez, C.J.; Petfield, J.; Lloyd, B.A.; Murray, C.K.; Stinner, D.; Aggarwal, D.; Shaikh, F.; et al. Early Complications and Outcomes in Combat Injury-Related Invasive Fungal Wound Infections: A Case-Control Analysis. J. Orthop. Trauma 2016, 30, e93–e99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez, C.; Weintrob, A.C.; Dunne, J.R.; Weisbrod, A.B.; Lloyd, B.; Warkentien, T.; Malone, D.; Wells, J.; Murray, C.K.; Bradley, W.; et al. Clinical relevance of mold culture positivity with and without recurrent wound necrosis following combat-related injuries. J. Trauma Acute Care Surg. 2014, 77, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lamaris, G.A.; Ben-Ami, R.; Lewis, R.E.; Samonis, G.; Kontoyiannis, D.P. Increased Virulence of Zygomycetes Organisms Following Exposure to Voriconazole: A Study Involving Fly and Murine Models of Zygomycosis. J. Infect. Dis. 2009, 199, 1399–1406. [Google Scholar]
- Rodriguez, C.J.; Tribble, D.R.; Malone, D.L.; Murray, C.K.; Jessie, E.M.; Khan, M.; Fleming, M.E.; Potter, B.K.; Gordon, W.T.; Shackelford, S.A. Treatment of Suspected Invasive Fungal Infection in War Wounds. Mil. Med. 2018, 183 (Suppl. 2), 142–146. [Google Scholar] [CrossRef]
- Andresen, D.; Donaldson, A.; Choo, L.; Knox, A.; Klaassen, M.; Ursic, C.; Vonthethoff, L.; Krilis, S.; Konecny, P. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet 2005, 365, 876–878. [Google Scholar] [CrossRef]
- Patiño, J.F.; Castro, D.; Valencia, A.; Morales, P. Necrotizing soft tissue lesions after a volcanic cataclysm. World J. Surg. 1991, 15, 240–247. [Google Scholar] [CrossRef]
- Schaal, J.; Leclerc, T.; Soler, C.; Donat, N.; Cirrode, A.; Jault, P.; Bargues, L. Epidemiology of filamentous fungal infections in burned patients: A French retrospective study. Burns 2015, 41, 853–863. [Google Scholar] [CrossRef]
- Christiaens, G.; Hayette, M.P.; Jacquemin, D.; Melin, P.; Mutsers, J.; De Mol, P. An outbreak of Absidia corymbifera infection associated with bandage contamination in a burns unit. J. Hosp. Infect. 2005, 61, 88. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Dromer, F.; Lortholary, O.; Lanternier, F.; Bitar, D. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54 (Suppl. 1), S35–S43. [Google Scholar]
- Taj-Aldeen, S.; Gamaletsou, M.N.; Rammaert, B.; Sipsas, N.V.; Zeller, V.; Roilides, E.; Kontoyiannis, D.P.; Henry, M.; Petraitis, V.; Moriyama, B.; et al. Bone and joint infections caused by Mucormycetes: A challenging osteoarticular mycosis of the 21st century. Med. Mycol. 2017, 55, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, B.; Weintrob, A.C.; Rodriguez, C.; Dunne, J.R.; Weisbrod, A.B.; Hinkle, M.; Warkentien, T.; Murray, C.K.; Oh, J.; Millar, E.V.; et al. Effect of Early Screening for Invasive Fungal Infections in U.S. Service Members with Explosive Blast Injuries. Surg. Infect. 2014, 15, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Gamaletsou, M.N.; Hayden, R.T.; Walsh, T.J.; McGinnis, M.R.; Kontoyiannis, D.P. Early Clinical and Laboratory Diagnosis of Invasive Pulmonary, Extrapulmonary, and Disseminated Mucormycosis (Zygomycosis). Clin. Infect. Dis. 2012, 54 (Suppl. 1), S55–S60. [Google Scholar]
- Kasai, M.; Harrington, S.M.; Francesconi, A.; Petraitis, V.; Petraitiene, R.; Schaufele, R.L.; Sein, T.; Cotton, M.P.; Hughes, J.E.; Beveridge, M.G.; et al. Detection of molecular biomarkers for Rhizopus spp., Mucor spp., and Cunninghamella spp. by quantitative PCR and melt curve analysis in plasma, bronchoalveolar lavage, and lung tissue in experimental pulmonary zygomycosis. J. Clin. Microbiol. 2008, 46, 3690–3702. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.; Herbrecht, R.; Grenouillet, F.; Morio, F.; Alanio, A.; Letscher-Bru, V.; Cassaing, S.; Chouaki, T.; Kauffmann-Lacroix, C.; Poirier, P.; et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 2016, 22, 810.e1–810.e8. [Google Scholar] [CrossRef]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, M.; Ferry, A.; Chaussard, M.; Hamane, S.; et al. Detection of Circulating Mucorales DNA in Critically Ill Burn Patients: Preliminary Report of a Screening Strategy for Early Diagnosis and Treatment. Clin. Infect. Dis. 2016, 63, 1312–1317. [Google Scholar] [CrossRef]
- Fréalle, E.; Rocchi, S.; Bacus, M.; Bachelet, H.; Pasquesoone, L.; Tavernier, B.; Mathieu, D.; Millon, L.; Jeanne, M. Real-time polymerase chain reaction detection of Lichtheimia species in bandages associated with cutaneous mucormycosis in burn patients. J. Hosp. Infect. 2018, 99, 68–74. [Google Scholar] [CrossRef]
- McDermott, N.E.; Shea, Y.R.; Walsh, T.J. Successful treatment of periodontal mucormycosis: Case report and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e64–e69. [Google Scholar] [CrossRef]
- Downie, M.L.; AlGhounaim, M.; Davidge, K.M.; Yau, Y.; Walsh, T.J.; Pope, E.; Somers, G.R.; Waters, V.; Robinson, L.A. Isolated cutaneous mucormycosis in a pediatric renal transplant recipient. Pediatr. Transplant. 2018, 22, e13172. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E.; Lotholary, O.; Spellberg, B.; Petrikkos, G.; Roillides, E.; Ibrahim, A.; Walsh, T.J. Future Directions in Mucormycosis Research. Clin. Infect. Dis. 2012, 54, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Skiada, A.; Cornely, O.A.; Roilides, E.; Ibrahim, A.; Zaoutis, T.; Groll, A.; Lortholary, O.; Kontoyiannis, D.P.; Petrikkos, G. Development of new strategies for early diagnosis of mucormycosis from bench to bedside. Mycoses 2014, 57, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Garcia-Hermoso, D.; Mercier-Delarue, S.; Lanternier, F.; Gits-Muselli, M.; Menotti, J.; Denis, B.; Bergeron, A.; Legrand, M.; Lortholary, O.; et al. Molecular identification of Mucorales in human tissues: Contribution of PCR electrospray-ionization mass spectrometry. Clin. Microbiol. Infect. 2015, 21, 594.e1–594.e5. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.; Scherer, E.; Rocchi, S.; Bellanger, A.-P. Molecular Strategies to Diagnose Mucormycosis. J. Fungi 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-Based Approach Targeting Mucorales-Specific Gene Family for Diagnosis of Mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, S.S.; Kontoyiannis, D.P. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev. Mol. Diagn. 2018, 18, 845–854. [Google Scholar] [CrossRef]
- Walsh, T.J.; McCarthy, M.W. The expanding use of matrix-assisted laser desorption/ionization-time of flight mass spectroscopy in the diagnosis of patients with mycotic diseases. Expert Rev. Mol. Diagn. 2019, 19, 241–248. [Google Scholar] [CrossRef]
- Di Pentima, M.C.; Chan, S.; Powell, J.; Napoli, J.A.; Walter, A.W.; Walsh, T.J. Topical amphotericin B in combination with standard therapy for severe necrotizing skin and soft-tissue mucormycosis in an infant with bilineal leukemia: case report and review. J. Pediatr. Hematol. Oncol. 2014, 36, e468–e470. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Kontoyiannis, D.P.; A Cornely, O.; Perfect, J.R.; Walsh, T.J. Novel Agents and Drug Targets to Meet the Challenges of Resistant Fungi. J. Infect. Dis. 2017, 216, S474–S483. [Google Scholar] [CrossRef]
- Thielen, B.K.; Barnes, A.M.T.; Sabin, A.P.; Huebner, B.; Nelson, S.; Wesenberg, E.; Hansen, G.T. Widespread Lichtheimia Infection in a Patient with Extensive Burns: Opportunities for Novel Antifungal Agents. Mycopathologia 2019, 184, 121–128. [Google Scholar] [CrossRef] [PubMed]
| Reference | Cause of Injury | Number of Patients | Organisms Recovered (n) | Location |
|---|---|---|---|---|
| Warkentien et al. 2015 [8] | Combat-related injury | 29 | Mucor spp. (15), Saksenaea vasiformis (5), Rhizopus spp. (1) | Afghanistan |
| Paolino et al. 2012 [13] | Combat-related injury | 2 | Mucor sp. (1), Absidia sp. (1) | Afghanistan |
| Warkentien et al. 2012 [9] | Combat-related injury | 16 | Mucor spp. (9), Saksenaea vasiformis (6), Apophysomyces spp. (2) | Afghanistan |
| Neblett Fanfair et al. 2011 [10] | Tornado, 2011 | 13 | Apophysomyces trapeziformis | Joplin, Missouri |
| Maegele et al. 2006 [11] | Tsunami, 2004 | 1 | Apophysomyces elegans | Southeast Asia |
| Andresen et al. 2005 [14] | Tsunami, 2004 | 1 | Apophysomyces elegans | Sri Lanka |
| Snell et al. 2007 [12] | Tsunami, 2004 | 1 | Apophysomyces elegans | Thailand |
| Kyriopoulos et al. 2015 [15] | Burn injuries and soft tissue automotive injury | 6 | Rhizopus spp. (3), Rhizomucor spp. (3) | Greece |
| Schaal et al. 2015 [16] | Burn injuries | 9 | NS | France |
| Christiaens et al. 2005 [17] | Burn injuries | 7 | Absidia (currently Lichtheimia) corymbifera | Belgium |
| Lelievre et al. 2014 [7] | Civilian industrial, agricultural, and automotive injuries | 16 | Apophysomyces elegans complex, Saksenaea vasiformis | France |
| Kordy et al. 2004 [18] | Automotive injury | 1 | Apophysomyces elegans | Saudi Arabia |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, T.J.; Hospenthal, D.R.; Petraitis, V.; Kontoyiannis, D.P. Necrotizing Mucormycosis of Wounds Following Combat Injuries, Natural Disasters, Burns, and Other Trauma. J. Fungi 2019, 5, 57. https://doi.org/10.3390/jof5030057
Walsh TJ, Hospenthal DR, Petraitis V, Kontoyiannis DP. Necrotizing Mucormycosis of Wounds Following Combat Injuries, Natural Disasters, Burns, and Other Trauma. Journal of Fungi. 2019; 5(3):57. https://doi.org/10.3390/jof5030057
Chicago/Turabian StyleWalsh, Thomas J., Duane R. Hospenthal, Vidmantas Petraitis, and Dimitrios P. Kontoyiannis. 2019. "Necrotizing Mucormycosis of Wounds Following Combat Injuries, Natural Disasters, Burns, and Other Trauma" Journal of Fungi 5, no. 3: 57. https://doi.org/10.3390/jof5030057
APA StyleWalsh, T. J., Hospenthal, D. R., Petraitis, V., & Kontoyiannis, D. P. (2019). Necrotizing Mucormycosis of Wounds Following Combat Injuries, Natural Disasters, Burns, and Other Trauma. Journal of Fungi, 5(3), 57. https://doi.org/10.3390/jof5030057






