Identification of Mucorales by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction Protocol for MALDI-TOF Mass Spectrometry Analysis
2.2. Evaluation of Parameters (Medium, Incubation Time)
2.3. Construction of the Database
2.4. Accuracy Test of the Database
3. Results
3.1. Evaluation of Parameters (Medium, Incubation Time)
3.1.1. Medium
3.1.2. Incubation time
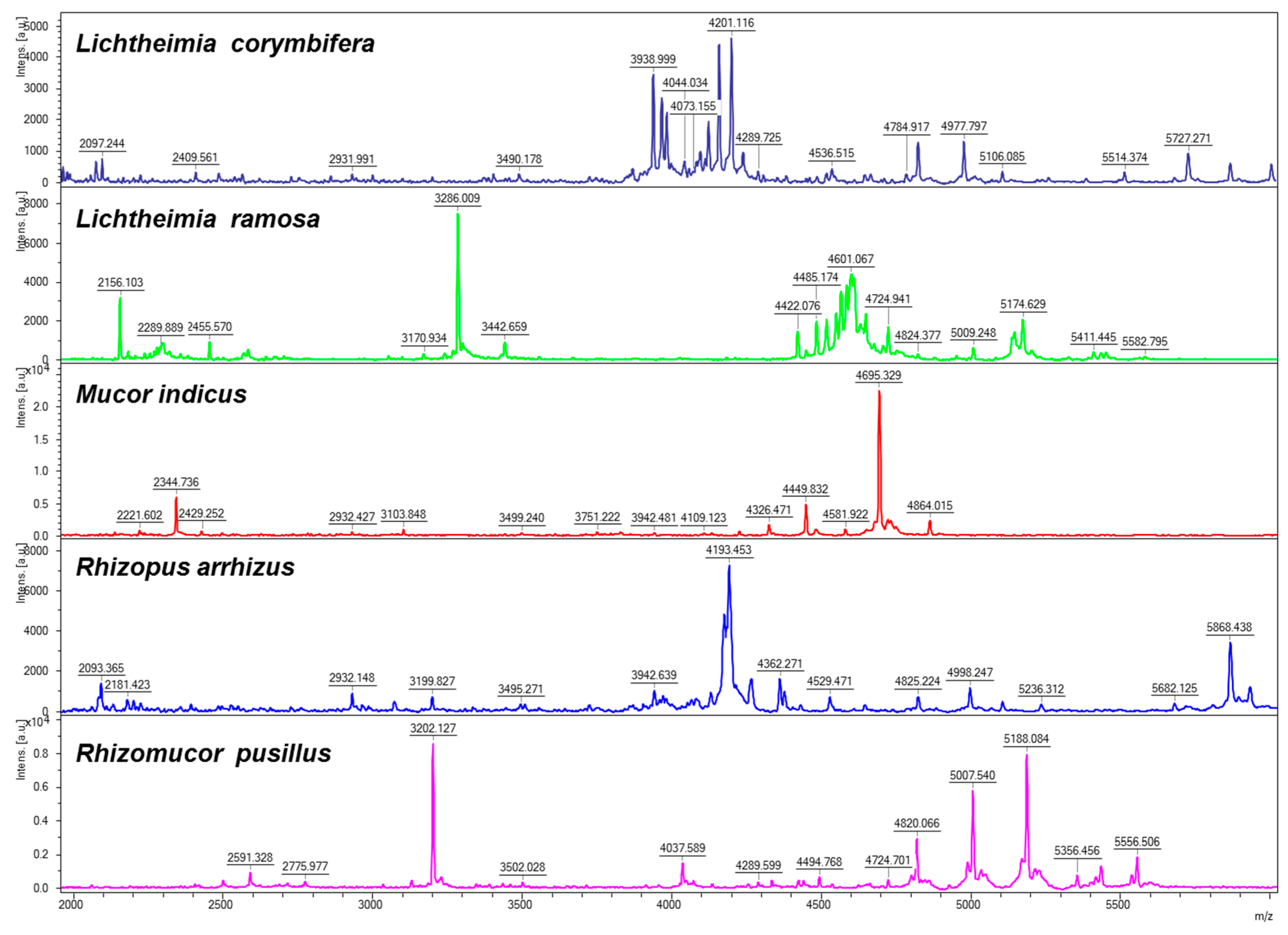
3.2. Construction of the Database
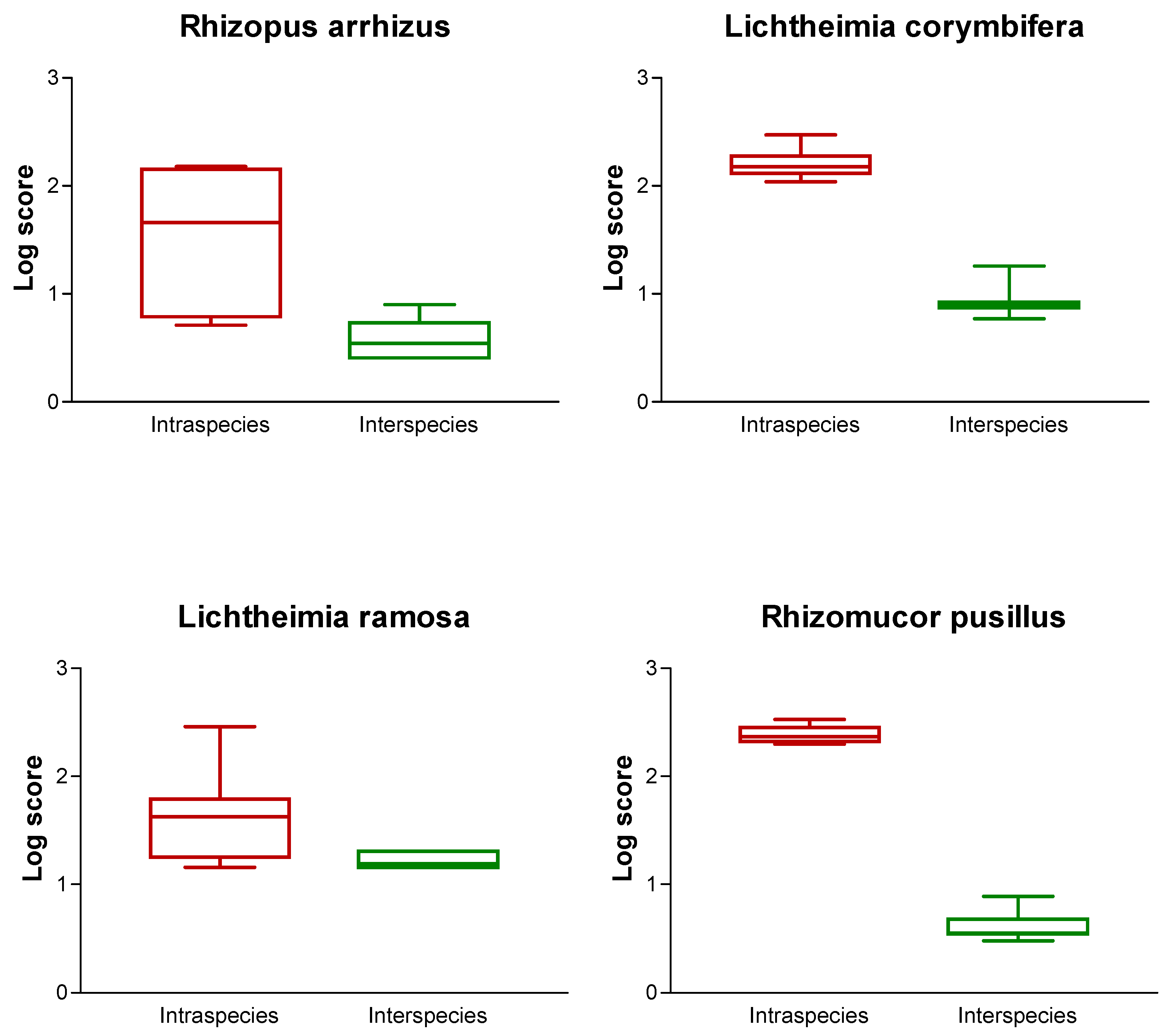
3.3. Accuracy Test of the Database
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef]
- Dannaoui, E.; Garcia-Hermoso, D. The Zygomycetes. In New Insights in Fungal Pathogenity; Kavanagh, K., Ed.; Springer Science: Dordrecht, The Netherlands, 2007; pp. 159–183. [Google Scholar]
- Bitar, D.; van Cauteren, D.; Lanternier, F.; Dannaoui, E.; Che, D.; Dromer, F.; Desenclos, J.C.; Lortholary, O. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009, 15, 1395–1401. [Google Scholar] [CrossRef]
- Lamoth, F.; Chung, S.J.; Damonti, L.; Alexander, B.D. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin. Infect. Dis. 2017, 64, 1619–1621. [Google Scholar] [CrossRef]
- Marty, F.M.; Cosimi, L.A.; Baden, L.R. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N. Engl. J. Med. 2004, 350, 950–952. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Castelli, M.V.; Cuesta, I.; Zaragoza, O.; Monzon, A.; Mellado, E.; Rodriguez-Tudela, J.L. In vitro activity of antifungals against Zygomycetes. Clin. Microbiol. Infect. 2009, 15 (Suppl. 5), 71–76. [Google Scholar] [CrossRef]
- Dannaoui, E. Antifungal resistance in Mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Dannaoui, E.; Meletiadis, J.; Mouton, J.W.; Meis, J.F.; Verweij, P.E. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 2003, 51, 45–52. [Google Scholar] [CrossRef]
- Vitale, R.G.; de Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; van de Sande, W.W.; Dolatabadi, S.; Meis, J.F.; et al. Antifungal susceptibility and phylogeny of opportunistic members of the order Mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: A case-control observational study of 27 recent cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef]
- CLSI. Interpretive Criteria for Identification of Bacteria and Fungi by Targeted DNA Sequencing; Approved Guideline MM18; Clinical and Laboratory Standards Institute: Alexandria, VA, USA, 2018. [Google Scholar]
- Balajee, S.A.; Borman, A.M.; Brandt, M.E.; Cano, J.; Cuenca-Estrella, M.; Dannaoui, E.; Guarro, J.; Haase, G.; Kibbler, C.C.; Meyer, W.; et al. Wickes, Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: Where are we and where should we go from here? J. Clin. Microbiol. 2009, 47, 877–884. [Google Scholar] [CrossRef]
- Schwarz, P.; Bretagne, S.; Gantier, J.C.; Garcia-Hermoso, D.; Lortholary, O.; Dromer, F.; Dannaoui, E. Molecular identification of zygomycetes from culture and experimentally infected tissues. J. Clin. Microbiol. 2006, 44, 340–349. [Google Scholar] [CrossRef]
- Cornely, O.A.; Arikan-Akdagli, S.; Dannaoui, E.; Groll, A.H.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.; Skiada, A.; Akova, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 5–26. [Google Scholar] [CrossRef]
- Schwarz, P.; Lortholary, O.; Dromer, F.; Dannaoui, E. Carbon assimilation profiles as a tool for identification of zygomycetes. J. Clin. Microbiol. 2007, 45, 1433–1439. [Google Scholar] [CrossRef]
- Buchan, B.W.; Ledeboer, N.A. Advances in identification of clinical yeast isolates by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 1359–1366. [Google Scholar] [CrossRef]
- Cassagne, C.; Normand, A.C.; L’Ollivier, C.; Ranque, S.; Piarroux, R. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses 2016, 59, 678–690. [Google Scholar] [CrossRef]
- Lau, A.F.; Drake, S.K.; Calhoun, L.B.; Henderson, C.M.; Zelazny, A.M. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 828–834. [Google Scholar] [CrossRef]
- Marinach-Patrice, C.; Lethuillier, A.; Marly, A.; Brossas, J.Y.; Gene, J.; Symoens, F.; Datry, A.; Guarro, J.; Mazier, D.; Hennequin, C. Use of mass spectrometry to identify clinical Fusarium isolates. Clin. Microbiol. Infect. 2009, 15, 634–642. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B. Identification of molds by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2017, 55, 369–379. [Google Scholar] [CrossRef]
- Becker, P.T.; De Bel, A.; Martiny, D.; Ranque, S.; Piarroux, R.; Cassagne, C.; Detandt, M.; Hendrickx, M. Identification of filamentous fungi isolates by MALDI-TOF mass spectrometry: Clinical evaluation of an extended reference spectra library. Med. Mycol. 2014, 52, 826–834. [Google Scholar] [CrossRef]
- Chen, Y.S.; Liu, Y.H.; Teng, S.H.; Liao, C.H.; Hung, C.C.; Sheng, W.H.; Teng, L.J.; Hsueh, P.R. Evaluation of the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry Bruker Biotyper for identification of Penicillium marneffei, Paecilomyces species, Fusarium solani, Rhizopus species, and Pseudallescheria boydii. Front. Microbiol. 2015, 6, 679. [Google Scholar] [CrossRef]
- De Carolis, E.; Posteraro, B.; Lass-Florl, C.; Vella, A.; Florio, A.R.; Torelli, R.; Girmenia, C.; Colozza, C.; Tortorano, A.M.; Sanguinetti, M.; et al. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 2012, 18, 475–484. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Kolecka, A.; Versteeg, M.; de Hoog, S.G.; Boekhout, T. Differentiation of clinically relevant Mucorales Rhizopus microsporus and R. arrhizus by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). J. Med. Microbiol. 2015, 64, 694–701. [Google Scholar]
- Schrödl, W.; Heydel, T.; Schwartze, V.U.; Hoffmann, K.; Grosse-Herrenthey, A.; Walther, G.; Alastruey-Izquierdo, A.; Rodriguez-Tudela, J.L.; Olias, P.; Jacobsen, I.D.; et al. Direct analysis and identification of pathogenic Lichtheimia species by matrix-assisted laser desorption ionization-time of flight analyzer-mediated mass spectrometry. J. Clin. Microbiol. 2012, 50, 419–427. [Google Scholar] [CrossRef]
- Schulthess, B.; Ledermann, R.; Mouttet, F.; Zbinden, A.; Bloemberg, G.V.; Bottger, E.C.; Hombach, M. Use of the Bruker MALDI Biotyper for identification of molds in the clinical mycology laboratory. J. Clin. Microbiol. 2014, 52, 2797–2803. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Li, R.; Yu, J. Species identification and delineation of pathogenic Mucorales by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2018, 56, e01886-17. [Google Scholar] [CrossRef]
- Jamal, W.; Albert, M.J.; Rotimi, V.O. Real-time comparative evaluation of bioMerieux VITEK MS versus Bruker Microflex MS, two matrix-assisted laser desorption-ionization time-of-flight mass spectrometry systems, for identification of clinically significant bacteria. BMC Microbiol. 2014, 14, 289. [Google Scholar] [CrossRef]
- Packeu, A.; de Bel, A.; l’Ollivier, C.; Ranque, S.; Detandt, M.; Hendrickx, M. Fast and accurate identification of dermatophytes by matrix-assisted laser desorption ionization-time of flight mass spectrometry: Validation in the clinical laboratory. J. Clin. Microbiol. 2014, 52, 3440–3443. [Google Scholar] [CrossRef]
- Sleiman, S.; Halliday, C.L.; Chapman, B.; Brown, M.; Nitschke, J.; Lau, A.F.; Chen, S.C. Performance of matrix-assisted laser desorption ionization-time of flight sass spectrometry for identification of Aspergillus, Scedosporium, and Fusarium spp. in the Australian clinical setting. J. Clin. Microbiol. 2016, 54, 2182–2186. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Hoffmann, K.; de Hoog, G.S.; Rodriguez-Tudela, J.L.; Voigt, K.; Bibashi, E.; Walther, G. Species recognition and clinical relevance of the zygomycetous genus Lichtheimia (syn. Absidia pro parte, Mycocladus). J. Clin. Microbiol. 2010, 48, 2154–2170. [Google Scholar]
| Species | Strain Number | Source | ITS Sequence Accession Number |
|---|---|---|---|
| Rhizopus oryzae | CBS 112.07T | Human, lung | DQ119031 |
| Rhizopus oryzae | IP 4.77 | Human, brain | DQ119024 |
| Rhizopus microsporus var. oligosporus | CBS 112589 | Environment, tempeh | DQ119011 |
| Rhizopus microsporus var. chinensis | CBS 631.72T | Environment, bread | DQ119009 |
| Lichtheimia corymbifera | CBS 429.75NT | Environment, soil | FJ719407 |
| Lichtheimia corymbifera | CBS 100.31 | Animal, aborted cow | FJ19398 |
| Lichtheimia ramosa | CBS 269.65 | Environment, hay | FJ19405 |
| Lichtheimia ramosa | CBS 270.65 | Unknown | FJ19406 |
| Lichtheimia ramosa | CBS 582.65NT | Seed, Theobroma cacao | GQ342909 |
| Lichtheimia ornata | CBS 291.66T | Animal, dung of bird | GQ342891 |
| Mucor circinelloides f. circinelloides | CBS 195.68NT | Environment, air | DQ118991 |
| Mucor circinelloides | CBS 384.95(T) | Human, skin | DQ119007 |
| Mucor indicus | CBS 226.29T | Unknown | DQ118994 |
| Rhizomucor miehei | CBS 182.67T | Environment, plant | DQ118995 |
| Rhizomucor pusillus | CBS 354.68NT | Environment, cornmeal | DQ119005 |
| Rhizomucor pusillus | IP 3.77 | Animal, brain of cat | DQ119001 |
| Syncephalastrum racemosum | CBS 441.59 | Animal, dung | HM999985 |
| Cunninghamella bertholletiae | CBS 190.84 | Human, heart | HM849701 |
| Isolate | Collection No. | Identified Species | Score |
|---|---|---|---|
| Rhizopus arrhizus | IP 1443.83 | Rhizopus arrhizus | 2.18 |
| Rhizopus arrhizus | CBS 120808 | Rhizopus arrhizus | 2.04 |
| Rhizopus arrhizus | CBS 120809 | Rhizopus arrhizus | 2.13 |
| Lichtheimia corymbifera | IP 1129.75 | Lichtheimia corymbifera | 2.17 |
| Lichtheimia corymbifera | IP 1279.81 | Lichtheimia corymbifera | 2.16 |
| Lichtheimia corymbifera | IP 1280.81 | Lichtheimia corymbifera | 2.09 |
| Lichtheimia corymbifera | CBS 101040 | Lichtheimia corymbifera | 2.21 |
| Lichtheimia corymbifera | CBS 120581 | Lichtheimia corymbifera | 2.04 |
| Lichtheimia corymbifera | CBS 120580 | Lichtheimia corymbifera | 2.45 |
| Lichtheimia corymbifera | BES 335 | Lichtheimia corymbifera | 2.2 |
| Lichtheimia corymbifera | PS 1.1 | Lichtheimia corymbifera | 2.07 |
| Lichtheimia ramosa | HEGP-3473 | Lichtheimia ramosa | 2.42 |
| Lichtheimia ramosa | BES 362 | Lichtheimia ramosa | 2.29 |
| Lichtheimia ramosa | BES 228 | Lichtheimia ramosa | 2.46 |
| Mucor indicus | CBS 120585 | Mucor indicus | 2.06 |
| Rhizomucor pusillus | IP 1127.75 | Rhizomucor pusillus | 2.42 |
| Rhizomucor pusillus | IP 1956.90 | Rhizomucor pusillus | 2.38 |
| Rhizomucor pusillus | CBS 120587 | Rhizomucor pusillus | 2.42 |
| Rhizomucor pusillus | CBS 120586 | Rhizomucor pusillus | 2.53 |
| Rhizomucor pusillus | CBS 120588 | Rhizomucor pusillus | 2.31 |
| Isolate Number and Identification | Log-Score Values for Strain | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| 1- C. bertholletiae CBS 190.84 | 3 | 0.57 | 0.42 | ND | 0.43 | 0.38 | ND | 0.38 | ND | ND | ND | 0.52 | 0.35 | ND | ND | ND | 0.28 | 0.58 |
| 2- L. corymbifera CBS 100.31 | 0.47 | 3 | 2.39 | 0.85 | ND | 0.18 | 0.66 | 0.6 | ND | 0.39 | ND | ND | ND | 0.23 | 0.35 | ND | ND | ND |
| 3- L. corymbifera CBS 429.75 | 0.44 | 2.41 | 3 | 1.14 | 0.12 | 0.6 | ND | ND | ND | ND | 0.48 | 0.37 | 0.25 | 0.26 | ND | ND | ND | ND |
| 4- L. ornata CBS 291.66 | ND | 0.8 | 1.13 | 3 | 0.35 | 0.67 | 0.67 | ND | ND | 0.91 | ND | ND | ND | 0.54 | ND | 0.4 | 0.38 | ND |
| 5- L. ramosa CBS 269.65 | 0.39 | ND | ND | 0.58 | 3 | 1.78 | 1.04 | 0.44 | 0.21 | 0.58 | ND | ND | ND | 0.51 | ND | ND | 0.37 | ND |
| 6- L. ramosa CBS 270.35 | 0.41 | ND | 0.58 | 0.6 | 1.76 | 3 | 0.87 | ND | ND | ND | 0.52 | ND | ND | 0.4 | 0.47 | ND | 0.44 | ND |
| 7- L. ramosa CBS 582.65 | ND | 0.42 | ND | 0.65 | 1.07 | 0.98 | 3 | ND | ND | 0.27 | ND | 0.7 | 0.34 | 0.38 | 0.32 | ND | ND | ND |
| 8- M. circinelloides CBS 195.68 | 0.58 | ND | ND | ND | 0.44 | 0.29 | ND | 3 | 0.87 | 0.98 | ND | ND | ND | 0.9 | 0.75 | 0.28 | ND | ND |
| 9- M. circinelloides CBS 384.95 | 0.14 | ND | ND | 0.1 | 0.1 | ND | ND | 0.9 | 3 | 0.63 | ND | ND | ND | 0.76 | 0.25 | ND | 0.31 | 0.13 |
| 10- M. indicus CBS 226.29 | ND | ND | ND | 0.95 | 0.55 | 0.66 | ND | 0.98 | 0.62 | 3 | ND | 0.59 | 0.98 | 0.53 | ND | 0.9 | ND | ND |
| 11- R. miehei CBS 182.67 | 0.23 | 0.16 | 0.04 | ND | ND | 0.54 | ND | ND | ND | 0.19 | 3 | 0.05 | 0.27 | 0.23 | ND | ND | ND | 0.22 |
| 12- R. pusillus CBS 354.68 | 0.48 | ND | ND | ND | ND | 0.27 | 0.71 | ND | ND | 0.57 | 0.09 | 3 | 2.82 | 0.38 | 0.64 | 0.31 | ND | ND |
| 13- R. pusillus IP 3.77 | 0.3 | ND | 0.33 | ND | ND | ND | 0.35 | ND | ND | 1.01 | 0.29 | 2.82 | 3 | 0.33 | 0.47 | 0.04 | ND | ND |
| 14- R. microsporus CBS 631.82 | ND | ND | ND | 0.61 | 0.53 | 0.38 | ND | 0.94 | 0.78 | 0.57 | ND | 0.38 | ND | 3 | 1.54 | ND | 0.43 | ND |
| 15- R. microsporus IP 1126.75 | ND | ND | ND | ND | ND | 0.47 | 0.31 | 0.81 | 0.24 | 0.42 | ND | 0.66 | 0.5 | 1.54 | 3 | ND | ND | 0.3 |
| 16- R. arrhizus CBS 112.07 | ND | ND | ND | 0.41 | ND | 0.66 | ND | 0.31 | ND | 0.92 | 0.05 | 0.21 | ND | ND | ND | 3 | 0.47 | 0.58 |
| 17- R. arrhizus IP 4.77 | 0.33 | ND | ND | 0.42 | 0.5 | 0.45 | 0.25 | ND | 0.3 | ND | ND | ND | ND | 0.45 | 0.11 | 0.41 | 3 | ND |
| 18- S. racemosum CBS 441.59 | 0.62 | ND | ND | ND | 0.16 | 0.3 | ND | ND | 0.14 | 0.3 | 0.27 | ND | ND | ND | 0.25 | 0.81 | 0.46 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, P.; Guedouar, H.; Laouiti, F.; Grenouillet, F.; Dannaoui, E. Identification of Mucorales by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. J. Fungi 2019, 5, 56. https://doi.org/10.3390/jof5030056
Schwarz P, Guedouar H, Laouiti F, Grenouillet F, Dannaoui E. Identification of Mucorales by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Journal of Fungi. 2019; 5(3):56. https://doi.org/10.3390/jof5030056
Chicago/Turabian StyleSchwarz, Patrick, Houssem Guedouar, Farah Laouiti, Frédéric Grenouillet, and Eric Dannaoui. 2019. "Identification of Mucorales by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry" Journal of Fungi 5, no. 3: 56. https://doi.org/10.3390/jof5030056
APA StyleSchwarz, P., Guedouar, H., Laouiti, F., Grenouillet, F., & Dannaoui, E. (2019). Identification of Mucorales by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Journal of Fungi, 5(3), 56. https://doi.org/10.3390/jof5030056







