A Ketoconazole Susceptibility Test for Malassezia pachydermatis Using Modified Leeming–Notman Agar
Abstract
:1. Introduction
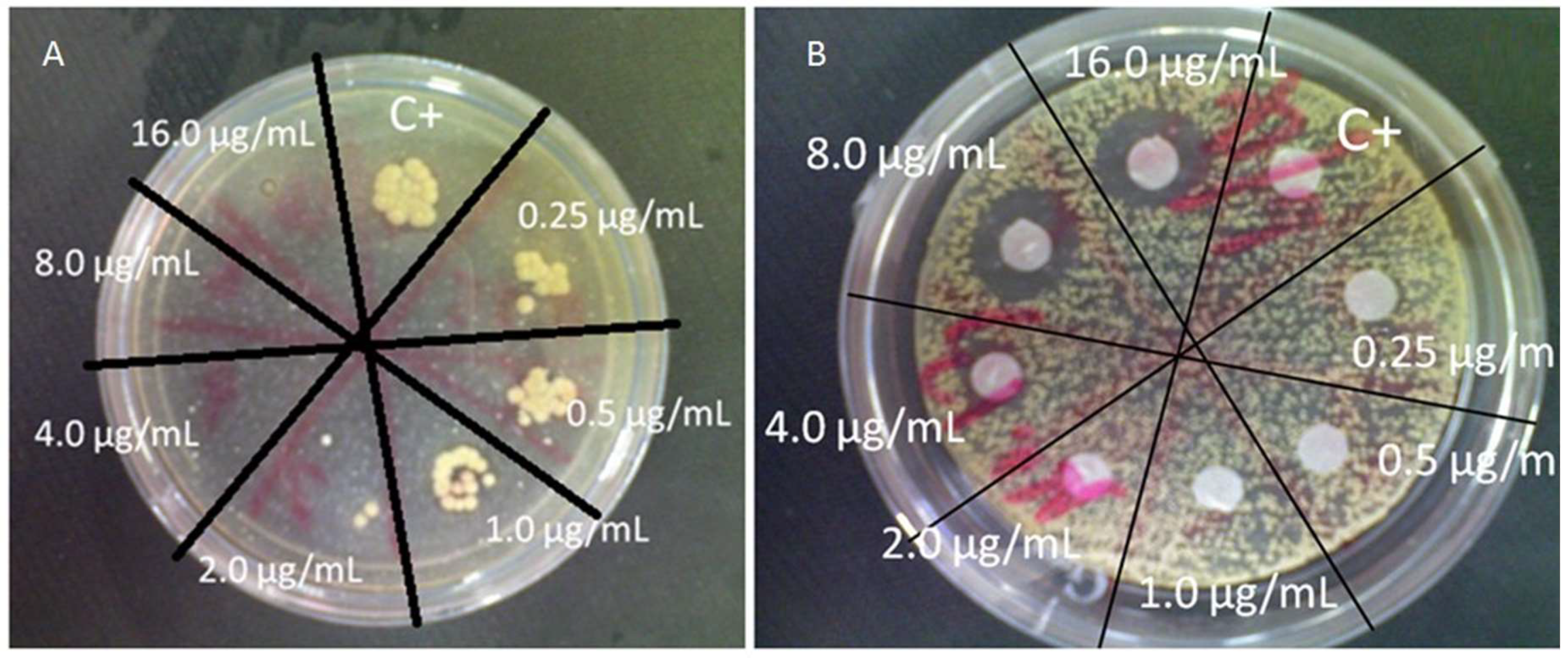
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cafarchia, C.; Gallo, S.; Capelli, G.; Otranto, D. Occurrence and population size of Malassezia spp. in the external ear canal of dogs and cats both healthy and with otitis. Mycopathologia 2005, 160, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Robson, D. Malassezia: Mechanisms of possible drug resistance. Aust. Coll. Vet. Sci. Dermatol. Chap. Sci. Week Proc. 2007, 63–67. [Google Scholar]
- Nijima, M.; Kano, R.; Nagata, M.; Hasegawa, A.; Kamata, H. An azole-resistant isolate of Malassezia pachydermatis. Vet. Microbiol. 2011, 149, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Munoz, A.J.; Rojas, F.; Tur-Tur, C.; de Los Angeles Sosa, M.; Diez, G.O.; Espada, C.M.; Payá, M.J.; Giusiano, G. In vitro antifungal activity of topical and systemic antifungal drugs against Malassezia species. Mycoses 2013, 56, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Koike, A.; Kano, R.; Nagata, M.; Chen, C.; Hwang, C.Y.; Hasegawa, A.; Kamata, H. In vitro susceptibility of Malassezia pachydermatis isolates from canine skin with atopic dermatitis to ketoconazole and itraconazole in East Asia. J. Vet. Med. Sci. 2014, 76, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Peano, A.; Pasquetti, M.; Tizzani, P.; Chiavassa, E.; Guillot, J.; Johnson, E. Methodological issues in antifungal susceptibility testing of Malassezia pachydermatis. J. Fungi 2017, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Figueredo, L.A.; Favuzzi, V.; Surico, M.R.; Colao, V.; Iatta, R.; Montagna, M.T.; Otranto, D. Assessment of the antifungal susceptibility of Malassezia pachydermatis in various media using a CLSI protocol. Vet. Microbiol. 2012, 159, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Pasquett, M.; Chiavassa, E.; Tizzani, P.; Danesi, P.; Peano, A. Agar diffusion procedures for susceptibility testing of Malassezia pachydermatis: Evaluation of Mueller-Hinton agar plus 2% glucose and 0.5 mg/mL methylene blue as the test medium. Mycopathologia 2015, 180, 153–158. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; CLSI document M27-A3; Clinical and Laboratory Standards Institutes: Wayne, PA, USA, 2008. [Google Scholar]
- Garau, M.; Pereiro, M., Jr.; del Palacio, A. In vitro susceptibilities of Malassezia species to a new triazole, albaconazole (UR-9825), and other antifungal compounds. Antimicrob. Agents Chemother. 2003, 47, 2342–2344. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.P.; Lautert, C.; Zanette, R.A.; Mahl, D.L.; Azevedo, M.I.; Machadod, M.L.; Dutrab, V.; Bottonc, S.A.; Alvesa, S.H.J.; Santurio, J.M. In vitro susceptibility of fluconazole-susceptible and -resistant isolates of Malassezia pachydermatis against azoles. Vet. Microbiol. 2011, 152, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.C.; de Araujo, C.R.; Costa, C.R.; Passos, X.S.; de Fatima Lisboa Fernandes, O.; do Rosário Rodrigues Silva, M. Antifungal activities of azole agents against the Malassezia species. Int. J. Antimicrob. Agents 2007, 29, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Rincon, S.; Cepero de Garcia, M.C.; Espinel-Ingroff, A. A modified Christensen’s urea and CLSI broth microdilution method for testing susceptibilities of six Malassezia species to voriconazole, itraconazole, and ketoconazole. J. Clin. Microbiol. 2006, 44, 3429–3431. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kano, R.; Murai, T.; Watanabe, S.; Hasegawa, A. Susceptibility testing of Malassezia species using the urea broth microdilution method. Antimicrob. Agents Chemother. 2000, 44, 2185–2186. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kohli, Y.; Li, A.; Faergemann, J.; Summerbell, R.C. In vitro susceptibility of the seven Malassezia species to ketoconazole, voriconazole, itraconazole and terbinafine. Br. J. Dermatol. 2000, 142, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Wanger, A.; Mills, K.; Nelson, P.W.; Rex, J.H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: Enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 1995, 39, 2520–2522. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Suto, H.; Unno, T.; Tsuboi, R.; Ogawa, H.; Shinoda, T.; Nishikawa, A. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J. Clin. Microbiol. 2001, 39, 3486–3490. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Matías, N.; Rodríguez-Medina, J.R. Fundamental concepts of azole compounds and triazole antifungals: A beginner’s review. P. R. Health Sci. J. 2018, 37, 135–142. [Google Scholar] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Silva, S.; Henriques, M. Candida glabrata biofilms: How far have we come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
| Samples | Ketoconazole Disks (μg/mL) | Modified M27-A3 (μg/mL) |
|---|---|---|
| M. pachydermatis (BCRC 21676) | 4.0 | 4.0 |
| Candida parapsilosis (ATCC 22019) | 0.5 | 0.5 |
| 1 | 2.0 | 2.0 |
| 2 | 2.0 | 1.0 |
| 3 | 2.0 | 0.5 |
| 4 | 2.0 | 2.0 |
| 5 | 4.0 | 2.0 |
| 6 | 2.0 | 2.0 |
| 7 | 2.0 | 2.0 |
| 8 | 4.0 | 4.0 |
| 9 | 2.0 | 4.0 |
| 10 | 2.0 | 2.0 |
| 11 | 4.0 | 4.0 |
| 12 | 4.0 | 2.0 |
| 13 | 16.0 | 16.0 |
| 14 | 2.0 | 4.0 |
| 15 | 2.0 | 2.0 |
| 16 | 8.0 | 4.0 |
| 17 | 8.0 | 8.0 |
| 18 | 2.0 | 2.0 |
| 19 | 2.0 | 4.0 |
| 20 | 2.0 | 8.0 |
| 21 | 16.0 | 4.0 |
| 22 | 16.0 | 8.0 |
| 23 | 8.0 | 4.0 |
| 24 | 4.0 | 4.0 |
| 25 | 4.0 | 4.0 |
| 26 | 16.0 | 16.0 |
| 27 | 16.0 | 16.0 |
| 28 | 16.0 | 16.0 |
| 29 | 16.0 | 16.0 |
| 30 | 2.0 | 2.0 |
| 31 | 2.0 | 2.0 |
| 32 | 2.0 | 2.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, B.-Y.; Chao, W.-H.; Xue, Y.-J.; Lai, J.-M. A Ketoconazole Susceptibility Test for Malassezia pachydermatis Using Modified Leeming–Notman Agar. J. Fungi 2018, 4, 126. https://doi.org/10.3390/jof4040126
Hsieh B-Y, Chao W-H, Xue Y-J, Lai J-M. A Ketoconazole Susceptibility Test for Malassezia pachydermatis Using Modified Leeming–Notman Agar. Journal of Fungi. 2018; 4(4):126. https://doi.org/10.3390/jof4040126
Chicago/Turabian StyleHsieh, Bo-Young, Wei-Hsun Chao, Yi-Jing Xue, and Jyh-Mirn Lai. 2018. "A Ketoconazole Susceptibility Test for Malassezia pachydermatis Using Modified Leeming–Notman Agar" Journal of Fungi 4, no. 4: 126. https://doi.org/10.3390/jof4040126





