Sporotrichosis In Immunocompromised Hosts
Abstract
1. Introduction
2. Epidemiology and Clinical Manifestations
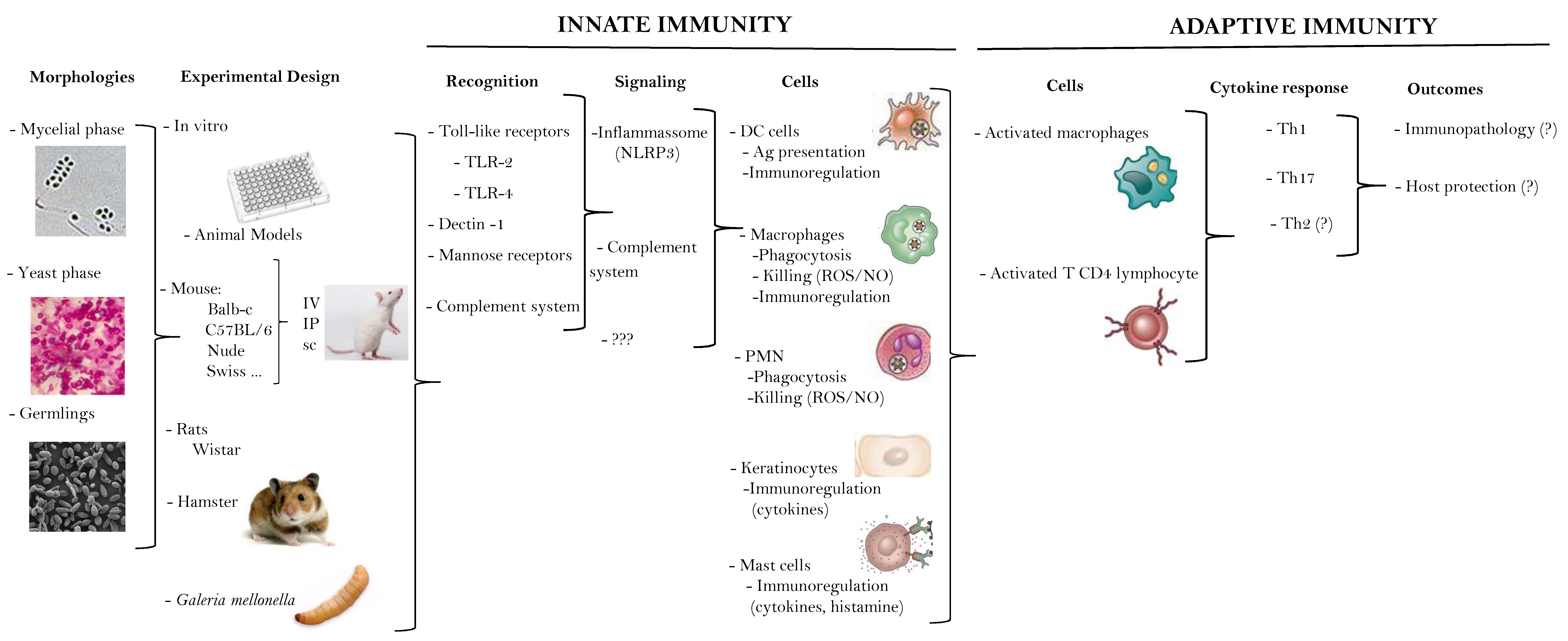
3. What We (Don´t) Know about the Immune Response in Human Sporotrichosis
4. Sporotrichosis in AIDS Patients
5. Sporotrichosis Associated with IRIS
Case Presentation
6. Comorbidities as Risk Factors for Sporotrichosis
Diabetes Mellitus and Alcoholism
7. Other Immunosuppressive Conditions
8. Laboratory Diagnosis
9. Treatment of Opportunistic Sporotrichosis
10. What Can Immunocompromised Patients with Sporotrichosis Teach Us?
11. Concluding Remarks and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.B.; de Almeida Paes, R.; Schubach, A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Telles, F.; Nucci, M.; Colombo, A.L.; Tobón, A.; Restrepo, A. Mycoses of implantation in Latin America: An overview of epidemiology, clinical manifestations, diagnosis and treatment. Med. Mycol. 2011, 49, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Aung, A.K.; Teh, B.M.; McGrath, C.; Thompson, P.J. Pulmonary sporotrichosis: Case series and systematic analysis of literature on clinico-radiological patterns and management outcomes. Med. Mycol. 2013, 51, 534–544. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix Species Causing Outbreaks in Animals and Humans Driven by Animal-Animal Transmission. PLoS Pathog. 2016, 12, e1005638. [Google Scholar] [CrossRef] [PubMed]
- Schubach, A.; Barros, M.B.; Wanke, B. Epidemic sporotrichosis. Curr. Opin. Infect. Dis. 2008, 21, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.; Menezes, R.C.; Schubach, T.M.; Figueiredo, A.B.; Cavalcanti, M.C.; Pereira, S.A. Feline sporotrichosis: Epidemiological and clinical aspects. Med. Mycol. 2015, 53, 15–21. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef]
- Fernández, N.; Iachini, R.; Farias, L.; Pozzi, N.; Tiraboschi, I. Esporotrichosis: Uma zoonosis em alerta. In Proceedings of the 13th Latin American Forum for Fungal Infections, Cordoba, Argentina, 5–7 November 2015; pp. 10–11. [Google Scholar]
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Zhang, Y.; Nino-Vega, G.; Rodrigues, A.M.; de Camargo, Z.P.; de Hoog, S. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med. Mycol. 2018, 56, 126–143. [Google Scholar] [CrossRef]
- Rios, M.E.; Suarez, J.; Moreno, J.; Vallee, J.; Moreno, J.P. Zoonotic Sporotrichosis Related to Cat Contact: First Case Report from Panama in Central America. Cureus 2018, 10, e2906. [Google Scholar] [CrossRef]
- Freitas, D.F.; de Siqueira Hoagland, B.; do Valle, A.C.; Fraga, B.B.; de Barros, M.B.; de Oliveira Schubach, A.; de Almeida-Paes, R.; Cuzzi, T.; Rosalino, C.M.; Zancope-Oliveira, R.M.; et al. Sporotrichosis in HIV-infected patients: Report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med. Mycol. 2012, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Tellez, I.; Deep, A.E.; Nolasco, D.; Holgado, W.; Bustamante, B. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin. Infect. Dis. 2000, 30, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, S.S.; Zhong, S.X.; Liu, Y.Y.; Yao, L.; Huo, S.S. Report of 457 sporotrichosis cases from Jilin province, northeast China, a serious endemic region. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.B. Disseminated cutaneous sporotrichosis in an immunocompetent individual. Int. J. Infect. Dis. 2011, 15, e727–e729. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cabello, R.; Bonifaz, A.; Romero-Feregrino, R.; Sánchez, C.J.; Linares, Y.; Zavala, J.T.; Romero, L.C.; Vega, J.T. Disseminated sporotrichosis. BMJ Case Rep. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Caligiorne, R.B.; Coutinho, D.M.; Gomes, R.R.; Rocha-Silva, F.; Machado, A.S.; Santrer, E.F.R.; Assuncao, C.B.; Guimaraes, C.F.; Laborne, M.S.; et al. A case of disseminated sporotrichosis caused by Sporothrix brasiliensis. Med. Mycol. Case Rep. 2018, 21, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.; Chander, R.; Jain, A.; Sanke, S.; Garg, T. Atypical Cutaneous Sporotrichosis in an Immunocompetent Adult: Response to Potassium Iodide. Indian J. Dermatol. 2016, 61, 236. [Google Scholar] [CrossRef]
- Hessler, C.; Kauffman, C.A.; Chow, F.C. The Upside of Bias: A Case of Chronic Meningitis Due to Sporothrix Schenckii in an Immunocompetent Host. Neurohospitalist 2017, 7, 30–34. [Google Scholar] [CrossRef]
- da Rosa, A.C.; Scroferneker, M.L.; Vettorato, R.; Gervini, R.L.; Vettorato, G.; Weber, A. Epidemiology of sporotrichosis: A study of 304 cases in Brazil. J. Am. Acad. Dermatol. 2005, 52, 451–459. [Google Scholar] [CrossRef]
- Itoh, M.; Okamoto, S.; Kariya, H. Survey of 200 cases of sporotrichosis. Dermatologica 1986, 172, 209–213. [Google Scholar] [CrossRef]
- Barros, M.B.; Schubach, A.E.O.; do Valle, A.C.; Gutierrez Galhardo, M.C.; Conceição-Silva, F.; Schubach, T.M.; Reis, R.S.; Wanke, B.; Marzochi, K.B.; Conceição, M.J. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: Description of a series of cases. Clin. Infect. Dis. 2004, 38, 529–535. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Bonfitto, M.; da Silva Junior, F.I.M.; de Ameida, M.T.G.; da Silva, R.C. Sporotrichosis in a liver transplant patient: A case report and literature review. Med. Mycol. Case Rep. 2017, 17, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Silva-Vergara, M.L.; de Camargo, Z.P.; Silva, P.F.; Abdalla, M.R.; Sgarbieri, R.N.; Rodrigues, A.M.; dos Santos, K.C.; Barata, C.H.; Ferreira-Paim, K. Disseminated Sporothrix brasiliensis infection with endocardial and ocular involvement in an HIV-infected patient. Am. J. Trop. Med. Hyg. 2012, 86, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Tiwari, S.C.; Dash, S.C.; Mehta, S.N.; Saxena, S.; Banerjee, U.; Kumar, R.; Bhunyan, U.N. Urinary sporotrichosis in a renal allograft recipient. Nephron 1994, 66, 485. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A. Sporotrichosis. Clin. Infect. Dis. 1999, 29, 231–236, quiz 237. [Google Scholar] [CrossRef] [PubMed]
- Alba-Fierro, C.A.; Pérez-Torres, A.; Toriello, C.; Pulido-Camarillo, E.; López-Romero, E.; Romo-Lozano, Y.; Gutiérrez-Sánchez, G.; Ruiz-Baca, E. Immune Response Induced by an Immunodominant 60 kDa Glycoprotein of the Cell Wall of Sporothrix schenckii in Two Mice Strains with Experimental Sporotrichosis. J. Immunol. Res. 2016, 2016, 6525831. [Google Scholar] [CrossRef] [PubMed]
- Conceição-Silva, F.; Morgado, F.N. Immunopathogenesis of Human Sporotrichosis: What We Already Know. J. Fungi 2018, 4, 89. [Google Scholar] [CrossRef]
- Castro, R.A.; Kubitschek-Barreira, P.H.; Teixeira, P.A.; Sanches, G.F.; Teixeira, M.M.; Quintella, L.P.; Almeida, S.R.; Costa, R.O.; Camargo, Z.P.; Felipe, M.S.; et al. Differences in cell morphometry, cell wall topography and gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS ONE 2013, 8, e75656. [Google Scholar] [CrossRef]
- de Almeida, J.R.F.; Jannuzzi, G.P.; Kaihami, G.H.; Breda, L.C.D.; Ferreira, K.S.; de Almeida, S.R. An immunoproteomic approach revealing peptides from Sporothrix brasiliensis that induce a cellular immune response in subcutaneous sporotrichosis. Sci. Rep. 2018, 8, 4192. [Google Scholar] [CrossRef]
- Manente, F.A.; Quinello, C.; Ferreira, L.S.; de Andrade, C.R.; Jellmayer, J.A.; Portuondo, D.L.; Batista-Duharte, A.; Carlos, I.Z. Experimental sporotrichosis in a cyclophosphamide-induced immunosuppressed mice model. Med. Mycol. 2018, 56, 711–722. [Google Scholar] [CrossRef]
- Kajiwara, H.; Saito, M.; Ohga, S.; Uenotsuchi, T.; Yoshida, S. Impaired host defense against Sporothrix schenckii in mice with chronic granulomatous disease. Infect. Immun. 2004, 72, 5073–5079. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.M.; Bulmer, G.S.; Rhoades, E.R. Phagocytosis and intracellular fate of Sporothrix schenckii. J. Infect. Dis. 1979, 140, 815–817. [Google Scholar] [CrossRef]
- Schaffner, A.; Davis, C.E.; Schaffner, T.; Markert, M.; Douglas, H.; Braude, A.I. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J. Clin. Investig. 1986, 78, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.S.; Coelho, A.L.; Lopes Bezerra, L.M.; Barja-Fidalgo, C. Virulence of Sporothrix schenckii conidia and yeast cells, and their susceptibility to nitric oxide. Immunology 2000, 101, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.S.; Neto, E.H.; Brito, M.M.; Silva, J.S.; Cunha, F.Q.; Barja-Fidalgo, C. Detrimental role of endogenous nitric oxide in host defence against Sporothrix schenckii. Immunology 2008, 123, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.N.; Schubach, A.O.; Barros, M.B.; Conceição-Silva, F. The in situ inflammatory profile of lymphocutaneous and fixed forms of human sporotrichosis. Med. Mycol. 2011, 49, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.N.; de Carvalho, L.M.V.; Leite-Silva, J.; Seba, A.J.; Pimentel, M.I.F.; Fagundes, A.; Madeira, M.F.; Lyra, M.R.; Oliveira, M.M.; Schubach, A.O.; et al. Unbalanced inflammatory reaction could increase tissue destruction and worsen skin infectious diseases—A comparative study of leishmaniasis and sporotrichosis. Sci. Rep. 2018, 8, 2898. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Beltran, S.; Perez-Torres, A.; Coronel-Cruz, C.; Torres-Guerrero, H. Phagocytic receptors on macrophages distinguish between different Sporothrix schenckii morphotypes. Microbes Infect. 2012, 14, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Madrid, I.M.; Xavier, M.O.; Mattei, A.S.; Fernandes, C.G.; Guim, T.N.; Santin, R.; Schuch, L.F.; Nobre, M.e.O.; Araújo Meireles, M.C. Role of melanin in the pathogenesis of cutaneous sporotrichosis. Microbes Infect. 2010, 12, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Verdan, F.F.; Faleiros, J.C.; Ferreira, L.S.; Monnazzi, L.G.; Maia, D.C.; Tansine, A.; Placeres, M.C.; Carlos, I.Z.; Santos-Junior, R.R. Dendritic cell are able to differentially recognize Sporothrix schenckii antigens and promote Th1/Th17 response in vitro. Immunobiology 2012, 217, 788–794. [Google Scholar] [CrossRef]
- Goncalves, A.C.; Maia, D.C.; Ferreira, L.S.; Monnazzi, L.G.; Alegranci, P.; Placeres, M.C.; Batista-Duharte, A.; Carlos, I.Z. Involvement of major components from Sporothrix schenckii cell wall in the caspase-1 activation, nitric oxide and cytokines production during experimental sporotrichosis. Mycopathologia 2015, 179, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.S.; Goncalves, A.C.; Portuondo, D.L.; Maia, D.C.; Placeres, M.C.; Batista-Duharte, A.; Carlos, I.Z. Optimal clearance of Sporothrix schenckii requires an intact Th17 response in a mouse model of systemic infection. Immunobiology 2015, 220, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Romo-Lozano, Y.; Hernandez-Hernandez, F.; Salinas, E. Sporothrix schenckii yeasts induce ERK pathway activation and secretion of IL-6 and TNF-alpha in rat mast cells, but no degranulation. Med. Mycol. 2014, 52, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Romo-Lozano, Y.; Hernandez-Hernandez, F.; Salinas, E. Mast cell activation by conidia of Sporothrix schenckii: Role in the severity of infection. Scand. J. Immunol. 2012, 76, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sassa, M.F.; Saturi, A.E.; Souza, L.F.; Ribeiro, L.C.; Sgarbi, D.B.; Carlos, I.Z. Response of macrophage Toll-like receptor 4 to a Sporothrix schenckii lipid extract during experimental sporotrichosis. Immunology 2009, 128, 301–309. [Google Scholar] [CrossRef]
- de, C.N.T.; Ferreira, L.S.; Arthur, R.A.; Alegranci, P.; Placeres, M.C.; Spolidorio, L.C.; Carlos, I.Z. Influence of TLR-2 in the immune response in the infection induced by fungus Sporothrix schenckii. Immunol. Investig. 2014, 43, 370–390. [Google Scholar] [CrossRef]
- Negrini Tde, C.; Ferreira, L.S.; Alegranci, P.; Arthur, R.A.; Sundfeld, P.P.; Maia, D.C.; Spolidorio, L.C.; Carlos, I.Z. Role of TLR-2 and fungal surface antigens on innate immune response against Sporothrix schenckii. Immunol. Investig. 2013, 42, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, Q.; Sun, J.; Shen, Y.; Liu, W. Inflammatory response of human keratinocytes triggered by Sporothrix schenckii via Toll-like receptor 2 and 4. J. Dermatol. Sci. 2012, 66, 80–82. [Google Scholar] [CrossRef]
- Jellmayer, J.A.; Ferreira, L.S.; Manente, F.A.; Goncalves, A.C.; Polesi, M.C.; Batista-Duharte, A.; Carlos, I.Z. Dectin-1 expression by macrophages and related antifungal mechanisms in a murine model of Sporothrix schenckii sensu stricto systemic infection. Microb. Pathog. 2017, 110, 78–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Lv, X.; Lin, J. Variation in genotype and higher virulence of a strain of Sporothrix schenckii causing disseminated cutaneous sporotrichosis. Mycopathologia 2011, 172, 439–446. [Google Scholar] [CrossRef]
- Goncalves, A.C.; Ferreira, L.S.; Manente, F.A.; de Faria, C.; Polesi, M.C.; de Andrade, C.R.; Zamboni, D.S.; Carlos, I.Z. The NLRP3 inflammasome contributes to host protection during Sporothrix schenckii infection. Immunology 2017, 151, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.C.; Gonçalves, A.C.; Ferreira, L.S.; Manente, F.A.; Portuondo, D.L.; Vellosa, J.C.; Polesi, M.C.; Batista-Duharte, A.; Carlos, I.Z. Response of Cytokines and Hydrogen Peroxide to Sporothrix schenckii Exoantigen in Systemic Experimental Infection. Mycopathologia 2016, 181, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.N.; Muchmore, H.G.; Fine, D.P. Activation of the alternative complement pathway by Sporothrix schenckii. Infect. Immun. 1986, 51, 6–9. [Google Scholar] [PubMed]
- Arrillaga-Moncrieff, I.; Capilla, J.; Mayayo, E.; Marimon, R.; Mariné, M.; Gené, J.; Cano, J.; Guarro, J. Different virulence levels of the species of Sporothrix in a murine model. Clin. Microbiol. Infect. 2009, 15, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Paes, R.; Bailao, A.M.; Pizzini, C.V.; Reis, R.S.; Soares, C.M.; Peralta, J.M.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M. Cell-free antigens of Sporothrix brasiliensis: Antigenic diversity and application in an immunoblot assay. Mycoses 2012, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Paes, R.; de Oliveira, L.C.; Oliveira, M.M.; Gutierrez-Galhardo, M.C.; Nosanchuk, J.D.; Zancope-Oliveira, R.M. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. Biomed. Res. Int. 2015, 2015, 212308. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.; León-Navarro, I.; Rodríguez-Brito, S.; Mendoza, M.; Niño-Vega, G.A. Molecular epidemiology of human sporotrichosis in Venezuela reveals high frequency of Sporothrix globosa. BMC Infect. Dis. 2015, 15, 94. [Google Scholar] [CrossRef]
- Teixeira, P.A.; de Castro, R.A.; Nascimento, R.C.; Tronchin, G.; Torres, A.P.; Lazéra, M.; de Almeida, S.R.; Bouchara, J.P.; Loureiro y Penha, C.V.; Lopes-Bezerra, L.M. Cell surface expression of adhesins for fibronectin correlates with virulence in Sporothrix schenckii. Microbiology 2009, 155, 3730–3738. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.A.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Martínez-Duncker, I.; Lópes-Bezerra, L.M.; Mora-Montes, H.M. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis Are Differentially Recognized by Human Peripheral Blood Mononuclear Cells. Front. Microbiol. 2017, 8, 843. [Google Scholar] [CrossRef]
- Shimonaka, H.; Noguchi, T.; Kawai, K.; Hasegawa, I.; Nozawa, Y.; Ito, Y. Immunochemical studies on the human pathogen Sporothrix schenckii: Effects of chemical and enzymatic modification of the antigenic compounds upon immediate and delayed reactions. Infect. Immun. 1975, 11, 1187–1194. [Google Scholar]
- Shiraishi, A.; Nakagaki, K.; Arai, T. Experimental sporotrichosis in congenitally athymic (nude) mice. J. Reticuloendothel. Soc. 1979, 26, 333–336. [Google Scholar]
- Shiraishi, A.; Nakagaki, K.; Arai, T. Role of cell-mediated immunity in the resistance to experimental sporotrichosis in mice. Mycopathologia 1992, 120, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Matsuyama, T.; Mitsuyama, M. Involvement of CD4+ T cells and macrophages in acquired protection against infection with Sporothrix schenckii in mice. Med. Mycol. 1999, 37, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, C.L.; Taylor, R.L.; Drutz, D.J. Susceptibility of congenitally athymic (nude) mice to sporotrichosis. Infect. Immun. 1983, 40, 417–420. [Google Scholar] [PubMed]
- Carlos, I.Z.; Sgarbi, D.B.; Angluster, J.; Alviano, C.S.; Silva, C.L. Detection of cellular immunity with the soluble antigen of the fungus Sporothrix schenckii in the systemic form of the disease. Mycopathologia 1992, 117, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Carlos, I.Z.; Sgarbi, D.B.; Placeres, M.C. Host organism defense by a peptide-polysaccharide extracted from the fungus Sporothrix schenckii. Mycopathologia 1998, 144, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Alegranci, P.; de Abreu Ribeiro, L.C.; Ferreira, L.S.; Negrini Tde, C.; Maia, D.C.; Tansini, A.; Goncalves, A.C.; Placeres, M.C.; Carlos, I.Z. The predominance of alternatively activated macrophages following challenge with cell wall peptide-polysaccharide after prior infection with Sporothrix schenckii. Mycopathologia 2013, 176, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Uenotsuchi, T.; Takeuchi, S.; Matsuda, T.; Urabe, K.; Koga, T.; Uchi, H.; Nakahara, T.; Fukagawa, S.; Kawasaki, M.; Kajiwara, H.; et al. Differential induction of Th1-prone immunity by human dendritic cells activated with Sporothrix schenckii of cutaneous and visceral origins to determine their different virulence. Int. Immunol. 2006, 18, 1637–1646. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Pimenta, M.A.; Pizzini, C.V.; Monteiro, P.C.; Peralta, J.M.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin. Vaccine Immunol. 2007, 14, 244–249. [Google Scholar] [CrossRef]
- Portuondo, D.L.; Batista-Duharte, A.; Ferreira, L.S.; Martínez, D.T.; Polesi, M.C.; Duarte, R.A.; de Paula E Silva, A.C.; Marcos, C.M.; Almeida, A.M.; Carlos, I.Z. A cell wall protein-based vaccine candidate induce protective immune response against Sporothrix schenckii infection. Immunobiology 2016, 221, 300–309. [Google Scholar] [CrossRef]
- Nascimento, R.C.; Espíndola, N.M.; Castro, R.A.; Teixeira, P.A.; Loureiro y Penha, C.V.; Lopes-Bezerra, L.M.; Almeida, S.R. Passive immunization with monoclonal antibody against a 70-kDa putative adhesin of Sporothrix schenckii induces protection in murine sporotrichosis. Eur. J. Immunol. 2008, 38, 3080–3089. [Google Scholar] [CrossRef] [PubMed]
- Franco Dde, L.; Nascimento, R.C.; Ferreira, K.S.; Almeida, S.R. Antibodies Against Sporothrix schenckii Enhance TNF-alpha Production and Killing by Macrophages. Scand. J. Immunol. 2012, 75, 142–146. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.R.; Kaihami, G.H.; Jannuzzi, G.P.; de Almeida, S.R. Therapeutic vaccine using a monoclonal antibody against a 70-kDa glycoprotein in mice infected with highly virulent Sporothrix schenckii and Sporothrix brasiliensis. Med. Mycol. 2015, 53, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Xu, X.G.; Zhang, M.; Jiang, P.; Zhou, X.Y.; Li, Z.Z.; Zhang, M.F. Sporotrichosis: Clinical and histopathological manifestations. Am. J. Dermatopathol. 2011, 33, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Quintella, L.P.; Passos, S.R.; do Vale, A.C.; Galhardo, M.C.; Barros, M.B.; Cuzzi, T.; Reis, R.O.S.; de Carvalho, M.H.; Zappa, M.B.; Schubach, A.E.O. Histopathology of cutaneous sporotrichosis in Rio de Janeiro: A series of 119 consecutive cases. J. Cutan. Pathol. 2011, 38, 25–32. [Google Scholar] [CrossRef]
- Morgado, F.N.; Schubach, A.O.; Pimentel, M.I.; Lyra, M.R.; Vasconcellos, É.; Valete-Rosalino, C.M.; Conceição-Silva, F. Is There Any Difference between the In Situ and Systemic IL-10 and IFN-γ Production when Clinical Forms of Cutaneous Sporotrichosis Are Compared? PLoS ONE 2016, 11, e0162764. [Google Scholar] [CrossRef]
- Plouffe, J.F.; Silva, J.; Fekety, R.; Reinhalter, E.; Browne, R. Cell-mediated immune responses in sporotrichosis. J. Infect. Dis. 1979, 139, 152–157. [Google Scholar] [CrossRef]
- Mialski, R.; de Oliveira, J.N., Jr.; da Silva, L.H.; Kono, A.; Pinheiro, R.L.; Teixeira, M.J.; Gomes, R.R.; de Queiroz-Telles, F.; Pinto, F.G.; Benard, G. Chronic Meningitis and Hydrocephalus due to Sporothrix brasiliensis in Immunocompetent Adults: A Challenging Entity. Open Forum Infect. Dis. 2018, 5, ofy081. [Google Scholar] [CrossRef]
- Lynch, P.J.; Voorhees, J.J.; Harrell, E.R. Systemic sporotrichosis. Ann. Intern. Med. 1970, 73, 23–30. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; de Oliveira, M.M.; Freitas, D.F.; do Valle, A.C.; Zancopé-Oliveira, R.M.; Gutierrez-Galhardo, M.C. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl. Trop. Dis. 2014, 8, e3094. [Google Scholar] [CrossRef]
- Lipstein-Kresch, E.; Isenberg, H.D.; Singer, C.; Cooke, O.; Greenwald, R.A. Disseminated Sporothrix schenckii infection with arthritis in a patient with acquired immunodeficiency syndrome. J. Rheumatol. 1985, 12, 805–808. [Google Scholar] [PubMed]
- Freitas, D.F.; Valle, A.C.; da Silva, M.B.; Campos, D.P.; Lyra, M.R.; de Souza, R.V.; Veloso, V.G.; Zancopé-Oliveira, R.M.; Bastos, F.I.; Galhardo, M.C. Sporotrichosis: An emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3110. [Google Scholar] [CrossRef]
- Moreira, J.A.; Freitas, D.F.; Lamas, C.C. The impact of sporotrichosis in HIV-infected patients: A systematic review. Infection 2015, 43, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Donabedian, H.; O'Donnell, E.; Olszewski, C.; MacArthur, R.D.; Budd, N. Disseminated cutaneous and meningeal sporotrichosis in an AIDS patient. Diagn. Microbiol. Infect. Dis. 1994, 18, 111–115. [Google Scholar] [CrossRef]
- Paixão, A.G.; Galhardo, M.C.G.; Almeida-Paes, R.; Nunes, E.P.; Gonçalves, M.L.C.; Chequer, G.L.; Lamas, C.D.C. The difficult management of disseminated Sporothrix brasiliensis in a patient with advanced AIDS. AIDS Res. Ther. 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.C.; Goldstein, E.; Bartholomew, W.R. Sporothrix schenckii meningitis in a patient with AIDS. Clin. Infect. Dis. 1992, 15, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Hardman, S.; Stephenson, I.; Jenkins, D.R.; Wiselka, M.J.; Johnson, E.M. Disseminated Sporothix schenckii in a patient with AIDS. J. Infect. 2005, 51, e73–e77. [Google Scholar] [CrossRef]
- Dong, J.A.; Chren, M.M.; Elewski, B.E. Bonsai tree: Risk factor for disseminated sporotrichosis. J. Am. Acad. Dermatol. 1995, 33, 839–840. [Google Scholar] [CrossRef]
- Silva-Vergara, M.L.; Maneira, F.R.; De Oliveira, R.M.; Santos, C.T.; Etchebehere, R.M.; Adad, S.J. Multifocal sporotrichosis with meningeal involvement in a patient with AIDS. Med. Mycol. 2005, 43, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Vilela, R.; Souza, G.F.; Fernandes Cota, G.; Mendoza, L. Cutaneous and meningeal sporotrichosis in a HIV patient. Rev. Iberoam. Micol. 2007, 24, 161–163. [Google Scholar] [CrossRef]
- Galhardo, M.C.; Silva, M.T.; Lima, M.A.; Nunes, E.P.; Schettini, L.E.; de Freitas, R.F.; Paes Rde, A.; Neves Ede, S.; do Valle, A.C. Sporothrix schenckii meningitis in AIDS during immune reconstitution syndrome. J. Neurol. Neurosurg. Psychiatry 2010, 81, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Rotz, L.D.; Slater, L.N.; Wack, M.F.; Boyd, A.L.; Nan, S.E.; Greenfield, R.A. Disseminated Sporotrichosis with Meningitis in a Patient with AIDS. Infect. Dis. Clin. Pract. 1996, 5, 566–568. [Google Scholar] [CrossRef]
- Callens, S.F.; Kitetele, F.; Lukun, P.; Lelo, P.; Van Rie, A.; Behets, F.; Colebunders, R. Pulmonary Sporothrix schenckii infection in a HIV positive child. J. Trop. Pediatr. 2006, 52, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Losman, J.A.; Cavanaugh, K. Cases from the Osler Medical Service at Johns Hopkins University. Diagnosis: P. carinii pneumonia and primary pulmonary sporotrichosis. Am. J. Med. 2004, 117, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Lupetti, A.; Moscato, G.; Parenti, M.; Lofaro, A. Pulmonary sporotrichosis with hyphae in a human immunodeficiency virus-infected patient. A case report. Acta Cytol. 1997, 41, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Reves, R. Invasive sinusitis due to Sporothrix schenckii in a patient with AIDS. Clin. Infect. Dis. 1996, 23, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, B.; Lama, J.R.; Mosquera, C.; Soto, L. Sporotrichosis in Human Immunodeficiency Virus Infected Peruvian Patients: Two Case Reports and Literature Review. Infect. Dis. Clin. Pract. 2009, 17, 78–83. [Google Scholar] [CrossRef]
- Buccheri, R.; Benard, G. Opinion: Paracoccidioidomycosis and HIV Immune Recovery Inflammatory Syndrome. Mycopathologia 2018, 183, 495–498. [Google Scholar] [CrossRef]
- Haddow, L.J.; Colebunders, R.; Meintjes, G.; Lawn, S.D.; Elliott, J.H.; Manabe, Y.C.; Bohjanen, P.R.; Sungkanuparph, S.; Easterbrook, P.J.; French, M.A.; et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: Proposed clinical case definitions. Lancet Infect. Dis. 2010, 10, 791–802. [Google Scholar] [CrossRef]
- Singh, N.; Perfect, J.R. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect. Dis. 2007, 7, 395–401. [Google Scholar] [CrossRef]
- Lyra, M.R.; Nascimento, M.L.; Varon, A.G.; Pimentel, M.I.; Antonio, L.E.F.; Saheki, M.N.; Bedoya-Pacheco, S.J.; Valle, A.C. Immune reconstitution inflammatory syndrome in HIV and sporotrichosis coinfection: report of two cases and review of the literature. Rev. Soc. Bras. Med. Trop. 2014, 47, 806–809. [Google Scholar] [CrossRef] [PubMed]
- de Lima Barros, M.B.; de Oliveira Schubach, A.; Galhardo, M.C.; Schubach, T.M.; dos Reis, R.S.; Conceição, M.J.; do Valle, A.C. Sporotrichosis with widespread cutaneous lesions: Report of 24 cases related to transmission by domestic cats in Rio de Janeiro, Brazil. Int. J. Dermatol. 2003, 42, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.M.; Peasley, E.D.; Crymes, T.P. Pulmonary sporotrichosis. Report of two cases with cavitation. N. Engl. J. Med. 1961, 265, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Mohr, J.A.; Patterson, C.D.; Eaton, B.G.; Rhoades, E.R.; Nichols, N.B. Primary pulmonary sporotrichosis. Am. Rev. Respir. Dis. 1972, 106, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hagen, F.; Wan, Z.; Liu, Y.; Wang, Q.; de Hoog, G.S.; Li, R.; Zhang, J. Two cases of sporotrichosis of the right upper extremity in right-handed patients with diabetes mellitus. Rev. Iberoam. Micol. 2016, 33, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Badrin, S.; Wan Abdullah, W.N.H. A Diabetic Elderly Man with Finger Ulcer. Korean J. Fam. Med. 2018, 39, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Soto, M.; Lizarraga-Trujillo, J. Granulomatous sporotrichosis: Report of two unusual cases. Rev. Chil. Infectol. 2013, 30, 548–553. [Google Scholar] [CrossRef]
- Nassif, P.W.; Granado, I.R.; Ferraz, J.S.; Souza, R.; Nassif, A.E. Atypical presentation of cutaneous sporotrichosis in an alcoholic patient. Dermatol. Online J. 2012, 18, 12. [Google Scholar]
- Castrejon, O.V.; Robles, M.; Zubieta Arroyo, O.E. Fatal fungaemia due to Sporothrix schenckii. Mycoses 1995, 38, 373–376. [Google Scholar] [CrossRef]
- Solorzano, S.; Ramirez, R.; Cabada, M.M.; Montoya, M.; Cazorla, E. Disseminated cutaneous sporotrichosis with joint involvement in a woman with type 2 diabetes. Rev. Peru. Med. Exp. Salud Publica 2015, 32, 187–190. [Google Scholar] [CrossRef]
- Agger, W.A.; Caplan, R.H.; Maki, D.G. Ocular sporotrichosis mimicking mucormycosis in a diabetic. Ann. Ophthalmol. 1978, 10, 767–771. [Google Scholar] [PubMed]
- Benvegnu, A.M.; Stramari, J.; Dallazem, L.N.D.; Chemello, R.M.L.; Beber, A.A.C. Disseminated cutaneous sporotrichosis in patient with alcoholism. Rev. Soc. Bras. Med. Trop. 2017, 50, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Ito, S.; Takano, Y.; Umeda, N.; Goto, M.; Horikoshi, M.; Hayashi, T.; Goto, D.; Matsumoto, I.; Sumida, T. A case of disseminated sporotrichosis treated with prednisolone, immunosuppressants, and tocilizumab under the diagnosis of rheumatoid arthritis. Intern. Med. 2012, 51, 2035–2039. [Google Scholar] [CrossRef]
- Yang, D.J.; Krishnan, R.S.; Guillen, D.R.; Schmiege, L.M.; Leis, P.F.; Hsu, S. Disseminated sporotrichosis mimicking sarcoidosis. Int. J. Dermatol. 2006, 45, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Mauermann, M.L.; Klein, C.J.; Orenstein, R.; Dyck, P.J. Disseminated sporotrichosis presenting with granulomatous inflammatory multiple mononeuropathies. Muscle Nerve 2007, 36, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.R.; El-Azhary, R.A.; Gibson, L.E.; Roberts, G.D. Sporotrichosis masquerading as pyoderma gangrenosum: Case report and review of 19 cases of sporotrichosis. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Russo, E.; Leporini, C.; Calabria, M.; Bruno, C.; Tripolino, C.; Naty, S.; Grembiale, R.D. Lymphocutaneous Sporotrichosis during Treatment with Anti-TNF-Alpha Monotherapy. Case Rep. Rheumatol. 2015, 2015, 614504. [Google Scholar] [CrossRef]
- Tochigi, M.; Ochiai, T.; Mekata, C.; Nishiyama, H.; Anzawa, K.; Kawasaki, M. Sporotrichosis of the face by autoinoculation in a patient undergoing tacrolimus treatment. J. Dermatol. 2012, 39, 796–798. [Google Scholar] [CrossRef]
- Severo, L.C.; Festugato, M.; Bernardi, C.; Londero, A.T. Widespread cutaneous lesions due to Sporothrix schenckii in a patient under a long-term steroids therapy. Rev. Inst. Med. Trop. Sao Paulo 1999, 41, 59–62. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Guimaraes, L.F.; Halpern, M.; de Lemos, A.S.; de Gouvea, E.F.; Goncalves, R.T.; da Rosa Santos, M.A.; Nucci, M.; Santoro-Lopes, G. Invasive Fungal Disease in Renal Transplant Recipients at a Brazilian Center: Local Epidemiology Matters. Transplant. Proc. 2016, 48, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Caroti, L.; Zanazzi, M.; Rogasi, P.; Fantoni, E.; Farsetti, S.; Rosso, G.; Bertoni, E.; Salvadori, M. Subcutaneous nodules and infectious complications in renal allograft recipients. Transplant. Proc. 2010, 42, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.H.; Jha, R.; Narayan, G.; Sinha, S. Opportunistic infections following renal transplantation. Indian J. Med. Microbiol. 2002, 20, 47–49. [Google Scholar]
- Gewehr, P.; Jung, B.; Aquino, V.; Manfro, R.C.; Spuldaro, F.; Rosa, R.G.; Goldani, L.Z. Sporotrichosis in renal transplant patients. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, e47–e49. [Google Scholar] [CrossRef] [PubMed]
- Bahr, N.C.; Janssen, K.; Billings, J.; Loor, G.; Green, J.S. Respiratory Failure due to Possible Donor-Derived Sporothrix schenckii Infection in a Lung Transplant Recipient. Case Rep. Infect. Dis. 2015, 2015, 925718. [Google Scholar] [CrossRef] [PubMed]
- Ewing, G.E.; Bosl, G.J.; Peterson, P.K. Sporothrix schenckii meningitis in a farmer with Hodgkin’s disease. Am. J. Med. 1980, 68, 455–457. [Google Scholar] [CrossRef]
- Bunce, P.E.; Yang, L.; Chun, S.; Zhang, S.X.; Trinkaus, M.A.; Matukas, L.M. Disseminated sporotrichosis in a patient with hairy cell leukemia treated with amphotericin B and posaconazole. Med. Mycol. 2012, 50, 197–201. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Gourley, W.K.; Alperin, J.B. Sporotrichosis as a presenting manifestation of hairy cell leukemia. Am. J. Hematol. 1994, 46, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, K.J.; Skelton, W.P.T.; Olson, A.; Trillo, C.A.; Lascano, J. A rare case of disseminated Sporothrix schenckii with bone marrow involvement in a patient with idiopathic CD4 lymphocytopenia. IDCases 2017, 9, 70–72. [Google Scholar] [CrossRef]
- Trotter, J.R.; Sriaroon, P.; Berman, D.; Petrovic, A.; Leiding, J.W. Sporothrix schenckii lymphadentitis in a male with X-linked chronic granulomatous disease. J. Clin. Immunol. 2014, 34, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, R.M.; Quintanilla, A.; Levin, M.L.; Williams, J.; Phair, J.P. Sporotrichosis: Recurrent cutaneous, articular, and central nervous system infection in a renal transplant recipient. Rev. Infect. Dis. 1987, 9, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Macedo, P.M.; Rodrigues, A.M.; Bernardes-Engemann, A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Sanchotene, K.O.; Madrid, I.M.; Klafke, G.B.; Bergamashi, M.; Della Terra, P.P.; Rodrigues, A.M.; de Camargo, Z.P.; Xavier, M.O. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses 2015, 58, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Tirado-Sánchez, A. Cutaneous Disseminated and Extracutaneous Sporotrichosis: Current Status of a Complex Disease. J. Fungi 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Sanchez, A.; Bonifaz, A. Nodular Lymphangitis (Sporotrichoid Lymphocutaneous Infections). Clues to Differential Diagnosis. J. Fungi 2018, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Bernardes-Engemann, A.R.; de Lima Barros, M.; Zeitune, T.; Russi, D.C.; Orofino-Costa, R.; Lopes-Bezerra, L.M. Validation of a serodiagnostic test for sporotrichosis: a follow-up study of patients related to the Rio de Janeiro zoonotic outbreak. Med. Mycol. 2015, 53, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ottonelli Stopiglia, C.D.; Magagnin, C.M.; Castrillon, M.R.; Mendes, S.D.; Heidrich, D.; Valente, P.; Scroferneker, M.L. Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med. Mycol. 2014, 52, 56–64. [Google Scholar] [CrossRef]
- Brilhante, R.S.; Rodrigues, A.M.; Sidrim, J.J.; Rocha, M.F.; Pereira, S.A.; Gremiao, I.D.; Schubach, T.M.; de Camargo, Z.P. In vitro susceptibility of antifungal drugs against Sporothrix brasiliensis recovered from cats with sporotrichosis in Brazil. Med. Mycol. 2016, 54, 275–279. [Google Scholar] [CrossRef]
- Kauffman, C.A.; Bustamante, B.; Chapman, S.W.; Pappas, P.G. Infectious Diseases Society of America. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 1255–1265. [Google Scholar] [CrossRef]
- de Lima Barros, M.B.; Schubach, A.O.; de Vasconcellos Carvalhaes de Oliveira, R.; Martins, E.B.; Teixeira, J.L.; Wanke, B. Treatment of cutaneous sporotrichosis with itraconazole—Study of 645 patients. Clin. Infect. Dis. 2011, 52, e200–e206. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; Valle, A.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Torok, M.E.; Yen, N.T.; Chau, T.T.; Mai, N.T.; Phu, N.H.; Mai, P.P.; Dung, N.T.; Chau, N.V.; Bang, N.D.; Tien, N.A.; et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)—Associated tuberculous meningitis. Clin. Infect. Dis. 2011, 52, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Meya, D.B.; Muzoora, C.; Rolfes, M.A.; Huppler Hullsiek, K.; Musubire, A.; Taseera, K.; Nabeta, H.W.; Schutz, C.; Williams, D.A.; et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 2014, 370, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Benson, C.; Holmes, K.K.; Brooks, J.T.; Pau, A.; Masur, H.; CDC; National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm. Rep. 2009, 58, 1–207, quiz CE201-204. [Google Scholar] [PubMed]
- Benard, G.; Duarte, A.J. Paracoccidioidomycosis: A model for evaluation of the effects of human immunodeficiency virus infection on the natural history of endemic tropical diseases. Clin. Infect. Dis. 2000, 31, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Hajjeh, R.A. Disseminated histoplasmosis in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 1995, 21 (Suppl. 1), S108–S110. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N. Coccidioidomycosis: Changes in clinical expression, serological diagnosis, and therapeutic options. Clin. Infect. Dis. 1992, 14 (Suppl. 1), S100–S105. [Google Scholar] [CrossRef]
- Szabo, G.; Saha, B. Alcohol’s Effect on Host Defense. Alcohol Res. 2015, 37, 159–170. [Google Scholar]
- Barr, T.; Helms, C.; Grant, K.; Messaoudi, I. Opposing effects of alcohol on the immune system. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 242–251. [Google Scholar] [CrossRef]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015, 37, 185–197. [Google Scholar]
- Pan, B.; Chen, M.; Pan, W.; Liao, W. Histoplasmosis: A new endemic fungal infection in China? Review and analysis of cases. Mycoses 2013, 56, 212–221. [Google Scholar] [CrossRef]
- Santelli, A.C.; Blair, J.E.; Roust, L.R. Coccidioidomycosis in patients with diabetes mellitus. Am. J. Med. 2006, 119, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Lemos, L.B.; Baliga, M.; Guo, M. Blastomycosis: The great pretender can also be an opportunist. Initial clinical diagnosis and underlying diseases in 123 patients. Ann. Diagn. Pathol. 2002, 6, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, B.I.; Schlesinger, L.S. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis 2013, 93, S10–S14. [Google Scholar] [CrossRef]
- Martinez, N.; Kornfeld, H. Diabetes and immunity to tuberculosis. Eur. J. Immunol. 2014, 44, 617–626. [Google Scholar] [CrossRef]
- Kumar Nathella, P.; Babu, S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017, 152, 13–24. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz-Telles, F.; Buccheri, R.; Benard, G. Sporotrichosis In Immunocompromised Hosts. J. Fungi 2019, 5, 8. https://doi.org/10.3390/jof5010008
Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis In Immunocompromised Hosts. Journal of Fungi. 2019; 5(1):8. https://doi.org/10.3390/jof5010008
Chicago/Turabian StyleQueiroz-Telles, Flavio, Renata Buccheri, and Gil Benard. 2019. "Sporotrichosis In Immunocompromised Hosts" Journal of Fungi 5, no. 1: 8. https://doi.org/10.3390/jof5010008
APA StyleQueiroz-Telles, F., Buccheri, R., & Benard, G. (2019). Sporotrichosis In Immunocompromised Hosts. Journal of Fungi, 5(1), 8. https://doi.org/10.3390/jof5010008




