Epidemiological and Mycological Aspects of Onychomycosis in Dakar (Senegal)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Mycological Examination
2.4. Data Entry and Analysis
2.5. Ethical Considerations
3. Results
3.1. Socio-Demographic Characteristics of Study Population
3.2. Clinical Characteristics of Lesions
3.3. Mycological Data
3.4. Variation of the Frequency of Onychomycosis According to the Socio-Demographic Characteristics of the Study Population
3.5. Variation of the Frequency of Onychomycosis Based on the Clinical Characteristics of the Lesions
3.6. Study of Onychomycosis Caused by Candida Albicans and Trichophyton Soudanense
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Scrivener, J.N. (Yannis). Onychomycoses: Épidémiologie et clinique. Rev. Fr. Lab. 2011, 432, 35. [Google Scholar] [CrossRef]
- Jain, S.; Sehgal, V. Onychomycosis: An epidemio-etiologic per- spective. Int. J. Dermatol. 2000, 39, 100. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Hajjeh, R.A.; Scher, R.; Konnikov, N.; Gupta, A.K.; Summerbell, R.; Sullivan, S.; Daniel, R.; Krusinski, P.; Fleckman, P.; et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 2000, 43, 641–648. [Google Scholar] [CrossRef]
- Delmas, V.; Bremond-Gignac, D. Anatomie Générale; Elsevier Masson: Paris, France, 2008; pp. 167–168. [Google Scholar]
- Chabasse, D. Place du laboratoire dans le diagnostic mycologique d’une onychomycose. Rev. Fr. Lab. 2011, 432, 45. [Google Scholar] [CrossRef]
- Rispail, P.; Bourgeois, N.; Lachaud, L. Diagnostic biologique des onychomycoses: Prééminence de l’examen direct. Rev. Fr. Lab. 2011, 432, 51. [Google Scholar] [CrossRef]
- Lachaud, L.; Sasso, M.; Rispail, P.; Bourgeois, N. Diagnostic biologique des onychomycoses. Examen direct après coloration PAS simplifiée. J. Myco. Med. 2014, 24, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Arrese, J.E.; Pierard-Franchimont, C.; Pierard, G.E. Facing up to the diagnostic uncertainty and management of onychomycoses. Int. J. Dermatol. 1999, 38, 1–6. [Google Scholar] [CrossRef]
- Diouf, K. Aspects épidémiologiques des onychomycoses diagnostiquées au laboratoire de parasitologie-mycologie du CHNU de FANN. Ph.D. Thesis, Université Cheikh Anta Diop, Dakar, Senegal, 2013. Available online: http://bibnum.ucad.sn/viewer.php?c=thm&d=THM-48384 (accessed on 10 April 2013).
- Seck, MC.; Ndiaye, D.; Diongue, K.; Ndiaye, M.; Badiane, AS.; Sow, D.; Sylla, K.; Tine, R.; Ndiaye, J.L.; Faye, B.; et al. Profil mycologique des onychomycoses à Dakar (Sénégal). J. Myco. Med. 2014, 24, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Konate, A.; Yavo, W.; Kassi, K.F.; Djohan, V.; Angora, K.E.; Bosson-Vanga, H.; Barro-Kiki, P.; Menan, E.I.H. Profil mycologique des onychomycoses à Abidjan (Côte d’Ivoire). J. Myco. Med. 2014, 24, 205–210. [Google Scholar] [CrossRef]
- Kouotou, E.A.; Kechia, F.A.; Somo, Y.I.; Feungue, U.N.; Nansseu, J.R.; Somo, R.M. Profil mycologique des onychomycoses vues en consultation de dermatologie à Yaoundé, Cameroun. J. Myco. Med. 2017, 22, 124–130. [Google Scholar] [CrossRef]
- Halim, I.; El Kadioui, F.; Soussi Abdallaoui, M. Les onychomycoses à Casablanca (Maroc). J. Myco. Med. 2012, 12, 1156–5233. [Google Scholar] [CrossRef]
- Farhi, D.; Savary, J.; Pansart, S.; Hesse, S. Étude prospective des onychomycoses des pieds en France: Prévalence, aspect clinique, impact et prise en charge en médecine générale. J. Myco. Med. 2011, 21, 266–272. [Google Scholar] [CrossRef]
- Roberts, D.T. Prevalence of dermatophyte onychomycosis in the United Kingdom: Results of an omnibus survey. Br. J. Dermatol. 1992, 126, 23–27. [Google Scholar] [CrossRef]
- Afène, S.N.; Ngoungou, E.B.; Mamfoumbi, M.M.; Akotet, M.B.; Mba, I.A.; Kombila, M. Les onychomycoses au Gabon: Aspects cliniques et mycologiques. J. Myco. Med. 2011, 21, 248–255. [Google Scholar] [CrossRef]
- Soltani, M.; Khosravi, A.R.; Shokri, H.; Sharifzadeh, A.; Balal, A. A study of onychomycosis in patients attending a dermatology center in Tehran, Iran. J. Myco. Med. 2015, 25, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Burzykowski, T.; Molenberghs, G.; Abeck, D.; Haneke, E.; Hay, R.; Katsambas, A.; Roseeuw, D.; van de Kerkhof, P.C.M.; van Aelst, R.; Marynissen, G. High prevalence of foot diseases in Europe: Results of the Achilles Project. Mycoses 2003, 46, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Sanae, A. Les onychomycoses: Étude rétrospective et particularités chez les diabétiques. M.D. Thesis, Université Sidi Mohammed Ben Abdallah, Fès, Maroc, 2013. Available online: http://scolarite.fmp-usmba.ac.ma/cdim/mediatheque/ (accessed on 22 April 2013).
- Sbay, A. Epidémiologie des onychomycoses à l’Hôpital Militaire d’Instruction Mohammed V de Rabat. Ph.D. Thesis, Université Mohamed V, Rabat, Maroc, 2010. Available online: http://ao.um5s.ac.ma/jspui/handle/123456789/1980.
- Heikkilä, H.; Stubb, S. The prevalence of onychomycosis in Finland. Br. J. Dermatol. 1995, 133, 699–703. [Google Scholar] [CrossRef]
- Chabasse, D. Peut-on chiffrer la fréquence des onychomycoses ? Ann. Dermatol. Venereol. 2003, 130, 1222–1230. [Google Scholar]
- Boukachabine, K.; Agoumi, A. Les onychomycoses au Maroc. Ann. Biol. Clin. 2005, 63, 639–642. [Google Scholar]
- Hajoui, F.Z.; Zeroual, Z.; Ghfir, B.; Moustachi, A.; Lyagoubi, M.; Aoufi, S. The mould onychomycosis in Morocco: About 150 isolated cases in 20 years. J. Myco. Med. 2012, 22, 221–224. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Number | Percentage (%) | 95% CI |
|---|---|---|---|
| Age group | |||
| <15 years | 49 | 10.5 | 7.7–13.8 |
| >15 years | 420 | 89.5 | 81.2–98.5 |
| Gender | |||
| Female | 309 | 65.9 | 58.74–73.6 |
| Male | 160 | 34.1 | 29.1–39.8 |
| Season | |||
| Dry | 351 | 74.8 | 67.2–83.1 |
| Rainy | 118 | 25.2 | 20.8–30.1 |
| Clinical Characteristic | Number | Percentage (%) | 95% CI |
|---|---|---|---|
| DLSO | 178 | 37.9 | 32.6–44 |
| WSO | 15 | 3.2 | 1.8–5.3 |
| Onyxis | 218 | 46.5 | 40.5–53.1 |
| Perionyxis | 11 | 2.4 | 1.2–4.2 |
| Onyxis/Perionyxis | 47 | 10 | 7.4–13.3 |
| Number | Percentage (%) | 95% CI | |
|---|---|---|---|
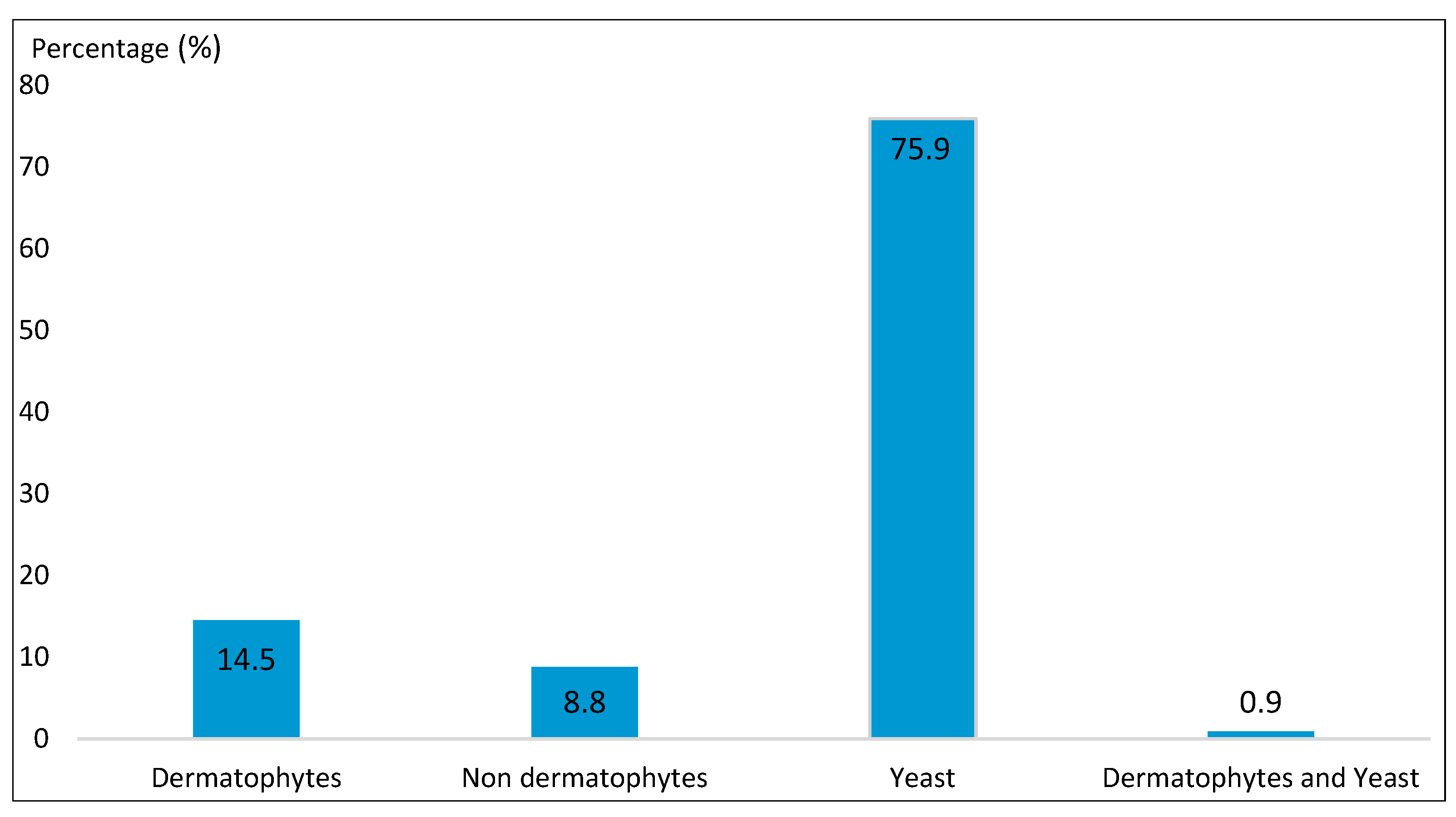
| Dermatophytes | |||
| Microsporum langeronii | 1 | 0.4 | 0.01–2.4 |
| Trichophyton mentagrophytes | 1 | 0.4 | 0.01–2.4 |
| Trichophyton rubrum | 3 | 1.3 | 0.2–3.8 |
| Trichophyton soudanense | 23 | 10.1 | 6.4–15.2 |
| Trichophyton verrucosum | 1 | 0.4 | 0.01–2.4 |
| Trichophyton violaceum | 3 | 1.3 | 0.2–3.8 |
| Trichophyton asahii | 1 | 0.4 | 0.01–2.4 |
| Non-dermatophytes | 20 | 8.8 | |
| Acremonium sp. | 1 | 0.4 | 0.01–2.4 |
| Aspergillus candidus | 1 | 0.4 | 0.01–2.4 |
| Aspergillus niger | 1 | 0.4 | 0.01–2.4 |
| Fusarium solani | 1 | 0.4 | 0.01–2.4 |
| Fusarium sp. | 12 | 5.3 | 2.7–9.2 |
| Onychocola sp. | 2 | 0.9 | 0.1–3.2 |
| Scedosporium sp. | 1 | 0.4 | 0.01–2.4 |
| Scytalidium sp. | 1 | 0.4 | 0.01–2.4 |
| Yeast | |||
| Candida albicans | 97 | 42.7 | 34.6–52.1 |
| Candida glabrata | 1 | 0.4 | 0.01–2.4 |
| Candida parapsilosis | 1 | 0.4 | 0.01–2.4 |
| Candida tropicalis | 6 | 2.6 | 0.9–5.7 |
| Candida sp. | 67 | 29.5 | 22.8–37,5 |
| Dermatophytes and Yeast | |||
| Trichophyton soudanense + Candida albicans | 2 | 0.9 | 0.1–3.2 |
| Parameters | Number | Percentage (%) | p Value |
|---|---|---|---|
| Age group | |||
| <15 years | 23 | 46.9 | 0.83 |
| >15 years | 204 | 48.6 | |
| Gender | |||
| Female | 158 | 51.1 | 0.1 |
| Male | 69 | 43.1 | |
| Season | |||
| Dry | 172 | 49 | 0.65 |
| Rainy | 55 | 46.6 |
| Results | DLSO | WSO | Onyxis | Onyxis/Perionyxis | Perionyxis | Total |
|---|---|---|---|---|---|---|
| Negative | 102 57.3% | 4 26.7% | 114 52.3% | 20 42.5% | 2 18.2% | 242 51.6% |
| Positive | 76 42.7% | 11 73.3% | 104 47.7% | 27 57.5% | 9 81.8% | 227 48.4% |
| Total | 178 100% | 15 100% | 218 100% | 47 100% | 11 100% | 469 100% |
| Total Positive | Number of C. albicans | Percentage (%) | 95% CI | Number of T. soudanense | Percentage (%) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Age group | |||||||
| <15 years | 23 | 8 | 34.8 | 15–68.5 | 4 | 17.4 | 4.7–44.5 |
| >15 years | 204 | 89 | 43.6 | 35–53.7 | 19 | 9.7 | 5.6–14.5 |
| Gender | |||||||
| Female | 158 | 71 | 45 | 35.1–56.7 | 13 | 8.2 | 4.4–14.1 |
| Male | 69 | 26 | 37.7 | 24.6–55.5 | 10 | 14.5 | 6.9–26.6 |
| Season | |||||||
| Dry | 172 | 77 | 44.8 | 35.3–56 | 17 | 10 | 5.7–15.8 |
| Rainy | 55 | 20 | 36.4 | 22.2–56.2 | 6 | 11 | 0.4–23.7 |
| Clinical Characteristic | Total Positive | Number of C. albicans | Percentage (%) | 95% CI | Number of T. soudanense | Percentage (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| DLSO | 76 | 33 | 43.4 | 30–61 | 6 | 7.9 | 2.9–17.2 |
| WSO | 11 | 1 | 9.1 | 0.2–50.6 | 1 | 9.1 | 0.2–50 |
| Onyxis | 104 | 40 | 38.5 | 27.5–52.3 | 15 | 14.4 | 8.9–23.7 |
| Perionyxis | 9 | 5 | 55.6 | 18.1–99.9 | 1 | 11.1 | 0.2–61.9 |
| Onyxis/Perionyxis | 27 | 18 | 66.7 | 39.6–99.9 | -- | -- | -- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sylla, K.; Tine, R.C.K.; Sow, D.; Lelo, S.; Dia, M.; Traoré, S.; Faye, B.; Dieng, T. Epidemiological and Mycological Aspects of Onychomycosis in Dakar (Senegal). J. Fungi 2019, 5, 35. https://doi.org/10.3390/jof5020035
Sylla K, Tine RCK, Sow D, Lelo S, Dia M, Traoré S, Faye B, Dieng T. Epidemiological and Mycological Aspects of Onychomycosis in Dakar (Senegal). Journal of Fungi. 2019; 5(2):35. https://doi.org/10.3390/jof5020035
Chicago/Turabian StyleSylla, Khadime, Roger C. K. Tine, Doudou Sow, Souleye Lelo, Mamadou Dia, Seyda Traoré, Babacar Faye, and Thérèse Dieng. 2019. "Epidemiological and Mycological Aspects of Onychomycosis in Dakar (Senegal)" Journal of Fungi 5, no. 2: 35. https://doi.org/10.3390/jof5020035
APA StyleSylla, K., Tine, R. C. K., Sow, D., Lelo, S., Dia, M., Traoré, S., Faye, B., & Dieng, T. (2019). Epidemiological and Mycological Aspects of Onychomycosis in Dakar (Senegal). Journal of Fungi, 5(2), 35. https://doi.org/10.3390/jof5020035





