Global Epidemiology of Mucormycosis
Abstract
:1. Introduction
2. Incidence of Mucormycosis
2.1. Population or Hospital-Based Estimates
2.2. Estimated Incidence
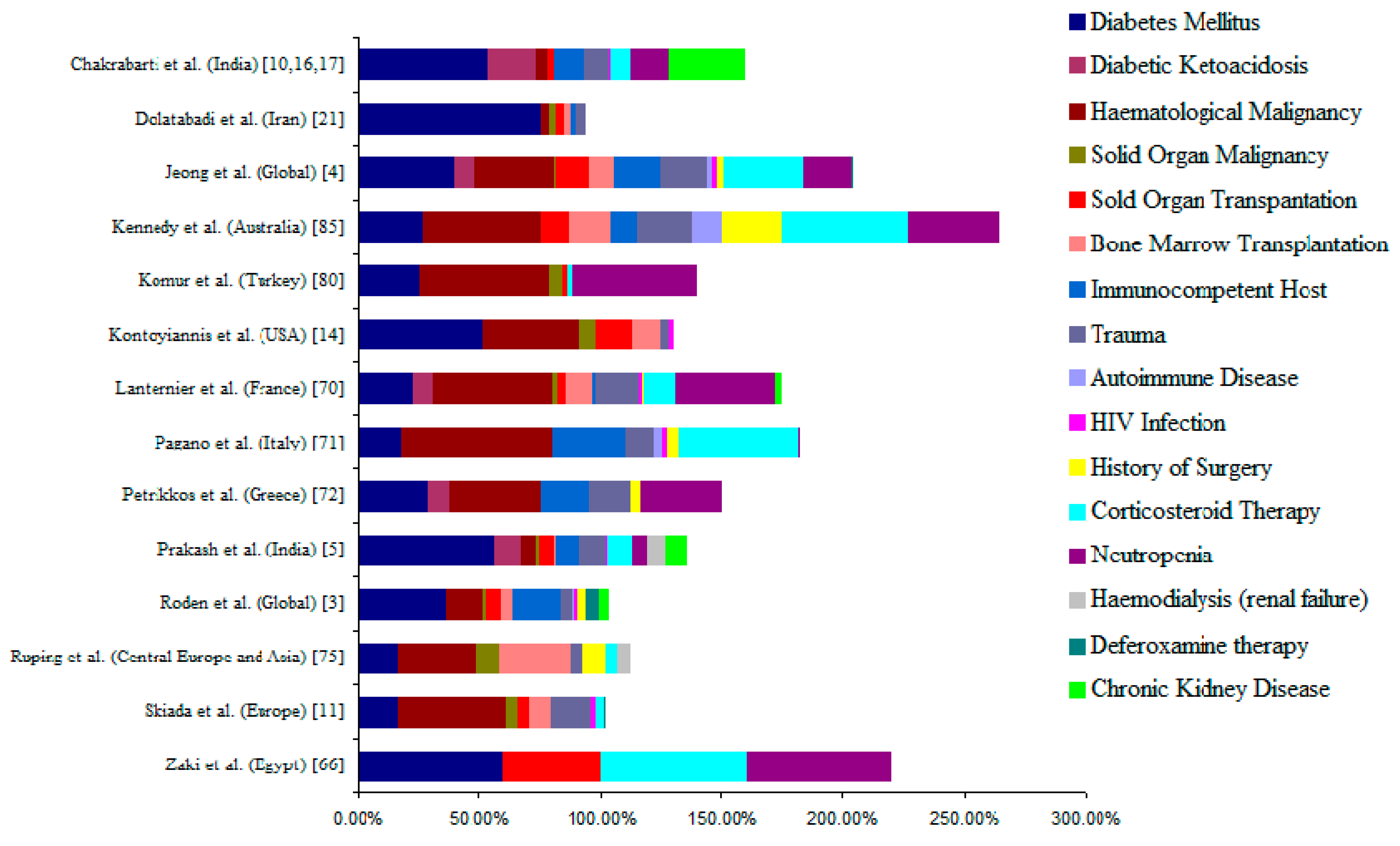
3. Underlying Disease/Predisposing Factors
4. Healthcare Associated Mucormycosis
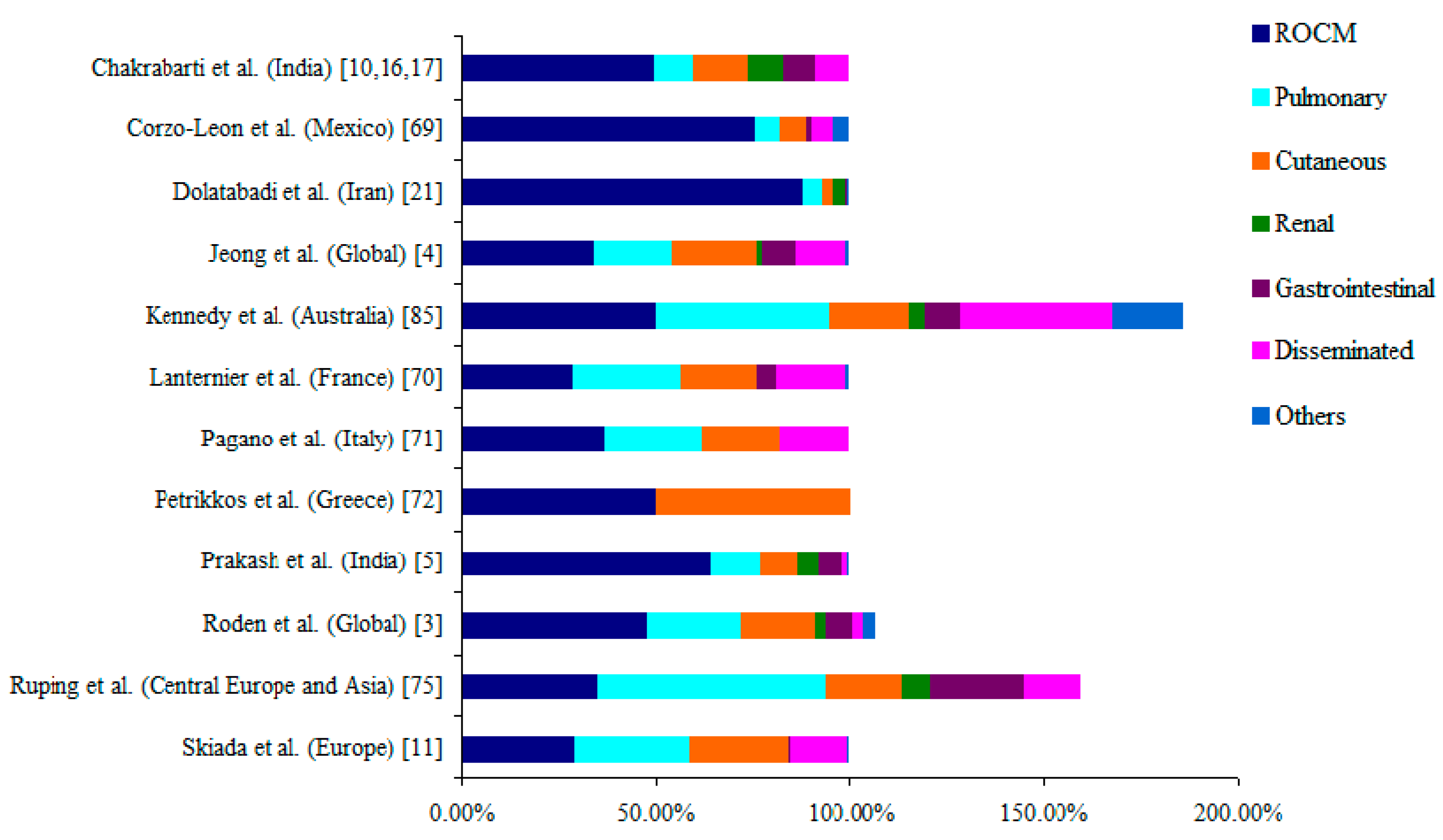
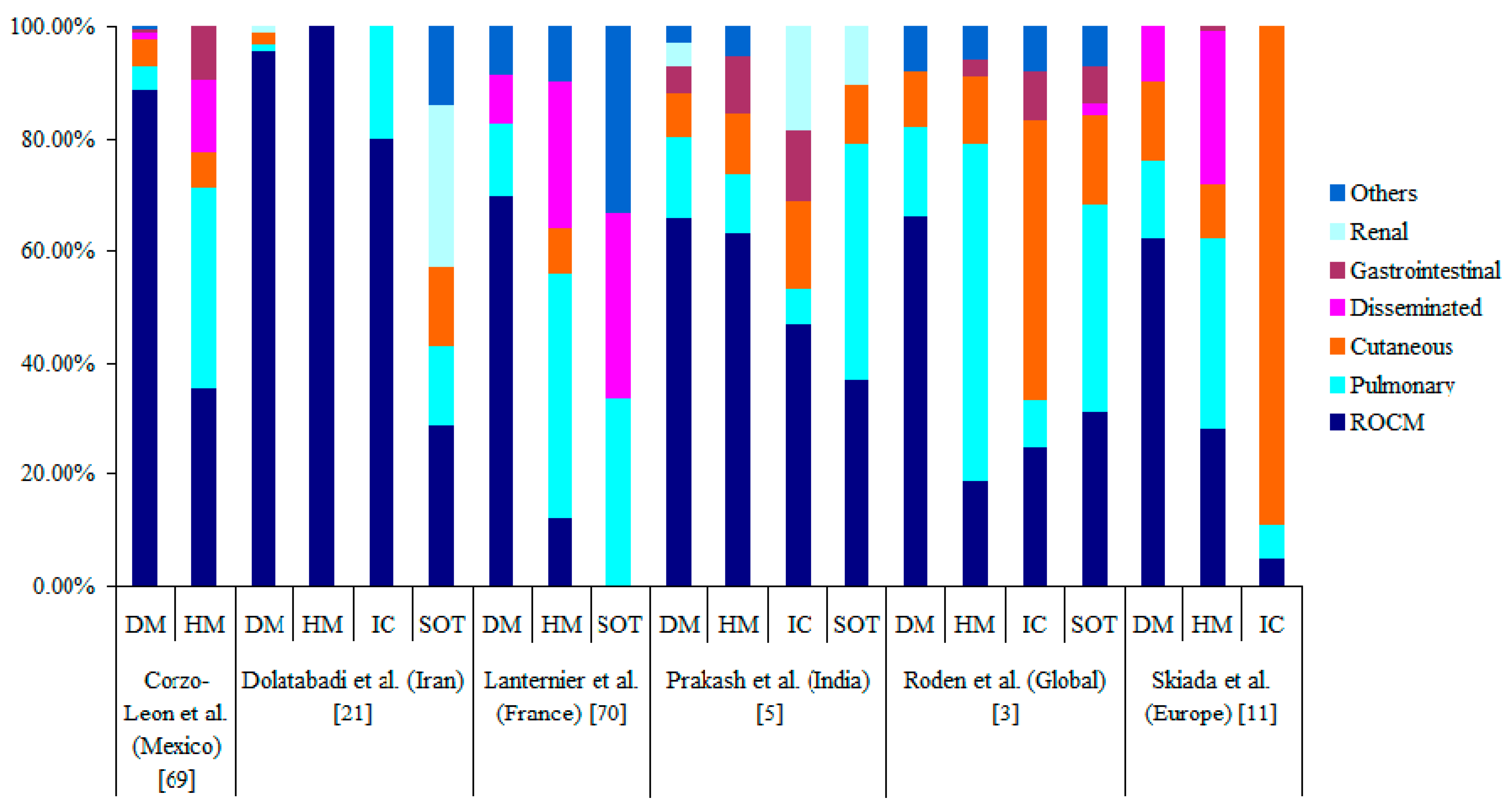
5. Clinical Forms of Mucormycosis
5.1. Rhino-Orbito-Cerebral Mucormycosis (ROCM)
5.2. Pulmonary Mucormycosis
5.3. Cutaneous Mucormycosis
5.4. Gastrointestinal Mucormycosis
5.5. Renal Mucormycosis
5.6. Disseminated Mucormycosis
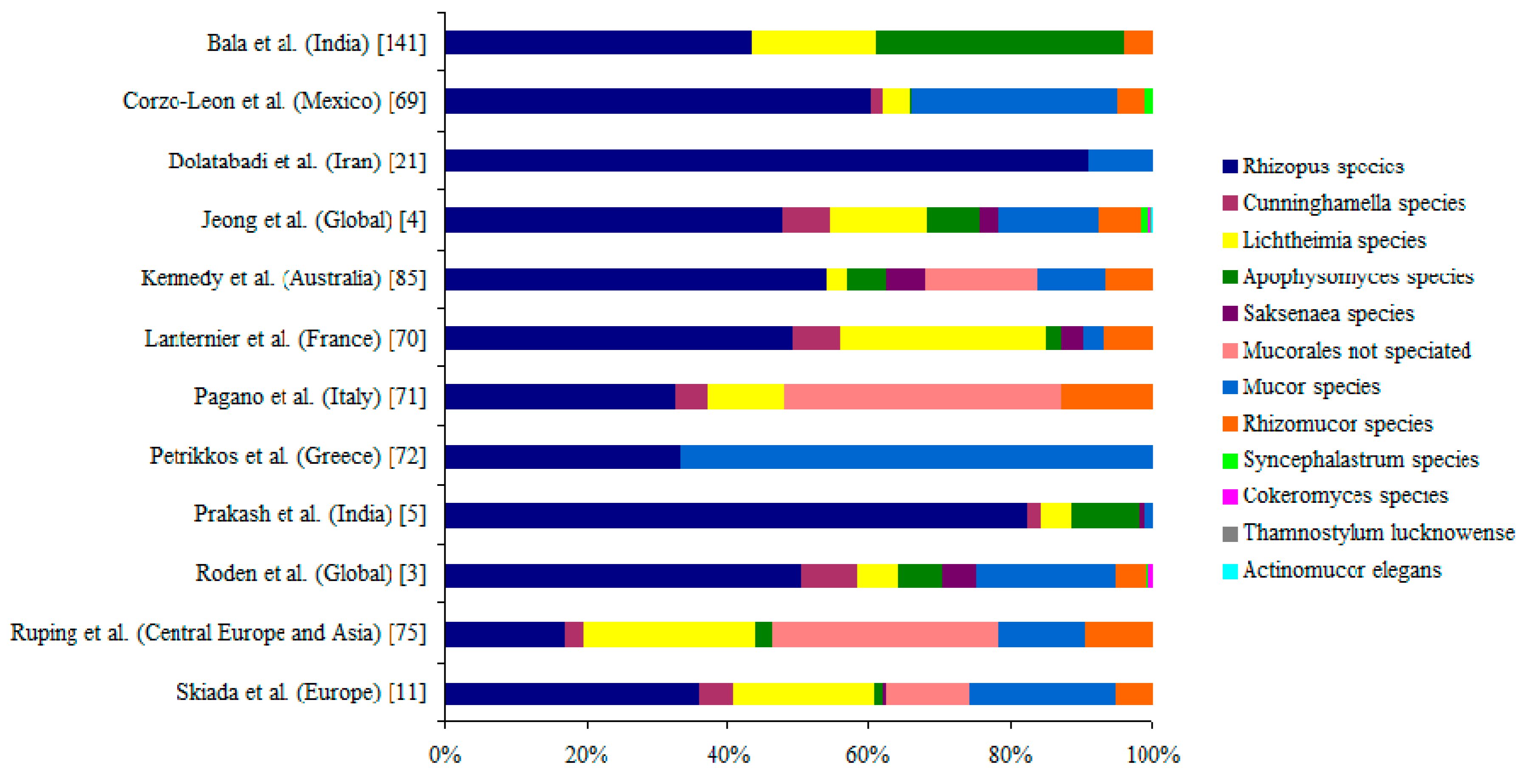
6. Causative Agents of Mucormycosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in Human Disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef]
- Richardson, M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin. Microbiol. Infect. 2009, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med. Mycol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Pinner, R.W.; Hajjeh, R.A.; Brandt, M.E.; Reingold, A.L. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: Results of population-based laboratory active surveillance. Clin. Infect. Dis. 1998, 27, 1138–1147. [Google Scholar] [CrossRef]
- Torres-Narbona, M.; Guinea, J.; Martínez-Alarcón, J.; Muñoz, P.; Gadea, I.; Bouza, E. Impact of zygomycosis on microbiology workload: A survey study in Spain. J. Clin. Microbiol. 2007, 45, 2051–2053. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D.; Van Cauteren, D.; Lanternier, F.; Dannaoui, E.; Che, D.; Dromer, F.; Desenclos, J.C.; Lortholary, O. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009, 15, 1395–1401. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Bouchuiguir-wafa, K.; Garbino, J. Emerging invasive zygomycosis in a tertiary care center: Epidemiology and associated risk factors. Int. J. Infect. Dis. 2010, 14, e100–e103. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Mandal, J.; Shivaprakash, M.R.; George, V.K.; Tarai, B.; Rao, P.; Panda, N.; Verma, S.C.; Sakhuja, V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med. Mycol. 2006, 44, 335–342. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Bouza, E.; Klimko, N.; Gaustad, P.; Lass-Florl, C.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef]
- Rammaert, B.; Lanternier, F.; Zahar, J.-R.; Dannaoui, E.; Bougnoux, M.-E.; Lecuit, M.; Lortholary, O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl. 1), S44–S54. [Google Scholar] [CrossRef]
- Saegeman, V.; Maertens, J.; Meersseman, W.; Spriet, I.; Verbeken, E.; Lagrou, K. Increasing incidence of Mucormycosis in university hospital, Belgium. Emerg. Infect. Dis. 2010, 16, 1456–1458. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: A retrospective study. BMC Infect. Dis. 2016, 16, 730. [Google Scholar] [CrossRef]
- Guinea, J.; Escribano, P.; Vena, A.; Muñoz, P.; Martínez-Jiménez, M.D.C.; Padilla, B.; Bouza, E. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS ONE 2017, 12, e0179136. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Sharma, A.; Panda, N.; Das, S.; Gupta, K.L.; Sakhuja, V. Ten Years’ Experience in Zygomycosis at a Tertiary Care Centre in India. J. Infect. 2001, 42, 261–266. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive zygomycosis in India: Experience in a tertiary care hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef]
- Lin, E.; Moua, T.; Limper, A.H. Pulmonary mucormycosis: Clinical features and outcomes. Infection 2017, 45, 443–448. [Google Scholar] [CrossRef]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Kaur, H.; Savio, J.; Rudramurthy, S.M.; Patel, A.; Shastri, P.; Pamidimukkala, U.; Karthik, R.; Bhattacharya, S.; Kindo, A.J.; et al. Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study). J. Crit. Care 2019, 5, 64–70. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Ahmadi, B.; Rezaei-Matehkolaei, A.; Zarrinfar, H.; Skiada, A.; Mirhendi, H.; Nashibi, R.; Niknejad, F.; Nazeri, M.; Rafiei, A.; et al. Mucormycosis in Iran: A six-year retrospective experience. J. Mycol. Med. 2018, 28, 269–273. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kume, H.; Murase, S.; Yamashita, E.; Arisawa, M. Epidemiology of visceral mycoses: Analysis of data in Annual of the Pathological Autopsy Cases in Japan. J. Clin. Microbiol. 1999, 37, 1732–1738. [Google Scholar]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Dhaliwal, M. Epidemiology of mucormycosis in India. Curr. Fungal Infect. Rep. 2013, 7, 287–292. [Google Scholar] [CrossRef]
- Chekiri-Talbi, M.; Denning, D.W. Burden of fungal infections in Algeria. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 999–1004. [Google Scholar] [CrossRef]
- Riera, F.; Caeiro, J.; Denning, D. Burden of Serious Fungal Infections in Argentina. J. Fungi 2018, 4, 51. [Google Scholar] [CrossRef]
- Chen, S.A.; Slavin, M.A.; Marriott, D.; Thursky, K.; Denning, D.; Sorrell, T.C.; Sorrell, T. Burden of Serious Fungal Infections in Australia. Available online: http://life-worldwide.org/assets/uploads/files/ICAAC%20poster%20burdenaustralia.pdf (accessed on 31 January 2019).
- Lagrou, K.; Maertens, J.; Van Even, E.; Denning, D.W. Burden of serious fungal infections in Belgium. Mycoses 2015, 58, 1–5. [Google Scholar] [CrossRef]
- Giacomazzi, J.; Baethgen, L.; Carneiro, L.C.; Millington, M.A.; Denning, D.W.; Colombo, A.L.; Pasqualotto, A.C. The burden of serious human fungal infections in Brazil. Mycoses 2016, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Aspergillus & Aspergillosis Website. The Burden of Severe Human Fungal Infections in Brazil. Available online: https://www.aspergillus.org.uk/content/burden-severe-human-fungal-infections-brazil (accessed on 31 January 2019).
- Mandengue, C.; Denning, D. The Burden of Serious Fungal Infections in Cameroon. J. Fungi 2018, 4, 44. [Google Scholar] [CrossRef]
- Dufresne, S.F.; Cole, D.C.; Denning, D.W.; Sheppard, D.C. Serious fungal infections in Canada. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 987–992. [Google Scholar] [CrossRef]
- Dufresne, S.F.; Sheppard, D.C.; Denning, D.W. Estimating the Burden of Serious Fungal Diseases in Canada. In Proceedings of the International Society of Human and Animal Mycology (ISHAM), Melbourne, Australia, 4–8 May 2015. [Google Scholar]
- Alvarez Duarte, E.; Denning, D.W.; Dufresne, S.F.; Cole, D.C.; Denning, D.W.; Sheppard, D.C. Serious fungal infections in Chile. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moreno, C.A.; Cortes, J.A.; Denning, D.W. Burden of Fungal Infections in Colombia. J. Fungi 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Chrdle, A.; Mallátová, N.; Vašáková, M.; Haber, J.; Denning, D.W. Burden of serious fungal infections in the Czech Republic. Mycoses 2015, 58, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.L.; Denning, D.W.; Arendrup, M.C. The burden of fungal disease in Denmark. Mycoses 2015, 58 (Suppl. 5), 15–21. [Google Scholar] [CrossRef]
- Gugnani, H.C.; Denning, D.W. Burden of serious fungal infections in the Dominican Republic. J. Infect. Public Health 2016, 9, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gangneux, J.; Bougnoux, M.; Hennequin, C.; Godet, C.; Chandenier, J.; Denning, D.W.; Dupont, B. An estimation of burden of serious fungal infections in France. J. Mycol. Med. 2016, 26, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Gamaletsou, M.N.; Drogari-Apiranthitou, M.; Denning, D.W.; Sipsas, N.V. An estimate of the burden of serious fungal diseases in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Dorgan, E.; Denning, D.W.; McMullan, R. Burden of fungal disease in Ireland. J. Med. Microbiol. 2015, 64, 423–426. [Google Scholar] [CrossRef]
- Aspergillus & Aspergillosis Website. The Burden of Serious Fungal Diseases in Japan. Available online: https://www.aspergillus.org.uk/content/burden-serious-fungal-diseases-japan (accessed on 30 January 2019).
- Wadi, J.; Denning, D. Burden of Serious Fungal Infections in Jordan. J. Fungi 2018, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Kemaykin, V.; Tabinbaev, N.; Khudaibergenova, M.; Olifirovich, A.; Abdrakhmanova, L.; Denning, D.; Klimko, N. An Estimate of Severe and Chronic Fungal Diseases in the Republic of Kazakhstan. J. Fungi 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Guto, J.A.; Bii, C.C.; Denning, D.W. Estimated burden of fungal infections in Kenya. J. Infect. Dev. Ctries. 2016, 10, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.; Ha, Y.E.; Denning, D.W.; Peck, K.R. Serious fungal infections in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Kalua, K.; Zimba, B.; Denning, D. Estimated Burden of Serious Fungal Infections in Malawi. J. Fungi 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Armstrong-James, D.; Denning, D.W. Burden of serious fungal infections in Mexico. Mycoses 2015, 58, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Oladele, R.O.; Denning, D.W. Burden of Serious Fungal Infection in Nigeria. West Afr. J. Med. 2014, 33, 107–114. [Google Scholar]
- Nordøy, I.; Hesstvedt, L.; Torp Andersen, C.; Mylvaganam, H.; Kols, N.; Falch, B.; Tofteland, S.; Müller, F.; Denning, D. An Estimate of the Burden of Fungal Disease in Norway. J. Fungi 2018, 4, 29. [Google Scholar] [CrossRef]
- Jabeen, K.; Farooqi, J.; Mirza, S.; Denning, D.; Zafar, A. Serious fungal infections in Pakistan. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Batac, M.C.R.; Denning, D. Serious fungal infections in the Philippines. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.; Verissímo, C.; Brandão, J.; Martins, C.; Alves, D.; Pais, C.; Denning, D.W. Serious fungal infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Taj-Aldeen, S.J.; Chandra, P.; Denning, D.W. Burden of fungal infections in Qatar. Mycoses 2015, 58, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mareș, M.; Moroti-Constantinescu, V.; Denning, D. The Burden of Fungal Diseases in Romania. J. Fungi 2018, 4, 31. [Google Scholar] [CrossRef]
- Klimko, N.; Kozlova, Y.; Khostelidi, S.; Shadrivova, O.; Borzova, Y.; Burygina, E.; Vasilieva, N.; Denning, D.W. The burden of serious fungal diseases in Russia. Mycoses 2015, 58, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Arsenijević, V.; Denning, D. Estimated Burden of Serious Fungal Diseases in Serbia. J. Fungi 2018, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Alastruey-Izquierdo, A.; Mellado, E.; Peláez, T.; Pemán, J.; Zapico, S.; Alvarez, M.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP study). Antimicrob. Agents Chemother. 2013, 57, 3380–3387. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Denning, D.W. Serious fungal infections in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Osmanov, A.; Denning, D.W. Burden of serious fungal infections in Ukraine. Mycoses 2015, 58, 94–100. [Google Scholar] [CrossRef]
- Pegorie, M.; Denning, D.W.; Welfare, W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J. Infect. 2017, 74, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.J.; Ferraro, J.P.; Rea, S.; Kaufusi, S.; Goodman, B.E.; Spalding, J. Epidemiology and Clinical Features of Invasive Fungal Infection in a US Health Care Network. Open Forum Infect. Dis. 2018, 5, ofy187. [Google Scholar] [CrossRef]
- Tilavberdiev, S.A.; Denning, D.W.; Klimko, N.N. Serious fungal diseases in the Republic of Uzbekistan. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Meis, J.F.; Chakrabarti, A.; Diseases, I.; Hospital, C.W. Changing epidemiology of an emerging infection: Zygomycosis. Clin. Microbiol. Infect. 2009, 15, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Nithyanandam, S.; Jacob, M.S.; Battu, R.R.; Thomas, R.K.; Correa, M.A.; D’Souza, O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J. Ophthalmol. 2003, 51, 231–236. [Google Scholar] [PubMed]
- Zaki, S.M.; Elkholy, I.M.; Elkady, N.A.; Abdel-Ghany, K. Mucormycosis in Cairo, Egypt: Review of 10 reported cases. Med. Mycol. 2014, 52, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bhansali, A.; Bhadada, S.; Sharma, A.; Suresh, V.; Gupta, A.; Singh, P.; Chakarbarti, A.; Dash, R.J. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad. Med. J. 2004, 80, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P. Decrease in the Number of Reported Cases of Zygomycosis among Patients with Diabetes Mellitus: A Hypothesis. Clin. Infect. Dis. 2007, 44, 1089–1090. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Chora-Hernández, L.D.; Rodríguez-Zulueta, A.P.; Walsh, T.J. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med. Mycol. 2018, 56, 29–43. [Google Scholar] [CrossRef]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O. A global analysis of mucormycosis in France: The RetroZygo study (2005–2007). Clin. Infect. Dis. 2012, 54, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Valentini, C.G.; Posteraro, B.; Girmenia, C.; Ossi, C.; Pan, A.; Candoni, A.; Nosari, A.; Riva, M.; Cattaneo, C.; et al. Zygomycosis in Italy: A survey of FIMUA-ECMM (Federazione Italiana di Micopatologia Umana ed Animale and European Confederation of Medical Mycology). J. Chemother. 2009, 21, 322–329. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Sambatakou, H.; Toskas, A.; Vaiopoulos, G.; Giannopoulou, M.; Katsilambros, N. Mucormycosis: Ten-Year Experience at a Tertiary-Care Center in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 753–756. [Google Scholar] [CrossRef]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Offidani, M.; Fianchi, L.; Nosari, A.; Candoni, A.; Picardi, M.; Corvatta, L.; D’Antonio, D.; Girmenia, C.; Martino, P.; et al. Mucormycosis in hematologic patients. Haematologica 2004, 89, 207–214. [Google Scholar] [PubMed]
- Rüping, M.J.G.T.; Heinz, W.J.; Kindo, A.J.; Rickerts, V.; Lass-Flörl, C.; Beisel, C.; Herbrecth, R.; Roth, Y.; Silling, G.; Ullmann, A.J.; et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J. Antimicrob. Chemother. 2009, 65, 296–302. [Google Scholar] [CrossRef]
- Xhaard, A.; Lanternier, F.; Porcher, R.; Dannaoui, E.; Bergeron, A.; Clement, L.; Lacroix, C.; Herbrecht, R.; Legrand, F.; Mohty, M.; et al. Mucormycosis after allogeneic haematopoietic stem cell transplantation: A French Multicentre Cohort Study (2003–2008). Clin. Microbiol. Infect. 2012, 18, E396–E400. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Caira, M.; Nosari, A.; Van Lint, M.T.; Candoni, A.; Offidani, M.; Aloisi, T.; Irrera, G.; Bonini, A.; Picardi, M.; et al. Fungal Infections in Recipients of Hematopoietic Stem Cell Transplants: Results of the SEIFEM B-2004 Study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin. Infect. Dis. 2007, 45, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Pappas, P.G.; Wannemuehler, K.A.; Alexander, B.D.; Anaissie, E.J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; Brumble, L.M.; Freifeld, A.G.; et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg. Infect. Dis. 2011, 17, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Kömür, S.; İnal, A.S.; Kurtaran, B.; Ulu, A.; Uğuz, A.; Aksu, H.S.Z.; Taşova, Y. Mucormycosis: A 10-year experience at a tertiary care center in Turkey. Turk. J. Med. Sci. 2016, 46, 58–62. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Almyroudis, N.G.; Sutton, D.A.; Linden, P.; Rinaldi, M.G.; Fung, J.; Kusne, S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am. J. Transplant. 2006, 6, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Aguado, J.M.; Bonatti, H.; Forrest, G.; Gupta, K.L.; Safdar, N.; John, G.T.; Pursell, K.J.; Muñoz, P.; Patel, R.; et al. Zygomycosis in Solid Organ Transplant Recipients: A Prospective, Matched Case-Control Study to Assess Risks for Disease and Outcome. J. Infect. Dis. 2009, 200, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.S. Rhinocerebral mucormycosis: Predisposing factors. Laryngoscope 1982, 92, 1140–1143. [Google Scholar] [CrossRef]
- Kennedy, K.J.; Daveson, K.; Slavin, M.A.; van Hal, S.J.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Chapman, B.; Halliday, C.L.; Hajkowicz, K.; et al. Mucormycosis in Australia: Contemporary epidemiology and outcomes. Clin. Microbiol. Infect. 2016, 22, 775–781. [Google Scholar] [CrossRef]
- Boelaert, J.R.; de Locht, M.; Van Cutsem, J.; Kerrels, V.; Cantinieaux, B.; Verdonck, A.; Van Landuyt, H.W.; Schneider, Y.J. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Investig. 1993, 91, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, J.R.; Fenves, A.Z.; Coburn, J.W. Deferoxamine Therapy and Mucormycosis in Dialysis Patients: Report of an International Registry. Am. J. Kidney Dis. 1991, 18, 660–667. [Google Scholar] [CrossRef]
- Spellberg, B.; Andes, D.; Perez, M.; Anglim, A.; Bonilla, H.; Mathisen, G.E.; Walsh, T.J.; Ibrahim, A.S. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob. Agents Chemother. 2009, 53, 3122–3125. [Google Scholar] [CrossRef]
- Chitasombat, M.N.; Niparuck, P. Deferiprone as adjunctive treatment for patients with invasive mucormycosis: A retrospective case series. Infect. Dis. Rep. 2018, 10, 7765. [Google Scholar] [CrossRef]
- Spellberg, B.; Ibrahim, A.S.; Chin-Hong, P.V.; Kontoyiannis, D.P.; Morris, M.I.; Perfect, J.R.; Fredricks, D.; Brass, E.P. The Deferasirox–AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: A randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2012, 67, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Siwek, G.T.; Dodgson, K.J.; De Magalhaes-Silverman, M.; Bartelt, L.A.; Kilborn, S.B.; Hoth, P.L.; Diekema, D.J.; Pfaller, M.A. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin. Infect. Dis. 2004, 39, 584–587. [Google Scholar] [CrossRef]
- Mattner, F.; Weissbrodt, H.; Strueber, M. Two case reports: Fatal absidia corymbifera pulmonary tract infection in the first postoperative phase of a lung transplant patient receiving voriconazole prophylaxis, and transient bronchial absidia corymbifera colonization in a lung transplant patient. Scand. J. Infect. Dis. 2004, 36, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a Tertiary-Care Cancer Center in the Era of Aspergillus- Active Antifungal Therapy: A Case-Control Observational Study of 27 Recent Cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Trifilio, S.M.; Bennett, C.L.; Yarnold, P.R.; McKoy, J.M.; Parada, J.; Mehta, J.; Chamilos, G.; Palella, F.; Kennedy, L.; Mullane, K.; et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007, 39, 425–429. [Google Scholar] [CrossRef]
- Wingard, J.R.; Carter, S.L.; Walsh, T.J.; Kurtzberg, J.; Small, T.N.; Baden, L.R.; Gersten, I.D.; Mendizabal, A.M.; Leather, H.L.; Confer, D.L.; et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010, 116, 5111–5118. [Google Scholar] [CrossRef]
- Auberger, J.; Lass-Florl, C.; Aigner, M.; Clausen, J.; Gastl, G.; Nachbaur, D. Invasive fungal breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: Real-life data from a single-centre institutional retrospective observational study. J. Antimicrob. Chemother. 2012, 67, 2268–2273. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Lee, D.-G.; Choi, S.-M.; Choi, J.-K.; Lee, H.-J.; Kim, S.-H.; Park, S.H.; Choi, J.-H.; Yoo, J.-H.; Kim, Y.-J.; et al. Posaconazole for primary antifungal prophylaxis in patients with acute myeloid leukaemia or myelodysplastic syndrome during remission induction chemotherapy: A single-centre retrospective study in Korea and clinical considerations. Mycoses 2015, 58, 565–571. [Google Scholar] [CrossRef]
- Lerolle, N.; Raffoux, E.; Socie, G.; Touratier, S.; Sauvageon, H.; Porcher, R.; Bretagne, S.; Bergeron, A.; Azoulay, E. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: A 4-year study. Eur. Soc. Clin. Infect. Dis. 2014, 20, O952–O959. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, S.; Kriengkauykiat, J.; Tegtmeier, B.; Ito, J. Breakthrough Invasive Fungal Infections in Patients with Hematologic Malignancy (HM) and Hematopoietic Cell Transplantation (HCT) Receiving Isavuconazole for Empiric or Directed Antifungal Therapy. Open Forum Infect. Dis. 2016, 3, 1580. [Google Scholar] [CrossRef]
- Fung, M.; Schwartz, B.S.; Doernberg, S.B.; Langelier, C.; Lo, M.; Graff, L.; Tan, M.; Logan, A.C.; Chin-Hong, P.; Babik, J.M. Breakthrough Invasive Fungal Infections on Isavuconazole Prophylaxis and Treatment: What Is Happening in the Real-World Setting? Clin. Infect. Dis. 2018, 67, 1142–1143. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.R.; DiPippo, A.J.; Bose, P.; Kontoyiannis, D.P. Breakthrough Fungal Infections in Patients with Leukemia Receiving Isavuconazole. Clin. Infect. Dis. 2018, 67, 1610–1613. [Google Scholar] [CrossRef]
- Phai Pang, K.-A.; Godet, C.; Fekkar, A.; Scholler, J.; Nivoix, Y.; Letscher-Bru, V.; Massias, L.; Kauffmann-Lacroix, C.; Elsendoorn, A.; Uzunov, M.; et al. Breakthrough invasive mould infections in patients treated with caspofungin. J. Infect. 2012, 64, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Van Burik, J.-A.H.; Ratanatharathorn, V.; Stepan, D.E.; Miller, C.B.; Lipton, J.H.; Vesole, D.H.; Bunin, N.; Wall, D.A.; Hiemenz, J.W.; Satoi, Y.; et al. Micafungin versus Fluconazole for Prophylaxis against Invasive Fungal Infections during Neutropenia in Patients Undergoing Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2004, 39, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.S.; Lewis, R.E.; Ippoliti, C.; Champlin, R.E.; Kontoyiannis, D.P. Caspofungin as Primary Antifungal Prophylaxis in Stem Cell Transplant Recipients. Pharmacotherapy 2007, 27, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Louis-Auguste, J.R.; Micallef, C.; Ambrose, T.; Upponi, S.; Butler, A.J.; Massey, D.; Middleton, S.J.; Russell, N.; Rutter, C.S.; Sharkey, L.M.; et al. Fatal breakthrough mucormycosis in a multivisceral transplant patient receiving micafungin: Case report and literature review. IDCases 2018, 12, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Varon, A.; Galhardo, M.C.; Santos, F.; Lyra, M.; Castro, R.; Oliveira, R.; Lamas, C.C. The burden of mucormycosis in HIV-infected patients: A systematic review. J. Infect. 2016, 73, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, F.; Kontoyiannis, D.P. The calcineurin pathway inhibitor tacrolimus enhances the in vitro activity of azoles against Mucorales via apoptosis. Eukaryot. Cell 2013, 12, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Revankar, S.G. In vitro interaction of caspofungin and immunosuppressives against agents of mucormycosis. J. Antimicrob. Chemother. 2011, 66, 2312–2314. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Ben-Ami, R.; Best, L.; Albert, N.; Walsh, T.J.; Kontoyiannis, D.P. Tacrolimus Enhances the Potency of Posaconazole Against Rhizopus oryzae In Vitro and in an Experimental Model of Mucormycosis. J. Infect. Dis. 2013, 207, 834–841. [Google Scholar] [CrossRef]
- Yu, J.; Yu Li, R. Primary renal zygomycosis due to Rhizopus oryzae. Med. Mycol. 2006, 44, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, A. Outbreaks of zygomycosis in hospitals Definition of a Hospital Outbreak. Clin. Microbiol. Infect. 2009, 15, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Alsuwaida, K. Primary cutaneous mucormycosis complicating the use of adhesive tape to secure the endotracheal tube. Can. J. Anesth. 2002, 49, 880–882. [Google Scholar] [CrossRef]
- Holzel, H.; Macqueen, S.; MacDonald, A.; Alexander, S.; Campbell, C.K.; Johnson, E.M.; Warnock, D.W. Rhizopus microsporus in wooden tongue depressors: A major threat or minor inconvenience? J. Hosp. Infect. 1998, 38, 113–118. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Gray, J.; Morgan, M.E.I.; Hocking, M.D.; Durbin, G.M. Nosocomial infection with Rhizopus microsporus in preterm infants: Association with wooden tongue depressors. Lancet 1996, 348, 441–443. [Google Scholar] [CrossRef]
- Verweij, P.E.; Voss, A.; Donnelly, J.P.; De Pauw, B.E.; Meis, J.F.G.M. Wooden sticks as the source of a pseudoepidemic of infection with Rhizopus microsporus var. rhizopodiformis among immunocompromised patients. J. Clin. Microbiol. 1997, 35, 2422–2423. [Google Scholar] [PubMed]
- LeMaile-Williams, M.; Burwell, L.A.; Salisbury, D.; Noble-Wang, J.; Arduino, M.J.; Lott, T.; Brandt, M.E.; Iiames, S.; Srinivasan, A.; Fridkin, S.K. Outbreak of cutaneous Rhizopus arrhizus infection associated with karaya ostomy bags. Clin. Infect. Dis. 2006, 43, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, G.; Hayette, M.P.; Jacquemin, D.; Melin, P.; Mutsers, J.; De Mol, P. An outbreak of Absidia corymbifera infection associated with bandage contamination in a burns unit. J. Hosp. Infect. 2005, 61, 88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.C.; Chan, J.F.W.; Ngan, A.H.Y.; To, K.K.W.; Leung, S.Y.; Tsoi, H.W.; Yam, W.C.; Tai, J.W.M.; Wong, S.S.Y.; Tse, H.; et al. Outbreak of intestinal infection due to Rhizopus microsporus. J. Clin. Microbiol. 2009, 47, 2834–2843. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.; Harris, J.; Gade, L.; Sehulster, L.; Newhouse, E.; O’Connell, H.; Noble-Wang, J.; Rao, C.; Balajee, S.A.; Chiller, T. Mucormycosis Outbreak Associated with Hospital Linens. Pediatr. Infect. Dis. J. 2014, 33, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards, J., Jr.; Filler, S.G.; Goldberg, R.; Spellberg, B. Combination Polyene-Caspofungin Treatment of Rhino-Orbital-Cerebral Mucormycosis. Clin. Infect. Dis. 2008, 47, 364–371. [Google Scholar] [CrossRef]
- Vaughan, C.; Bartolo, A.; Vallabh, N.; Leong, S.C. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis—has anything changed in the past 20 years? Clin. Otolaryngol. 2018, 43, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 1994, 39, 3–22. [Google Scholar] [CrossRef]
- Sun, H.Y.; Forrest, G.; Gupta, K.L.; Aguado, J.M.; Lortholary, O.; Julia, M.B.; Safdar, N.; Patel, R.; Kusne, S.; Singh, N. Rhino-orbital-cerebral zygomycosis in solid organ transplant recipients. Transplantation 2010, 90, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wali, U.; Balkhair, A.; Al-Mujaini, A. Cerebro-rhino orbital mucormycosis: An update. J. Infect. Public Health 2012, 5, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Therakathu, J.; Prabhu, S.; Irodi, A.; Sudhakar, S.V.; Yadav, V.K.; Rupa, V. Imaging features of rhinocerebral mucormycosis: A study of 43 patients. Egypt. J. Radiol. Nucl. Med. 2018, 49, 447–452. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012, 54, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tedder, M.; Spratt, J.A.; Anstadt, M.P.; Hegde, S.S.; Tedder, S.D.; Lowe, J.E. Pulmonary mucormycosis: Results of medical and surgical therapy. Ann. Thorac. Surg. 1994, 57, 1044–1050. [Google Scholar] [CrossRef]
- Lee, F.Y.; Mossad, S.B.; Adal, K.A. Pulmonary mucormycosis: The last 30 years. Arch. Intern. Med. 1999, 159, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Sun, X. Characteristics of pulmonary mucormycosis and predictive risk factors for the outcome. Infection 2018, 46, 503–512. [Google Scholar] [CrossRef]
- Skiada, A.; Rigopoulos, D.; Larios, G.; Petrikkos, G.; Katsambas, A. Global epidemiology of cutaneous zygomycosis. Clin. Dermatol. 2012, 30, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Petrikkos, G. Cutaneous zygomycosis. Clin. Microbiol. Infect. 2009, 15 (Suppl. 5), 41–45. [Google Scholar] [CrossRef]
- Simbli, M.; Hakim, F.; Koudieh, M.; Tleyjeh, I.M. Nosocomial post-traumatic cutaneous mucormycosis: A systematic review. Scand. J. Infect. Dis. 2008, 40, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ghosh, A.; Rudramurthy, S.M.; Chakrabarti, A. Gastrointestinal mucormycosis in apparently immunocompetent hosts—A review. Mycoses 2018, 61, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Dioverti, M.V.; Cawcutt, K.A.; Abidi, M.; Sohail, M.R.; Walker, R.C.; Osmon, D.R. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses 2015, 58, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Jianhong, L.; Xianliang, H.; Xuewu, J. Isolated renal mucormycosis in children. J. Urol. 2004, 171, 387–388. [Google Scholar] [CrossRef]
- Bhadauria, D.; Etta, P.; Chelappan, A.; Gurjar, M.; Kaul, A.; Sharma, R.K.; Gupta, A.; Prasad, N.; Marak, R.S.; Jain, M.; et al. Isolated bilateral renal mucormycosis in apparently immunocompetent patients—a case series from India and review of the literature. Clin. Kidney J. 2018, 11, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.Z.R.; Lewis, R.E.; Kontoyiannis, D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 2011, 24, 411–445. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Ghosh, A.; Prasad, G.S.; David, J.K.; Gupta, S.; Das, A.; Sakhuja, V.; Panda, N.K.; Singh, S.K.; Das, S.; et al. Apophysomyces elegans: An emerging zygomycete in India. J. Clin. Microbiol. 2003, 41, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Bala, K.; Chander, J.; Handa, U.; Punia, R.S.; Attri, A.K. A prospective study of mucormycosis in north India: Experience from a tertiary care hospital. Med. Mycol. 2015, 53, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Singh, G.; Agarwal, R.; Dabas, Y.; Jyotsna, V.P.; Kumar, R.; Xess, I. Emerging Rhizopus microsporus Infections in India. J. Clin. Microbiol. 2018, 56, e00433-18. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Marak, R.S.K.; Shivaprakash, M.R.; Gupta, S.; Garg, R.; Sakhuja, V.; Singhal, S.; Baghela, A.; Dixit, A.; Garg, M.K.; et al. Cavitary pulmonary zygomycosis caused by Rhizopus homothallicus. J. Clin. Microbiol. 2010, 48, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Kokkayil, P.; Pandey, M.; Agarwal, R.; Kale, P.; Singh, G.; Xess, I. Rhizopus homothallicus Causing Invasive Infections: Series of Three Cases from a Single Centre in North India. Mycopathologia 2017, 182, 921–926. [Google Scholar] [CrossRef]
- Compain, F.; Aït-Ammar, N.; Botterel, F.; Gibault, L.; Le Pimpec Barthes, F.; Dannaoui, E. Fatal Pulmonary Mucormycosis due to Rhizopus homothallicus. Mycopathologia 2017, 182, 907–913. [Google Scholar] [CrossRef]
- Hemashettar, B.M.; Patil, R.N.; O’Donnell, K.; Chaturvedi, V.; Ren, P.; Padhye, A.A. Chronic rhinofacial mucormycosis caused by Mucor irregularis (Rhizomucor variabilis) in India. J. Clin. Microbiol. 2011, 49, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-L.; Najafzadeh, M.J.; Dolatabadi, S.; Ran, Y.-P.; Gerrits van den Ende, A.H.G.; Shen, Y.-N.; Li, C.-Y.; Xi, L.-Y.; Hao, F.; Zhang, Q.-Q.; et al. Taxonomy and epidemiology of Mucor irregularis, agent of chronic cutaneous mucormycosis. Persoonia 2013, 30, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Cano, J.; Stchigel, A.M.; Sutton, D.A.; Fothergill, A.W.; Salas, V.; Rinaldi, M.G.; Guarro, J. Two new species of Mucor from clinical samples. Med. Mycol. 2011, 49, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Xess, I.; Mohapatra, S.; Shivaprakash, M.R.; Chakrabarti, A.; Benny, G.L.; O’Donnell, K.; Padhye, A.A. Evidence implicating Thamnostylum lucknowense as an etiological agent of rhino-orbital mucormycosis. J. Clin. Microbiol. 2012, 50, 1491–1494. [Google Scholar] [CrossRef]
- Prakash, H.; Ghosh, A.; Rudramurthy, S.; Paul, R.; Gupta, S.; Negi, V.; Chakrabarti, A. The environmental source of emerging apophysomyces variabilis infection in India. Med. Mycol. 2016, 54, 567–575. [Google Scholar] [CrossRef] [PubMed]
| Country | Total Population (in millions) | Total Estimated Fungal Burden | Mucormycosis | Invasive Aspergillosis | ||
|---|---|---|---|---|---|---|
| Total Burden | Rate/100K | Total Burden | Rate/100K | |||
| Algeria [25] | 40.4 | 568,942 | 79 | 0.2 | 2865 | 7.1 |
| Argentina [26] | 43.8 | 881,023 | 75 | 0.17 | 2536 | 5.8 |
| Australia [27] | 23.57 | 693,708 | 21 | 0.06 | 560 | 3–29% |
| Belgium [13,28] | 11.1 | 233,000 | 31 | 0.58 | 675 | 6.08 |
| Brazil [29,30] | 194.0 | 3,800,000 | 243 | 0.2 | 8664 | 4.47 |
| Cameroon [31] | 24.2 | 1,126,332 | 5 | 0.2 | 1175 | 5.3 |
| Canada [32,33] | 35.5 | 652,932 | 43 | 0.12 | 566 | 1.59 |
| Chile [34] | 17.5 | 325,036 | 35 | 0.2 | 296 | 1.7 |
| Colombia [35] | 49.3 | 760,808 | 99 | 0.2 | 2820 | 5.7 |
| Czech Republic [36] | 10.5 | 176,073 | 22 | 0.2 | 297 | 2.8 |
| Denmark [37] | 5.6 | 894,430 | 1 | 0.02 | 294 | 5.3 |
| Dominican Republic [38] | 10.9 | 2,293,681 | 20 | 0.2 | 61 | 0.8 |
| France [39] | 65.8 | 968,143 | 79 | 0.12 | 1185 | 1.8 |
| Greece [40] | 10.8 | 194,067 | 7 | 0.06 | 1125 | 10.4 |
| India [24] | 1300.0 | NA | 171,504 | 14 | NA | NA |
| Ireland [41] | 6.4 | 117,384 | 13 | 0.2 | 445 | 7 |
| Japan [42] | 127.0 | 2,370,314 | 254 | 0.2 | 1308 | 1 |
| Jordan [43] | 6.3 | 119,153 | 1 | 0.02 | 84 | 1.34 |
| Kazakhstan [44] | 17.7 | 300,824 | 16 | 0.09 | 511 | 2.8 |
| Kenya [45] | 43.6 | 3,186,766 | 80 | 0.2 | 239 | 0.6 |
| Korea [46] | 48.0 | 985,079 | 68 | 0.14 | 2150 | 4.48 |
| Malawi [47] | 17.7 | 1,338,523 | 30 | 0.2 | 1186 | 6.7 |
| Mexico [48] | 112.3 | 2,749,159 | 134 | 0.12 | 4510 | 4 |
| Nigeria [49] | 155 | 17,983,517 | 300 | 0.2 | 928 | 0.6 |
| Norway [50] | 5.2 | 839,087 | 7 | 0.1 | 278 | 5.3 |
| Pakistan [51] | 184.5 | 3,280,554 | 25,830 | 14 | 10,949 | 5.9 |
| Philippines [52] | 98.4 | 1,852,137 | 20 | 0.02 | 3085 | 3 |
| Portugal [53] | 10.6 | 1,695,514 | 10 | 9.5 | 240 | 2.3 |
| Qatar [54] | 1.9 | 33,448 | 23 | 1.23 | 11 | 0.6 |
| Romania [55] | 19.7 | 436,230 | 7 | 0.04 | 1524 | 7.7 |
| Russia [56] | 142.9 | 3,082,907 | 232 | 0.16 | 3238 | 2.27 |
| Serbia [57] | 7.1 | 156,825 | 23 | 0.33 | 619 | 8.8 |
| Spain [58] | 47.0 | 8,144,605 | 20 | 0.04 | 1293 | 2.75 |
| Thailand [59] | 65.1 | 1,254,562 | 130 | 0.2 | 941 | 1.4 |
| Ukraine [60] | 45.5 | 999,152 | 90 | 0.1975 | 1233 | 2.7067 |
| United Kingdom [61] | 63.18 | 241,525–662,987 | 57 | 0.09 | 2901–2912 | 4.59–4.61 |
| USA [62] | NA | NA | 36 | 0.3 | 301 | 2.4 |
| Republic of Uzbekistan [63] | 30.7 | 536,978 | 27 | 0.08 | 1521 | 4.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. https://doi.org/10.3390/jof5010026
Prakash H, Chakrabarti A. Global Epidemiology of Mucormycosis. Journal of Fungi. 2019; 5(1):26. https://doi.org/10.3390/jof5010026
Chicago/Turabian StylePrakash, Hariprasath, and Arunaloke Chakrabarti. 2019. "Global Epidemiology of Mucormycosis" Journal of Fungi 5, no. 1: 26. https://doi.org/10.3390/jof5010026
APA StylePrakash, H., & Chakrabarti, A. (2019). Global Epidemiology of Mucormycosis. Journal of Fungi, 5(1), 26. https://doi.org/10.3390/jof5010026





