Endophytic Fungi from Terminalia Species: A Comprehensive Review
Abstract
1. Introduction
2. Biodiversity of Endophytic Fungi Isolated from Different Terminalia Species
2.1. Diversity of Fungal Genera Isolated from Terminalia spp. as a Function of Locations and Seasons
2.2. Influence of Plant Species and Plant Organs on Fungi Colonization
3. Biological Activity of Endophytic Fungi from Terminalia Species
3.1. Antimicrobial Activities of Fungal Endophytes
3.2. Antioxidant Potential of Endophytes
3.3. Anticancer Potential of Endophytes
3.4. Other Biological Activities
4. Brief Outline for the Isolation, Identification and Metabolic Profiling of Endophytic Fungi from Terminalia Species
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Deines, P.; Lachnit, T.; Bosch, T.C.G. Competing forces maintain the Hydra metaorganism. Immunol. Rev. 2017, 279, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Petrini, O.; Fisher, P. Occurrence of fungal endophytes in twigs of Salix fragilis and Quercus robur. Mycological Res. 1990, 94, 1077–1080. [Google Scholar] [CrossRef]
- Bacon, C.W.; White, J.F. Microbial Endophytes; Marcel Dekker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Molecul. Biol. Rev. 2003, 491–502. [Google Scholar] [CrossRef]
- Strobel, G. The Emergence of Endophytic Microbes and Their Biological Promise. J. Fungi 2018, 4, 57. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Li, L.; Zheng, C.; Guo, L.; Li, W.; Sun, P.; Qin, L. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol. Res. 2010, 165, 437–449. [Google Scholar] [CrossRef]
- JI, Y.; BI, J.; YAN, B.; ZHU, X. Taxol-producing Fungi: A New Approach to Industrial Production of Taxol. Chin. J. Biotechnol. 2006, 22, 1–6. [Google Scholar] [CrossRef]
- Venieraki, A.; Dimou, M.; Katinakis, P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hellenic Plant Protect. J. 2017, 10, 51–66. [Google Scholar] [CrossRef]
- Stace, C.A. Combretaceae. Springer 2007, 9, 67–82. [Google Scholar]
- Dwivedi, S. Terminalia arjuna Wright & Arn—a useful drug for cardiovascular disorders. J. Ethnopharmacol. 2007, 114, 114–129. [Google Scholar] [PubMed]
- Li, K.; Diao, Y.; Zhang, H.; Wang, S.; Zhang, Z.; Yu, B.; Huang, S.; Yang, H. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. Promote cutaneous wound healing in rats. BMN. Compliment. Altern. Med. 2011, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Rabe, T.; Sparg, S.G.; Ja¨ger, A.K.; Ejoff, J.N.; Van Staden, J. An investigation on the biological activity of Combretum species. J Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef]
- Eloff, J.N.; Katerere, D.R.; McGaw, L.J. The biological activity and chemistry of the southern African Combretaceae. J. Ethnopharmacol. 2008, 119, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Aneja, K.R.; Sharma, C.; Joshi, R. Antimicrobial activity of Terminalia arjuna Wight & Arn: An ethnomedicinal plant against pathogens causing ear infection. Braz. J. Otorhinolaryngol. 2012, 78, 68–74. [Google Scholar] [PubMed]
- Cock, I.E.; Van Vuuren, S.F. A comparison of the antimicrobial activity and toxicity of six Combretum and two Terminalia species from Southern Africa. Pharmacogn. Mag. 2015, 11, 208–218. [Google Scholar] [CrossRef]
- Ngouana, T.K.; Mbouna, C.D.J.; Kuipou, R.M.T.; Tchuenmogne, M.A.T.; Zeuko’o, E.M.; Ngouana, V.; Mallié, M.; Bertout, S.; Boyom, F.F. Potent and Synergistic Extract Combinations from Terminalia Catappa, Terminalia Mantaly and Monodora tenuifolia Against Pathogenic Yeasts. Medicines 2015, 2, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Alayande, L.B.; Alayande, M.O.; Mohammed, A.A.; Adamu, T.; Abubakar, U.; Daneji, A.; Ajagbonna, O.P. Efficacy of Terminalia avicennoides and its combination with diminazene aceturate (Berenil) in rats experimentally infected with Trypanosoma brucei brucei. Sokoto J. Vet. Sci. 2011, 9, 11–15. [Google Scholar]
- Rayan, P.; Matthews, B.; McDonnell, P.A.; Cock, I.E. Terminalia ferdinandiana extracts as inhibitors of Giardia duodenalis proliferation: A new treatment for giardiasis. Parasitol. Res. 2015, 114, 2611–2620. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Lin, C.C.; Lin, T.C. Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antivir. Res. 2002, 55, 447–455. [Google Scholar] [CrossRef]
- Sumithira, P.; Dhivya, M.S.; Sophie, A.M.; Padma, L.C. Antiviral and antioxidant activities of two medicinal plants. Int. J. Curr. Sci. 2012, 256–261. [Google Scholar]
- Fyhrquist, P.; Mwasumbi, L.; Haeggstrom, C.A.; Vuorela, H.; Hiltunen, R.; Vuorela, P. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J. Ethnopharmacol. 2002, 79, 169–177. [Google Scholar] [CrossRef]
- Dongmo, A.B.; Beppe, J.G.; Nole, T.; Kamanyi, A. Analgesic activities of the stem bark extract of Terminalia superba Engl. Et Diels (Combreaaceae). Pharmacologyonline 2006, 2, 171–177. [Google Scholar]
- Mareges, S.; Van Miert, S.; Pannecouque, C.; Feiz Haddad, M.H.; Hermans, N.; Wright, C.W.; Vlietinck, A.J.; Apers, S.; Pieters, L. Screening of Tanzanian medicinal plants against Plasmodium falciparum and human immunodeficiency virus. Planta Med. 2010, 76, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mbouna, J.C.D.; Kouipou, R.M.T.; Keumoe, R.; Tchokouaha, L.R.Y.; Fokou, P.V.T.; Tali, B.M.T.; Sahal, D.; Boyom, F.F. Potent antiplasmodial extracts and fractions from Terminalia mantaly and Terminalia superba. Malaria J. 2018, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Mety, S.S.; Mathad, P. Antioxidative and free radical scavenging activities of Terminalia species. Int. J. Biotechnol. 2011, 2, 119–127. [Google Scholar]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS induced inflammation by suppressing NK-rB and MAPK avtivation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef]
- Biswas, M.; Biswas, K.; Karan, T.K.; Bhattacharya, S.; Ghosh, A.K.; Haldar, P.K. Evaluation of analgesic and anti-inflammatory activities of Terminalia arjuna leaf. J. Phytol. 2011, 3, 33–38. [Google Scholar]
- Das, N.D.; Jung, K.H.; Park, J.H.; Choi, M.R.; Lee, H.T.; Kim, M.S.; Lee, S.R.; Chai, Y.G. Proteomic analysis of Terminalia chebula extract-dependent changes in human lymphoblastic T cell protein expression. J. Med. Food 2012, 15, 651–657. [Google Scholar] [CrossRef]
- Momo, C.E.N.; Ngwa, A.F.; Dongmo, I.F.; Oben, J.E. Antioxidant properties and a-amylase inhibition of Terminalia superba, Albizia sp., Cola nitida, Cola odorata and Harungana madagascarensis used in the management of diabetes in Cameroon. J. Health Sci. 2009, 55, 732–738. [Google Scholar] [CrossRef][Green Version]
- Kannan, M.; Dasari, A.; Karthikeyan, M.; Ashokkumar, J.; Rajasekar, S. Effect of Terminalia bellerica fruit Roxb on Alloxan induced diabetic related atherosclerosis on Wistar albino rats. Int. J. Phytopharmacol. 2012, 3, 5–9. [Google Scholar]
- Latha, R.; Daisy, P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chemico-Biological Interact. 2011, 189, 112–118. [Google Scholar] [CrossRef]
- Israni, D.A.; Patel, K.V.T.R.G. Anti-hyperlipidemic activity of aqueous extract of Terminalia chebula and Gaumutra in high cholesterol diet fed rats. Int. J. Pharm. Pharm. Sci. 2010, 1, 48–59. [Google Scholar]
- Pinmai, K.; Chunlaratthanabhorn, S.; Ngamkitidechakul, C.; Soonthornchareon, N.; Hahnvajanawong, C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: Doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J. Gastroenterol. 2008, 14, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sahu, R.P.; Srivastava, S.K. Triphala inhibits both in vitro and in vivo xenograft growth of pancreatic tumor cells by inducing apoptosis. BMC Cancer 2008, 8, 294. [Google Scholar] [CrossRef]
- Mohanty, S.; Cock, I.E. The chemotherapeutic potential of Terminalia ferdinandiana: Phytochemistry and bioactivity. Pharmacogn. Rev. 2012, 6, 29–36. [Google Scholar] [PubMed]
- Martino, V.S.; Lo´pez, P.; Martinez, I.J.J.; Sanroma´n, M.; Cuevas, M.T.; Santiago, E.; Lasarte, J.J.; Font, M.; Coussio, J.D.; Mongue, A. Inhibitory effect against polymerase and ribonuclease activities of HIV-reverse transcriptase of the aqueous leaf extract of Terminalia triflora. Phytother. Res. 2002, 16, 778–780. [Google Scholar] [CrossRef]
- Arnold, A.E.; Maynard, Z.; Gilbert, G.S.; Coley, P.D.; Kursar, T.A. Are tropical fungal endophytes hyperdiverse? Ecol. Lett. 2000, 3, 267–274. [Google Scholar] [CrossRef]
- Persoh, D. Factors shaping community structure of endophytic fungi-evidence from the Pinus-Viscum-system. Fungal Divers. 2013, 60, 55–69. [Google Scholar] [CrossRef]
- Arnold, A.E.; Maynard, Z.; Gilbert, G.S. Fungal endophytes in dicotyledonous neotropical trees: Patterns of abundance and diversity. Mycol. Res. 2001, 105, 1502–1507. [Google Scholar] [CrossRef]
- Prathyusha, P.; Rajitha Sri, A.B.; Ashokvardhan, T.; Satya, P.K. Antimicrobial and Siderophore Activity of the Endophytic Fungus Acremonium sclerotigenum Inhabiting Terminalia bellerica Roxb. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 84–87. [Google Scholar]
- Phaopongthai, J.; Wiyakrutta, S.; Meksuriyen, D.; Sriubolmas, N.; Suwanborirux, K. Azole-synergistic Anti-Candidal Activity of Altenusin, a Biphenyl Metabolite of the Endophytic Fungus Alternaria alternata Isolated from Terminalia chebula Retz. J. Microbiol. 2013, 51, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Patil, R.H.; Patil, S.G.; Maheshwari, V.L. Endophytic Mycoflora of Indian medicinal plant, Terminalia arjuna and their biological activities. Int. J. Biotechnol. Wellness Ind. 2014, 3, 53. [Google Scholar]
- Basha, N.S.; Ogbaghebriel, A.; Yemane, K.; Zenebe, M. Isolation and screening of endophytic fungi from Eritrean traditional medicinal plant Terminalia brownii leaves for antimicrobial activity. Int. J. Green Pharm. 2012, 6, 40–44. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Tate, R.; Abbott, G.; Young, L.; Viegelmann, C.; Schumacher, M.; Diederich, M.; Edrada-Ebel, R. Metabolomic Tools to Assess the Chemistry and Bioactivity of Endophytic Aspergillus Strain. Chem. Biodivers. 2017, 14, e1700040. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Zeuko’o Menkem, E.; Mbekou Kanko, M.I.; Jesus Marie, A.; Ngo Mback, N.; Eke, P.; Vázquez de Aldana, B.R.; Íñigo, Z.; Fekam, B.F. Antimicrobial and antiradical activities of ethyl acetate extracts from endophytic fungi isolated from Cameroonian medicinal plants. J. Med. Studies 2016, 4, 290–295. [Google Scholar]
- Tejesvi, M.V.; Mahesh, B.; Nalini, M.S.; Prakash, H.S.; Kini, K.R.; Shetty, H.S. Fungal endophyte assemblages from ethnopharmaceutically important medicinal trees. Can. J. Microbiol. 2006, 52, 427–435. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Zabalgogeazcoa, I.; Vázquez de Aldana, B.R.; Boyom, F.F. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. South African J. Bot. 2017, 109, 146–153. [Google Scholar] [CrossRef]
- Tejesvi, M.V.; Basavanna, M.; Monnanda, S.N.; Harishchandra, S.P.; Kukkundoor, R.K.; Ven, S.; Hunthrike, S.S. Endophytic fungal assemblages from inner bark and twig of Terminalia arjuna W. & A. (Combretaceae). World J. Microbiol. Biotech. 2005, 21, 1535–1540. [Google Scholar]
- Suryanarayanan, T.S.; Devarajan, P.T.; Girivasan, K.P.; Govindarajulu, M.B.; Kumaresan, V.; Murali, T.S.; Rajamani, T.; Thirunavukkarasu, N.; Venkatesan, G. The host range of multi-host endophytic fungi. Curr. Science 2018, 115. [Google Scholar]
- Tawfike, A.F.; Abbott, G.; Young, L.; Edrada-Ebel, R. Metabolomic-Guided Isolation of Bioactive Natural Products from Curvularia sp., an Endophytic Fungus of Terminalia laxiflora. Planta Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Begoude, B.A.D.; Slippers, B.; Wingfield, M.J.; Roux, J. The pathogenic potential of endophytic Botryosphaeriaceous fungi on Terminalia species in Cameroon. For. Path. 2011, 41, 281–292. [Google Scholar] [CrossRef][Green Version]
- Krittapong, O.; Sophon, R.; Prakitsin, S. Diversity of Endophytic Fungi Isolated from Plant Leaves of Deciduous Dipterocarp Forest in Tak Province. Kasetsart J. (Nat. Sci.) 2009, 43, 182–188. [Google Scholar]
- Mookherjee, A.; Dineshkumar, R.; Kutty, N.N.; Agarwal, T.; Sen, R.; Mitra, A.; Maiti, K.T.; Maiti, K.M. Quorum sensing inhibitory activity of the metabolome from endophytic Kwoniella sp. PY016: Characterization and hybrid model-based optimization. Appl. Microbiol. Biotechnol. 2018, 102, 7389–7406. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Spang, S.; Kluck, K.; Hess, W.M.; Sears, J.; Livinghouse, T. Synergism among volatile organic compounds resulting in increased antibiosis in Oidium sp. FEMS Microbiol. Lett. 2008, 283, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Haque, M.; Nahar, N. Bioactive compounds from endophytic fungus Penicillium thiomii isolated from Terminalia chebula Retz. J. Nat. Prod. Plant Resour. 2014, 4, 65–70. [Google Scholar]
- Tejesvi, M.V.; Kini, K.R.; Prakash, H.S.; Subbiah, V.; Shetty, H.S. Genetic diversity and antifungal activity of species of Pestalotiopis isolated as endophytes from medicinal plants. Fungal Divers. 2007, 24, 37–54. [Google Scholar]
- Womersley, J.S. Handbooks of the Flora of Papua New Guinea; Melbourne University Press: Melbourne, Australia.
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D. The metacommunity concept: A framework for multi-scale community ecology. Ecology Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Singh, D.K.; Sharma, V.K.; Kumar, J.; Mishra, A.; Verma, S.K.; Sieber, T.N.; Kharwar, R.N. Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn.f.: Spatiotemporal and tissue type effects. Sci. Rep. 2017, 7, 3745. [Google Scholar] [CrossRef]
- Herre, E.A.; Mejia, L.C.; Kyllo, D.A.; Rojas, E.; Maynard, Z.; Butler, A.; Van Bael, S.A. Ecological implication of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology 2007, 88, 550–558. [Google Scholar]
- Yadav, M.; Yadav, A.; Kumar, S.; Yadav, J.P. Spatial and seasonal influences on culturable endophytic mycobiota associated with different tissues of Eugenia jambolana Lam. and their antibacterial activity against MDR strains. BMC microbiology 2016, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Gond, S.K.; Kumar, A.; Sharma, V.K.; Verma, S.K.; Kharwar, R.N.; Sieber, T.N. Season and tissue type affect fungal endophyte communities of the Indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microb. Ecol. 2012, 64, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Samsampour, D.; Seyahooei, M.A.; Bagheri, A.; Soltani, J. Diversity and Spatiotemporal Distribution of Fungal Endophytes Associated with Citrus reticulata cv. Siyahoo. Curr. Microbiol. 2019, 76, 279. [Google Scholar] [CrossRef] [PubMed]
- Göre, M.E.; Bucak, C. Geographical and seasonal influences on the distribution of fungal endophytes in Laurus nobilis. Forest Pathology 2007, 37, 281–288. [Google Scholar] [CrossRef]
- Compant, S.; Van der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol.Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Worchel, E.R.; Giauque, H.E.; Kivlin, S.N. Fungal symbionts alter plant drought response. Microbial. Ecol. 2013, 65, 671–678. [Google Scholar] [CrossRef]
- De Abreu, L.M.; Almeida, A.R.; Salgado, M.; Pfenning, L.H. Fungal endophytes associated with the mistletoe Phoradendron perrottettii and its host tree Tapirira guianensis. Mycol. Prog. 2010, 9, 559–566. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; Hyde, K.D. Endophytic fungi of wild banana (Musa acuminata) at Doi Suthep Pui National Park, Thailand. Mycol Res. 2001, 105, 1508–1513. [Google Scholar] [CrossRef]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of grapevine flowers, berries, and seeds: Identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Cardinale, M. Scanning a microhabitat: Plant-microbe interactions revealed by confocal laser microscopy. Front. Microbiol. 2014, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ding, Q.; Hyde, H.D.; Guo, L.D. Community structure and preference of endophytic fungi of three woody plants in a mixed forest. Fungal Ecol. 2012. [Google Scholar] [CrossRef]
- Felber, C.A.; Orlandelli, C.R.; Rhoden, S.A.; Garcia, A.; Costa, A.T.; Azevedo, J.L.; Pamphile, J.A. Bioprospecting foliar endophytic fungi of Vitis labrusca L., cv. Bordô and Concord. Annals Microbiol. 2015, 1, 11. [Google Scholar] [CrossRef]
- Duong, L.M.; Jeewon, R.; Lumyong, S.; Hyde, K.D. DGGE coupled with ribosomal DNA gene phylogenies reveal uncharacterized fungal phylotypes. Fungal Divers. 2006, 23, 121–138. [Google Scholar]
- Strobel, G.A. Rainforest endophytes and bioactive products. Crit. Rev. Biotechnol. 2002, 22, 315–333. [Google Scholar] [CrossRef]
- Tejesvi, M.V.; Nalini, M.S.; Mahesh, B.; Parkash, S.H.; Kinni, R.K.; Shetty, H.S. New hopes from endophytic fungal secondary metabolite, Bol. Soc. QuimMex. 2007, 1, 19–26. [Google Scholar]
- Strobel, G.A.; Ford, E.; Worapong, J.; Harper, J.K.; Arif, A.M.; Grant, D.M.; Fung, P.C.W.; Chan, K. Ispoestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry 2002, 60, 179–183. [Google Scholar] [CrossRef]
- Harper, J.K.; Ford, E.J.; Strobel, G.A.; Arif, A.; Grant, D.M.; Porco, J.; Tomer, D.P.; Oneill, K. Pestacin: A 1,3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron 2003, 59, 2471–2476. [Google Scholar] [CrossRef]
- Patil, M.; Patil, R.; Mohammad, S.; Maheshwari, V. Bioactivities of phenolics-rich fraction from Diaporthe arengae TATW2, an endophytic fungus from Terminalia arjuna (Roxb.). Biocatal. Agr. Biotech. 2017, 10, 396–402. [Google Scholar] [CrossRef]
- Tejesvi, V.M.; Kini, R.K.; Prakash, S.H.; Subbiah, V.; Shetty, S.H. Antioxidant, antihypertensive, and antibacterial properties of endophytic Pestalotiopsis species from medicinal plants. Can. J. Microbiol. 2008, 54, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Ezra, D.; Hess, W.M.; Strobel, A.G. New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiol. 2004, 150, 4023–4031. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Thoo-Lin, K.P.; Nahar, N. Anti-colon cancer activity of endophytic fungal strains from Terminalia chebula Rezt. Bangladesh J. Pharmacol. 2012, 7, 47–49. [Google Scholar] [CrossRef][Green Version]
- Gangadevi, V.; Muthumary, J. Taxol production by Pestalotiopsis terminaliae, an endophytic fungus of Terminalia arjuna (arjun tree). Biotechnol. Appl. Biochem. 2009, 52, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gangadevi, V.; Muthumary, J. A novel endophytic Taxol-producing fungus Chaetomella raphigera isolated from a medicinal plant, Terminalia arjuna. Appl. Biochem. Biotechnol. 2009, 158, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Kesting, J.R.; Staerk, D.; Tejesvi, M.V.; Kini, K.R.; Prakash, H.S.; Jaroszewski, J.W. HPLC-SPE-NMR Identification of a Novel Metabolite Containing the Benzo[c]oxepin Skeleton from the Endophytic Fungus Pestalotiopsis virgatula Culture. Planta Med. 2009, 75, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Toghueo, R.M.K.; Dinkar, S.; Inigo, Z.; Baker, B.; Boyom, F.F. Conditioned media and organic elicitors underpin the production of potent antiplasmodial metabolites by endophytic fungi from Cameroonian medicinal plants. Parasitol. Res. 2018, 117, 2473–2485. [Google Scholar] [CrossRef]
- Kesting, R.J.; Olsen, L.; Staerk, D.; Tejesvi, M.V.; Kini, K.R.; Prakash, H.S.; Jaroszewski, J.W. Production of Unusual Dispiro Metabolites in Pestalotiopsis virgatula Endophyte Cultures: HPLC-SPE-NMR, Electronic Circular Dichroism, and Time-Dependent Density-Functional Computation Study. J. Nat. Prod. 2011, 74, 2206–2215. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Ejiya, I.E.; Sahal, D.; Yazdani, S.S.; Boyom, F.F. Production of Cellulolytic Enzymes by Endophytic Fungi Isolated from Cameroonian Medicinal Plants. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1264–1271. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Eke, P.; Zabalgogeazcoa, I.; Vázquez de Aldana, B.R.; Nana, W.L.; Fekam, B.F. Biocontrol and growth enhancement potential of two endophytic Trichoderma spp. from Terminalia catappa against the causative agent of Common Bean Root Rot (Fusarium solani). Biol. Control 2016, 96, 8–20. [Google Scholar] [CrossRef]
- Hay, S.I.; Rao, P.C.; Dolecek, C.; Day, N.; Stergachis, A.; Lopez, A.D.; Murray, C. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, K.; Puri, S. Free radicals and antioxidants in health and disease. EMHJ. 1998, 4, 350–360. [Google Scholar]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef]
- Tamimi, R.M.; Hankinson, S.E.; Campos, H.; Spiegelman, D.; Zhang, S.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am. J. Epidemiol. 2005, 161, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, F. Antioxidant vitamins newsletter. Nutr. Rev. 1997, 14, 234–236. [Google Scholar]
- Davì, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef]
- Hitchon, C.A.; El-Gabalawy, H.S. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004, 6, 265–278. [Google Scholar] [CrossRef]
- Cookson, M.R.; Shaw, P.J. Oxidative stress and motor neurone disease. Brain Pathol. 1999, 9, 165–186. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are antioxidants helpful for disease prevention? Res. Pharm. Sci. 2010, 5, 1–8. [Google Scholar]
- Rob, M.; Rahman, R.; Mosihuzzaman, M.; Rahman, K.M.; Sultana, N.J. Bangladesh Chem. Soc. 2005, 18, 64–69.
- Arnone, A.; Nasini, G.; Camarda, L.; Assante, G. Secondary mould metabolites. The structure of rubellins C and D, two novelanthraquinone metabolites from Mycosphaerella rubella. Gazzett a Chimica Italiana 1989, 119, 35–39. [Google Scholar]
- Arnone, A.; Assante, G.; Nasini, G.; De Pava, O.V. Spirolaxine and sporotricale: Two long-chain phthalides produced by Sporotrichum laxum. Phytochemistry 1990, 29, 613–616. [Google Scholar] [CrossRef]
- Asakawa, Y.; Takikawa, K.; Tori, M.; Campbell, E.O. Isotachin C and balantiolide, two aromatic compounds from the New Zealand liverwort Balantiopsis rosea. Phytochemistry 1986, 25, 2543–2546. [Google Scholar] [CrossRef]
- Kraut, L.; Mues, R.; Sim-Sim, M. Sesquiterpene lactones and 3- benzylphthalides from Frullania muscicola. Phytochemistry 1994, 37, 1337–1346. [Google Scholar] [CrossRef]
- Dekker, K.A.; Inagaki, T.; Gootz, T.D.; Kaneda, K.; Nomura, E.; Sakakibara, T.; Sakemi, S.; Sugie, Y.; Yamauchi, Y.; Yoshikawa, N.; et al. CJ-12,954 and its congeners, new anti-Helicobacter pylori compounds produced by Phanerochaete velutina: Fermentation, isolation, structural elucidation and biological activities. J. Antibiot. 1997, 50, 833–839. [Google Scholar] [CrossRef]
- IARC Global Cancer Observatory. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Press Release N° 263, 12 September 2018. [Google Scholar]
- Argoudelis, A.D.; Dietz, A.; Johnson, L.E. Zervamicins I and II, polypeptide antibiotics produced by Emericellopsis salmosynnemata. J. Antibiot. 1974, 27, 321–328. [Google Scholar] [CrossRef]
- Argoudelis, A.D.; Mizsak, S.A.; Baczynskyj, L. N-acetyl-l-phenylalanyl-l-phenylalaninol a metabolite of Emericellopsis salmosynnemata. J. Antibiot. 1975, 28, 733–736. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: Discovery to clinic--thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1995, 55, 753–760. [Google Scholar]
- Cragg, G.M.; Snader, K.M. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Gangadevi, V.; Murugan, M.; Muthumary, J. Taxol determination from Pestalotiopsis pauciseta, a fungal endophyte of a medicinal plant. Chinese J. Biotechnol. 2008, 24, 1433–1438. [Google Scholar] [CrossRef]
- Li, J.Y.; Strobel, G.; Sidhu, R.; Hess, W.M.; Ford, E.J. Endophytic taxol-producing fungi from bald cypress, Taxodium distichum. Microbiol. 1996, 142, 2223–2226. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, E.M.K.; Paranagama, P.A.; Marron, M.T.; Gunatilaka, M.K.; Arnold, A.E.; Gunatilaka, A.A.L. Sesquiterpene quinones and related metabolites from Phyllosticta spinarum, a fungal strain endophytic in Platycladus orientalis, of the sonoran desert. J. Nat.Prod. 2008, 71, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Gangadevi, V.; Muthumary, J. Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J. Microbiol. Biotech. 2008, 24, 717–724. [Google Scholar] [CrossRef]
- Schulz, B.U.; Wanke, U.; Drager, S.; Aust, H.J. Endophytes from herbaceous plants and shrubs: Effectiveness of surface sterilization methods. Mycology Res. 1993, 97, 1447–1450. [Google Scholar] [CrossRef]
- Ainsworth, G.C.; Sparrow, F.K.; Sussman, A.S. The Fungi: An Advanced Treatise; Academic Press: New York, NY, USA, 1973; Volume 4A. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: St. Paul, MN, USA, 1998. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfland, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Amagata, T. Natural Products Structural Diversity-II Secondary Metabolites: Sources, Structures and Chemical Biology. Comprehensive Natural Products II 2010, 2, 581–621. [Google Scholar]
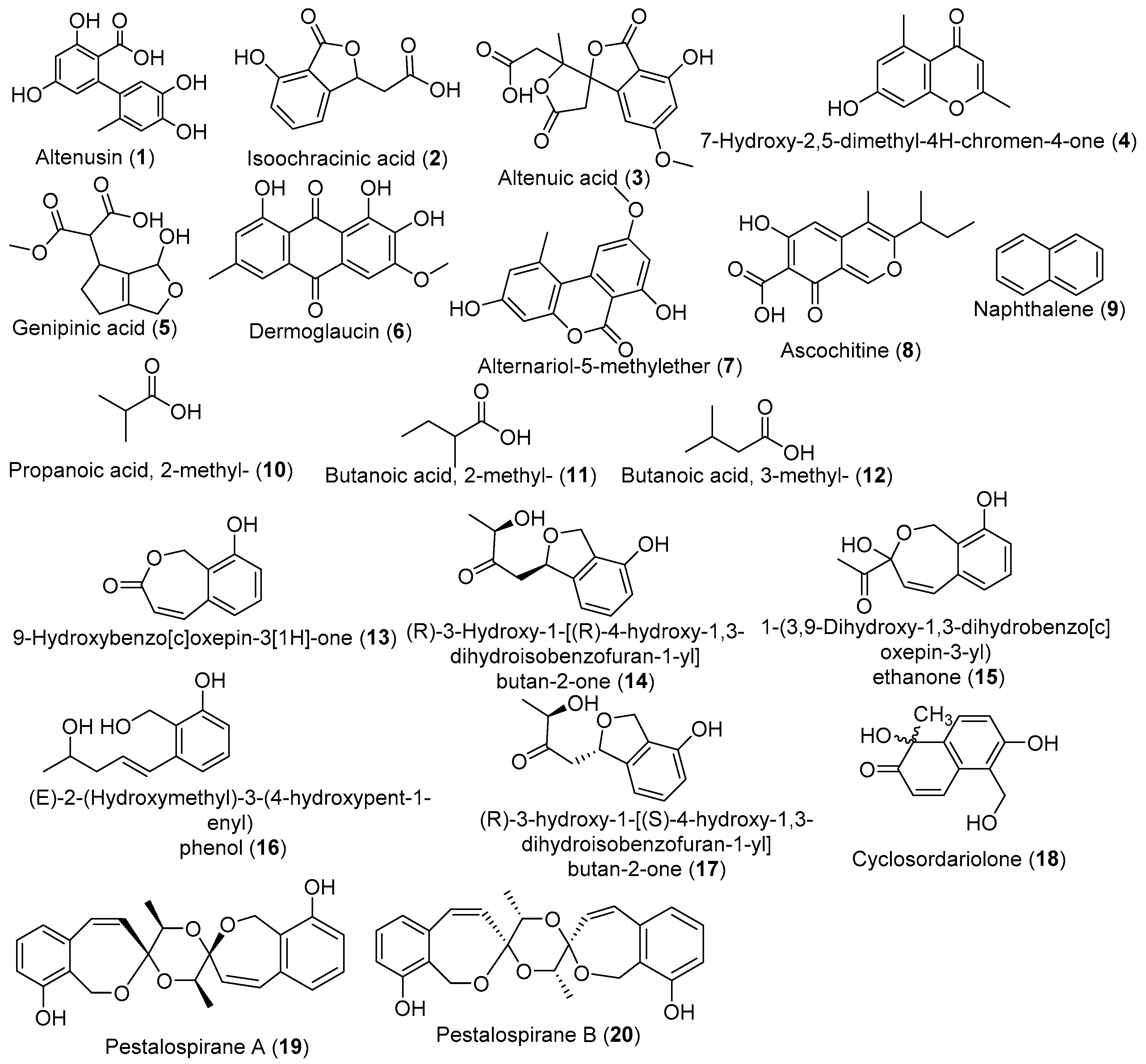
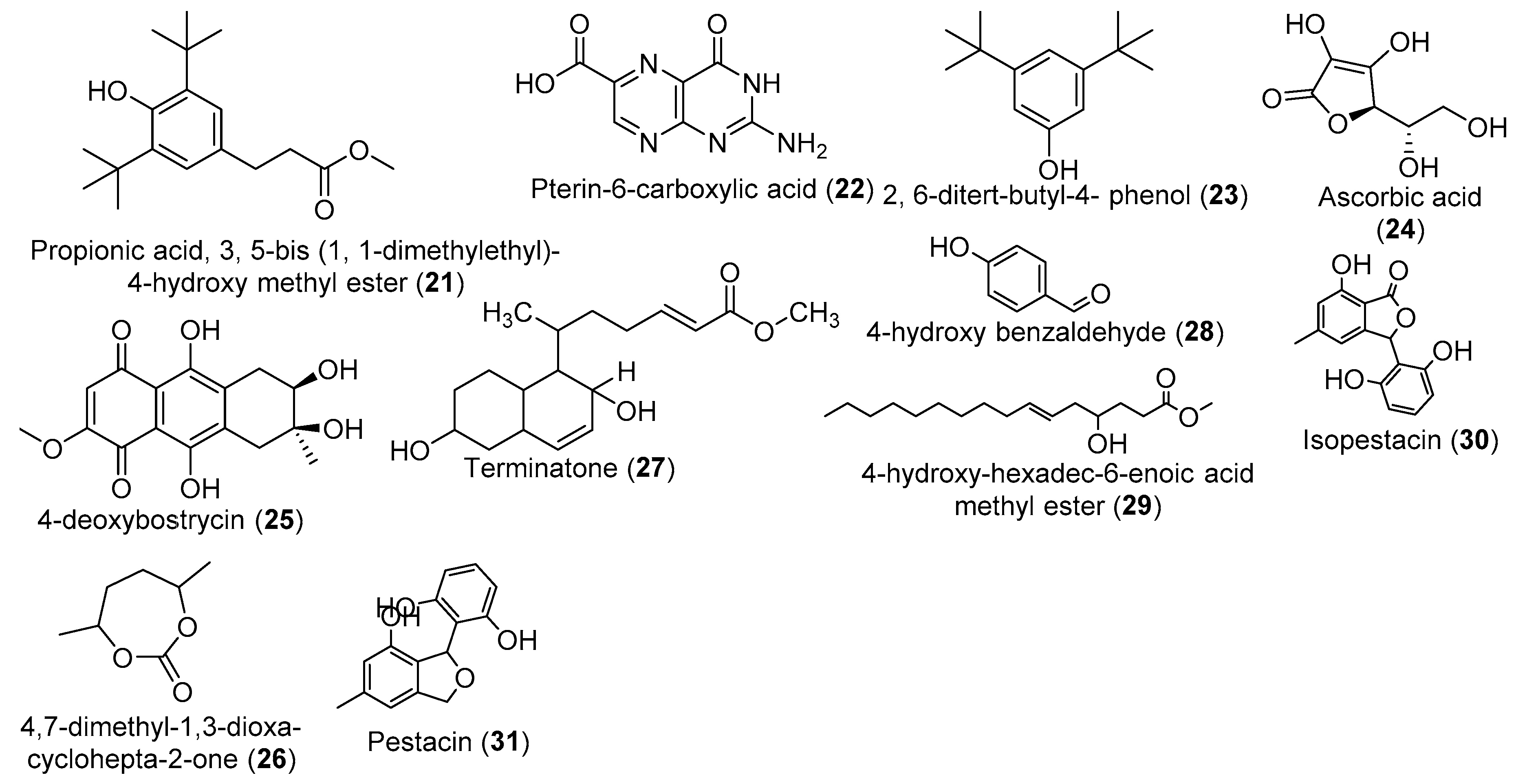
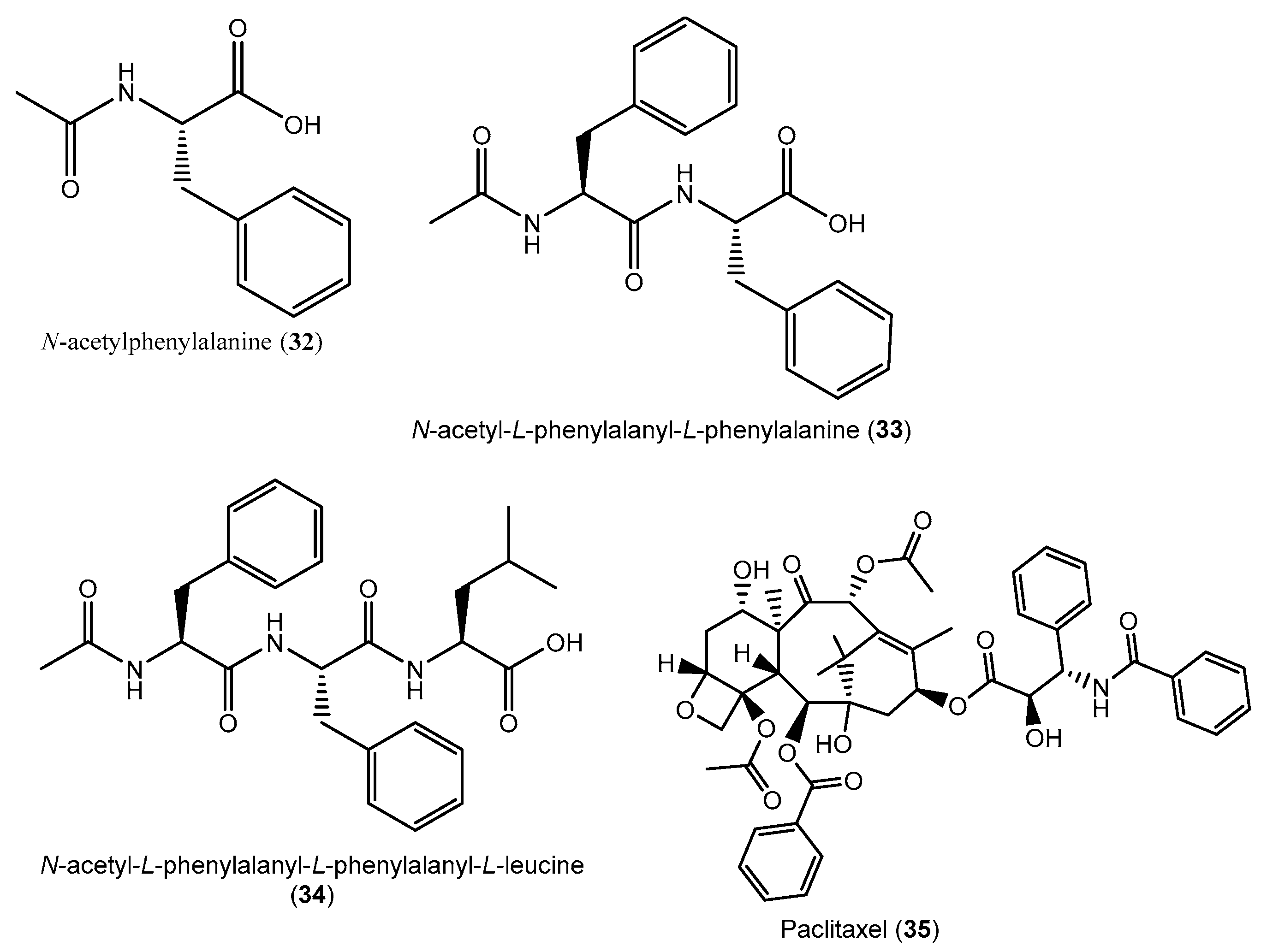
| No. | Fungal Genus | Medicinal Plants | Organs of Isolation | Regions/Countries | Refs. |
|---|---|---|---|---|---|
| 1 | Acremonium | T. bellerica | Leaves | Telangana, India | [43] |
| 2 | Alternaria | T. chebula | Leaves | Bangkok, Thailand | [44] |
| T. arjuna | Leaves, bark, twig | Shirpur, India | [45] | ||
| 3 | Aspergillus | T. arjuna | Leaves, bark, twig | Shirpur, India | [45] |
| T. brownie | Leaves | Ghindae, Eritrea | [46] | ||
| T. laxiflora | Leaves | Giza, Egypt | [47] | ||
| T. catappa | Bark | Yaoundé, Cameroon | [48] | ||
| 4 | Botryodiplodia | T. arjuna | Bark | Srirangapatra, India | [49] |
| 5 | Botryosphaeria | T. catappa | Bark | Yaoundé, Cameroon | [50] |
| 6 | Cercospora | T. catappa | Leaves, stem, twig, bark | Yaoundé, Cameroon | [50] |
| T. mantaly | Leaves | ||||
| 7 | Chaetomium | T. arjuna | Bark | Mysore region, India | [49] |
| Bark, twig | Riparian, India | [51] | |||
| 8 | Chloridium | T. arjuna | Bark | Srirangapatra, India | [49] |
| 9 | Cladosporium | T. arjuna | Bark | Mysore region, India | [49] |
| T. catappa | Leaves | Yaoundé, Cameroon | [50] | ||
| 10 | Cochlonema | T. arjuna | Bark | Mysore region, India | [49] |
| 11 | Colletotrichum | T. alata, T. arjuna ,T. catappax, T. chebula T. crenulate | Leaves | Kargudi, Ronohills, and Masinagudi, India | [52] |
| T. mantaly | Leaves | Yaoundé, Cameroon | [50] | ||
| 12 | Corynescora | T. catappa | Leaves | Yaoundé, Cameroon | [50] |
| 13 | Cryptococcus | T. mantaly | Leaves | Yaoundé, Cameroon | [50] |
| 14 | Curvularia | T. laxiflora | Leaves | Giza, Egypt | [53] |
| 15 | Diaporthe | T. arjuna | Leaves, bark | Shirpur, India | [45] |
| T. catappa | Stem | Yaoundé, Cameroon | [50] | ||
| T. mantaly | Bark, leaves, stem | Yaoundé, Cameroon | |||
| 16 | Endomelanconiopsis | T. mantaly, T. ivorensis T. superba | Bark | Belabo, Cameroon | [54] |
| 17 | Fusarium | T. mantaly | Stem | Yaounde, Cameroon | [50] |
| 18 | Gliocladium | T. arjuna | Bark | Nanjangud, India | [49] |
| 19 | Glomerella | T. chebula | Leaves | Tak province, Thailand, | [55] |
| 20 | Guignardia | T. catappa | Bark, leaves, stem | Yaoundé, Cameroon | [50] |
| 21 | Humicola | T. arjuna | Bark | Mysore region, India | [49] |
| 22 | Kwoniella | T. bellerica | Fruit | Kharagpur, India | [56] |
| 23 | Lasiodiplodia | T. chebula | Leaves | Kargudi, Ronohills, and Masinagudi, India | [52] |
| T. arjuna | Leaves, bark | Shirpur, India | [45] | ||
| T. catappa, T. mantaly | Bark | Yaounde, Cameroon | [50] | ||
| T. mantaly, T. ivorensis, T. superba | Bark | Belabo, Cameroon | [54] | ||
| 24 | Memnoniella | T. arjuna | Bark | Nanjangud, India | [49] |
| 25 | Monocillium | T. arjuna | Bark | Srirangapatra, India | [49] |
| 26 | Mycosphaerella | T. bellerica | Fruit | Kharagpur, India | [56] |
| 27 | Myrothecium | T. arjuna | Bark, twig | Riparian, India | [51] |
| T. arjuna | Bark | Mysore region, India | [49] | ||
| 28 | Nigrospora | T. arjuna | Bark | Mysore region, India | [49] |
| T. mantaly | Leaves | Yaoundé, Cameroon | [50] | ||
| 29 | Oidium | T. catappa | - | Costa Rica | [57] |
| 30 | Ophioceras | T. catappa | Leaves, stem, twig, bark | Yaoundé, Cameroon | [50] |
| 31 | Paecilomyces | T. chebula | Leaves | Tak province, Thailand | [55] |
| 32 | Paraconiothyrium | T. catappa | Stem | Yaoundé, Cameroon | [50] |
| 33 | Penicillium | T. chebula | - | Dhaka, Bangladesh | [58] |
| 34 | Pestalotiopsis | T. alata, T. chebula, T. crenulata | Leaves | Kargudi, Ronohills, and Masinagudi, India | [52] |
| T. arjuna | Bark, twig | Riparian, India | [51] | ||
| T. chebula | Bark | Gopalaswamy hills, India | [49,59] | ||
| T. arjuna | Bark | Nanjangud and Srirangapatra, India | [49] | ||
| T. catappa | Stem | Yaoundé, Cameroon | [50] | ||
| T. morobensis | Stem | Papua New Guinea | [60] | ||
| 35 | Phialophora | T. arjuna | Bark | Mysore region, India | [49] |
| 36 | Phoma | T. mantaly | Leaves | Yaoundé, Cameroon | [50] |
| 37 | Phomopsis | T. alata, T. arjuna, T. catappa, T. chebula, T. crenulata | Leaves | Kargudi, Ronohills, and Masinagudi, India | [52] |
| T. chebula | Leaves | Tak province, Thailand | [55] | ||
| 38 | Phyllosticata | T. alata, T. catappa, T. chebula, T. crenulata | Leaves | Kargudi, Ronohills, and Masinagudi, India | [52] |
| 39 | Pseudocercospora | T. catappa | Leaves, stem, twig, bark | Yaoundé, Cameroon | [50] |
| 40 | Pseudofusicoccum | T. mantaly T. catappa | Stem | Yaoundé, Cameroon | [50] |
| 41 | Rhizophus | T. brownie | Leaves | Ghindae, Eritrea | [46] |
| 42 | Septoria | T. mantaly | Leaves | Yaoundé, Cameroon | [50] |
| 43 | Sporomiella | T. chebula | Leaves | Kargudi, Ronohills, and Masinagudi, India | [52] |
| 44 | Stemphylium | T. arjuna | Bark | Mysore region, India | [49] |
| 45 | Trichoderma | T. arjuna | Bark | Nanjangud and Srirangapatra, india | [49] |
| T. catappa | Bark | Yaoundé, Cameroon | [50] | ||
| 46 | Tubercularia | T. arjuna | Bark, twig | Riparian, India | [49,51] |
| 47 | Xylaria | T. chebula T. crenulata | Leaves | Kargudi, Ronohills, and Masinagudi, India | [52] |
| T. catappa | Leaves, stem, twig, bark | Yaoundé, Cameroon | [50] |
| Endophytic Fungal | Host Plant | Culture Conditions | Compound/Extract | Activities | References |
|---|---|---|---|---|---|
| P. microspore | T. morobensis | MID, 35 days, 23 °C (no shaking) | MeCl extract | Antimicrobial, Antioxidant, Antimalarial | [80] |
| Isopestacin | Antioxidant, | [80] | |||
| Pestacin | Antioxidant, | [81] | |||
| D. arengae, | T. arjuna | SBM, 28 °C, 16 days, shaking (150) rpm | EtOAc extract | Antimicrobial, Antioxidant, | [45] |
| Benzene propionic acid, 3, 5–bis (1, 1-dimethylethyl)-4-hydroxy methyl ester; Pterin-6-carboxylic acid; 2, 6-ditert-butyl-4- phenol | Anti-inflammatory, Anti-hypercholesterolemic | [82] | |||
| Pestalotiopsis spp. | T. arjuna | PDB, 21 days, 23 °C (no shaking) | EtOAc extracts | Antifungal | [59] |
| Antibacterial, Antioxidant, Antihypertensive | [83] | ||||
| Acremonium sclerotigenum | T. bellerica | Rice, 25 °C, 30 days (No shaking) | EtOAc extract | Antimicrobial, Siderophore production | [43] |
| Muscudor albus | T. prostrata | PDA, 4 days, 23 °C (no shaking) | naphthalene, and naphthalene, 1,19-oxybis- | Antimicrobial | [84] |
| Oidium sp. | T. catappa | PDA,10 days, 23 °C (no shaking) | Volatile organic compounds | Antifungal | [57] |
| Penicillium thiomii | T. chebula | PDA, 21 days, 25 °C (no shaking) | EtOAc extract | Anticancer | [85] |
| Terminatone | Antioxidant | [58] | |||
| P. terminaliae, | T. arjuna | MID, 21 days, 26 °C (no shaking) | Paclitaxel/ MeCl extract | Anticancer | [86] |
| C. raphigera | T. arjuna | MID, 21 days, 26 °C (no shaking) | Paclitaxel/MeCl extract | Anticancer | [87] |
| Aspergillus spp. | T. brownii | SDB, 9 days, 30 °C (no shaking) | - | Antimicrobial | [46] |
| A. alternata | T. chebula | YES, 21 days, 25 °C (no shaking) | EtOAc extract | Antifungal | [44] |
| Altenusin | |||||
| Isoochracinic acid | |||||
| Altenuic acid | |||||
| 2,5-dimethyl-7-hydroxychromone | |||||
| A. aculeatus, A. oryzae | T. laxiflora | RM, (no shaking) | EtOAc extract | Anticancer | [47] |
| Curvularia sp. | T. laxiflora | RM, (no shaking) | EtOAc extract | Anticancer | [53] |
| Pestalotiopsis virgatula | T. chebula | M1D, 21 days, 23 °C (no shaking) | 9-Hydroxybenzo[c]oxepin-3[1H]-one/ EtOAc extract | Antimicrobial | [88] |
| Aspergillus niger | T.catappa | PDB, MEB and CDB, 6 days, 25 °C (no shaking) | EtOAc extract | Antiplasmodial | [89] |
| Phomopsis sp. Xylaria sp. | T.mantaly | ||||
| Pestalotiopsis virgatula | T. chebula | PDB, 21 days, 23 °C (no shaking) | EtOAc extract | - | [90] |
| Cyclosordariolone | |||||
| (R)-3-Hydroxy-1-[(R)-4-hydroxy-1,3-dihydroisobenzofuran-1-yl]butan-2-one; | |||||
| (R)-3-Hydroxy-1-[(S)-4-hydroxy-1,3-dihydroisobenzofuran-1-yl]butan-2-one; | |||||
| (E)-2-(Hydroxymethyl)-3-(4-hydroxypent-1-enyl) phenol; | |||||
| 1-(3,9-Dihydroxy-1,3-dihydrobenzo[c]oxepin-3-yl) ethanone; | |||||
| Pestalospirane A; | |||||
| Pestalospirane B | |||||
| 9-Hydroxybenzo[c]oxepin-3[1H]-one | |||||
| T. atroviride P. chermesinum | T.catappa | RM, 40 days, 25 °C, (No shaking) | EtOAc extract | Antimicrobial | [48] |
| Diaporthe spp. | T.mantaly | Antioxidant | |||
| Penicilium spp. | T.catappa | CMC, 6 days, 25 °C (No shaking) | - | Cellulase activity | [91] |
| Trichoderma spp. | T.catappa | PDA, 10 days, 25 °C (No shaking) | - | Biocontrol | [92] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouipou Toghueo, R.M.; Boyom, F.F. Endophytic Fungi from Terminalia Species: A Comprehensive Review. J. Fungi 2019, 5, 43. https://doi.org/10.3390/jof5020043
Kouipou Toghueo RM, Boyom FF. Endophytic Fungi from Terminalia Species: A Comprehensive Review. Journal of Fungi. 2019; 5(2):43. https://doi.org/10.3390/jof5020043
Chicago/Turabian StyleKouipou Toghueo, Rufin Marie, and Fabrice Fekam Boyom. 2019. "Endophytic Fungi from Terminalia Species: A Comprehensive Review" Journal of Fungi 5, no. 2: 43. https://doi.org/10.3390/jof5020043
APA StyleKouipou Toghueo, R. M., & Boyom, F. F. (2019). Endophytic Fungi from Terminalia Species: A Comprehensive Review. Journal of Fungi, 5(2), 43. https://doi.org/10.3390/jof5020043





