Abstract
Echinocandin antifungals represent one of the most important drug classes for the treatment of invasive fungal infections. The mode of action of the echinocandins relies on inhibition of the β-1,3-glucan synthase, an enzyme essentially required for the synthesis of the major fungal cell wall carbohydrate β-1,3-glucan. Depending on the species, echinocandins may exert fungicidal or fungistatic activity. Apparently independent of this differential activity, a surprising in vitro phenomenon called the “paradoxical effect” can be observed. The paradoxical effect is characterized by the ability of certain fungal isolates to reconstitute growth in the presence of higher echinocandin concentrations, while being fully susceptible at lower concentrations. The nature of the paradoxical effect is not fully understood and has been the focus of multiple studies in the last two decades. Here we concisely review the current literature and propose an updated model for the paradoxical effect, taking into account recent advances in the field.
1. Introduction
Invasive fungal infections contribute significantly to the morbidity and mortality of immunocompromised patients. Unfortunately, the arsenal of antimycotic drugs is very limited. Current therapy of invasive fungal infections relies on primarily four antifungal drug classes: the polyenes (i.e., amphotericin B), the azoles (e.g., fluconazole, posaconazole, and voriconazole), the pyrimidine analogues (i.e., flucytosin), and the echinocandins (e.g., caspofungin, micafungin, and anidulafungin). While polyenes and azoles directly or indirectly target the fungal membrane and the antimetabolite flucytosin disturbs DNA and RNA synthesis, echinocandins interfere with the fungal cell wall biogenesis by noncompetitively inhibiting the β-1,3-glucan synthase (β-GS, Fks1). This enzyme is responsible for the synthesis of the major fungal cell wall carbohydrate β-1,3-glucan.
The β-GS is a multi-pass membrane protein that is located in the plasma membrane [1]. It is assumed that the enzyme synthesizes a linear β-1,3-glucan polymer using UDP-glucose monomers as a substrate [2,3]. Specialized enzymes may subsequently rearrange the β-1,3-glucan polymers, such as by forming β-1,6-glucan linkages [4], which significantly contribute to the rigidity and dynamics of the cell wall. Depending on the fungal species, approximately one-third to four-fifths of the cell wall consists of β-1,3-glucan and its rearrangement derivatives [5].
Chemical inhibition of the β-GS or deletion of the underlying genes may have fatal consequences for the organism. Yeasts that are lacking the enzymatic activity are typically not viable [6,7,8,9]. Because of this, echinocandins are usually considered fungicidal for susceptible (pathogenic) yeasts. Resistance may occur, but, so far, was always associated with mutation in the β-GS itself [10]. It was proposed that these mutations do not impair the ability of echinocandins to bind to the enzyme but instead reduce the synthesis velocity [10,11,12]. The situation with filamentous fungi in the genus Aspergillus is somewhat different. Echinocandins show only a fungistatic activity against these molds [13]. Under in vitro conditions, this is reflected by incomplete growth inhibition, accompanied by significant morphological changes in the mold’s hyphae. The lowest echinocandin concentration that is able to provoke this morphological alteration is therefore called the minimal effective concentration (MEC), which is in contrast to the minimal inhibitory concentration (MIC) usually determined for antimicrobial agents. The reason for the limited activity of echinocandins against Aspergillus has not been understood as for a long time β-1,3-glucan was believed to be essential in all fungi. However, we could recently show that the pathogenic mold Aspergillus fumigatus is able to survive and grow without β-1,3-glucan. Nevertheless, deletion of the β-GS in A. fumigatus has severe consequences for the growth rate, morphotype, and conidiation (sporulation) [14].
In clinical settings, echinocandins show excellent antifungal activity against the most relevant pathogenic Candida species and good antifungal activity against most Aspergillus species. The frequency of echinocandin resistance is generally low, thereby making echinocandins a first choice for empiric therapy of invasive candidiasis [15,16]. Moreover, echinocandins are recommended for second-line therapy of invasive aspergillosis [16]. Phenotypic antifungal susceptibility testing is routinely performed to avoid potential therapy failure. When performing susceptibility testing under standardized conditions, e.g., with the broth microdilution method, a surprising echinocandin-specific phenomenon called the “paradoxical effect” can be observed. This paradoxical effect is characterized by the ability of certain strains to grow in the presence of higher echinocandin concentrations (approximately 4 to 32 µg mL−1) while being fully susceptible at lower concentrations (approximately 0.03 to 1 µg mL−1). In the presence of very high concentrations (approximately >64 µg mL−1), the fungal species showing the paradoxical effect become susceptible again [17,18,19]. The phenomenon was observed with multiple species in the genera Candida and Aspergillus [20,21,22]. Within this review we will summarize and discuss the current knowledge and recent advances related to the paradoxical effect of the echinocandins.
2. The Paradoxical Effect: Dependence on Species, Strain, and Echinocandin Derivative
A paradoxical effect of an echinocandin was described for the first time in 1988. Hall and colleagues reported for the nonclinical echinocandin cilofungin that many isolates of Candida albicans and Candida tropicalis continued to grow in concentrations above that of partial inhibition [17]. Later on, this phenomenon was also observed for other echinocandins and further fungal species, including molds of the genus Aspergillus [20,21,22]. Interestingly, not every echinocandin derivative seems to have the same capability to evoke the paradoxical effect. Of the three derivatives in clinical use, namely caspofungin, micafungin, and anidulafungin, caspofungin was most frequently associated with paradoxical growth in Candida spp. [18,21,22,23,24,25]. For example, of approximately 120 clinical Candida dubliniensis isolates that were tested by Fleischhacker et al., 90% showed paradoxical growth with caspofungin, 63% with micafungin, but none with anidulafungin [22]. Similar observations were made with different isolates of Aspergillus spp. [20,26]. Of 11 clinical isolates six showed paradoxical growth with caspofungin, two with micafungin, and five with anidulafungin [20,26]. However, it has to be stated that the frequency of the paradoxical effect reported for the individual echinocandins but also for isolates of different fungal species differs greatly between different studies (Table 1). For example, paradoxical growth of C. albicans in the presence of caspofungin was observed by Chamilos et al. in 12 of 20 clinical isolates (60%) and by Fleischhacker et al. in only 14 of 101 clinical isolates (14%) [21,22]. A recently published study systematically assessed the occurrence of the paradoxical effect for a large number of Candida isolates (n = 602) treated with caspofungin, anidulafungin, and micafungin [24]. Again, the results regarding the frequency and drug dependency differed in some cases for the individual species when compared to previous studies (Table 1). The authors of this study proposed that the discrepancies in results could be related to methodological differences between the studies such as the chosen definition of paradoxical growth or the in vitro testing protocols, which were either EUCAST (European Committee on Antimicrobial Susceptibility Testing)- or CLSI (Clinical and Laboratory Standards Institute)-based and apply different media [24].

Table 1.
Manifestation frequency of the paradoxical effect in vitro in percent (%) of caspofungin (CS), micafungin (MI), and anidulafungin (AN) among clinical isolates of selected Candida and Aspergillus species in different studies. The numbers of tested isolates (n) are given in brackets. Paradoxical growth was determined with CLSI (Clinical and Laboratory Standards Institute)-[21,22,23], EUCAST (European Committee on Antimicrobial Susceptibility Testing)-[24], or metabolism-based [20] broth microdilution methods in RPMI 1640-based media.
3. Factors That Influence the Manifestation of the Paradoxical Effect
Apart from the chosen echinocandin and the examined species and strain, several additional external factors influence the manifestation of the paradoxical effect. These include the media composition, media additives but also the exact culturing conditions such as steadiness of the echinocandin concentration, the presence of additive chemicals or physical stress, or growth in a planktonic versus sessile phase. For example, Stevens et al. have shown that paradoxical growth of C. albicans is more pronounced in synthetic amino acid medium-fungal (SAAMF) compared to RPMI 1640 medium, and less pronounced in yeast nitrogen base medium (YNB). Since RPMI 1640 and SAAMF are both buffered to physiologic conditions, the differences do not solely depend on the pH [18]. Supplementation of RPMI 1640 with a high concentration of serum (50%) was shown to abolish the paradoxical effect of caspofungin. Interestingly, a lower serum concentration (10%) did not abolish but rather drastically increased the concentration required to induce the paradoxical effect [23]. At the same time, the MIC of caspofungin was not (10%) or only moderately (50%) affected by serum [18,23]. Lewis and colleagues reported that supplementation of RPMI with 5% mouse serum can suppress the paradoxical effect of caspofungin on A. fumigatus. However, these results should be considered with some caution because they are solely based on measuring the metabolic activity of Aspergillus mycelium [27]. In another study, 50% serum was able to suppress paradoxical growth of A. fumigatus and other Aspergillus species [28]. Notably, the effect of serum on the MIC or MEC (in the case of Aspergillus spp.) of echinocandins is controversial and was summarized and discussed elsewhere [29].
Another factor that was repeatedly reported to affect the occurrence of a paradoxical effect is sessile growth within a biofilm as compared to conventional growth as planktonic cells [25,30,31]. Melo et al. analyzed the manifestation of the paradoxical effect for several isolates of C. albicans, C. tropicalis, C. parapsilosis, C. orthopsilosis, and C. metapsilosis. Interestingly, paradoxical growth was more frequently observed when the isolates were grown as biofilms (24 of 30 isolates; 80%) compared to planktonic cells culturing conditions (12 of 30 isolates; 40%). Besides this, individual isolates also had a different concentration range for the occurrence of the paradoxical effect when grown as a biofilm [30]. These results were confirmed by Ferreira et al. [31]. Later, Walraven et al. performed similar experiments with clinical Candida isolates that harbor defined echinocandin resistance mutations in the fks1 gene, which encodes the β-GS. As expected, the echinocandin MICs of the different isolates were significantly increased when compared to susceptible strains. At the same time, the majority of the isolates still demonstrated paradoxical growth in the presence of extremely high echinocandin concentrations. Again, manifestation of the paradoxical effect of individual isolates depended significantly on the culturing condition (biofilm vs. planktonic) but also on the applied echinocandin derivative [25].
An interesting factor that was reported to significantly influence the appearance of the paradoxical effect is the steadiness of the echinocandin concentration. Stevens et al. already observed that inconstant drug concentration, which may occur when testing the susceptibility with a disc diffusion-like method on solid medium (agar), seems to suppress the paradoxical effect [18]. Similar observations were made by Shields et al. when investigating the postantifungal effect of caspofungin. They found that removal of caspofungin after exposure in a time-kill assay abolishes paradoxical growth. Interestingly, the intermittent exposure not only suppressed paradoxical growth but additionally increased the fungicidal activity of caspofungin when compared to unaltered or renewed drug exposure [32,33].
One important factor that determines the manifestation of the paradoxical effect is the echinocandin concentration itself. Apparently, the effect is only manifest in a certain high concentration range (approximately 4 to 32 µg mL−1) [17,18,19]. The reason for the absence of paradoxical growth at concentrations below this range but above the MIC or MEC (approximately 0.03 to 1 µg mL−1) is not known. However, we recently answered the question of why the paradoxical effect seems to disappear at very high concentrations (approximately >64 µg mL−1). We could show that the echinocandin caspofungin exerts additional antifungal activity against A. fumigatus besides inhibition of the β-GS at very high concentrations [34]. This additional activity suppresses the growth of otherwise paradoxically growing colonies and thereby explains the visual disappearance of the effect [34]. The exact mechanism behind the additional antifungal activity, however, remains unknown. Although possible alternative modes of action of echinocandins were proposed previously—for example, inhibition of β-1,6-glucan synthesis or induction of apoptosis [35,36,37]—none has been further elaborated or validated in successive studies.
4. The Clinical Relevance of the Paradoxical Effect
The question of whether the paradoxical effect is a mere in vitro phenomenon or could be of relevance in a clinical setting for the treatment of fungal infections was repeatedly raised and debated [18,33,38,39,40]. Unfortunately, this question still lacks a conclusive answer. So far, no clinical data directly support the hypothesis that the paradoxical effect could interfere with antifungal chemotherapy of candidiasis in humans [41,42,43,44,45]. Similar, the results of experimental studies in animal models that explored the potential benefit of high-dose echinocandin therapy against Candida species did not confirm the clinical relevance of the phenomenon [46,47]. On the contrary, several other experimental studies in murine or rabbit invasive aspergillosis models that focused on the efficacy of echinocandin dose escalation suggested a possible role of the paradoxical effect, mostly reflected by a moderate increase in the fungal burden at higher doses [27,48,49,50]. A similar result was obtained in a murine intraperitoneal candidiasis model in a study that specifically addressed the role of paradoxical growth of a C. tropicalis isolate treated with caspofungin [51]. Interestingly, one study that investigated the paradoxical effect in a neutropenic murine model of invasive aspergillosis specifically associated the phenomenon with caspofungin and not with micafungin, which would be in good agreement with the differing in vitro data for these two echinocandins [20,27]. Nevertheless, the repeatedly described paradoxical effect in animal models finally appeared to not significantly affect the overall survival [27,48,49,50,51].
Taken together, the evidence obtained with animal experiments suggests the occurrence of the paradoxical effect under in vivo conditions. However, the clinical significance remains unknown. It has to be noted that several of the paradoxical effect influencing the conditions mentioned above specifically play a role under in vivo conditions. Serum or similar factors could possibly suppress the manifestation of the effect. Continuous undulation of blood echinocandin levels during therapy as well as the chosen echinocandin derivative (caspofungin vs. micafungin/anidulafungin) could hinder manifestation as well. On the other hand, factors such as growth as a sessile pathogen in a microbial biofilm, a condition typically associated with catheter or implant infections, could facilitate a paradoxical effect that subsequently promotes the persistence of the infection in a niche under high-dose echinocandin therapy. Furthermore, clinically measurable significance might also be restricted to certain fungal species. For example, while C. dubliniensis showed paradoxical growth in up to 90% of the tested isolates [22], C. glabrata isolates almost never show the phenomenon [21,23,24]. Finally, the introduction of new echinocandin derivatives with exceptionally long-lived pharmacokinetic profiles, such as the echinocandin CD101 [52,53,54,55], could open up the question of whether the paradoxical effect manifests in vivo under constantly high drug levels.
5. Mechanistic Insights and Possible Signaling Pathways Involved
Despite great efforts to determine the factors that promote or repress paradoxical growth, the genetic and mechanistic basis of this phenomenon remains largely elusive. Many initial hypotheses to explain the paradoxical effect could be excluded: It has been demonstrated that paradoxical growth is not a result of resistance-associated mutations in the β-GS, upregulation of β-GS activity, inactivation, precipitation or degradation of the drug, or selection of a resistant subpopulation [18,19,56].
It was repeatedly reported that exposure to inhibitory echinocandin concentrations triggers a massive increase of cell wall chitin in Candida as well as in Aspergillus [14,34,56,57,58,59,60]. A similar drastic increase in cell wall chitin was observed after inhibition of β-GS expression in A. fumigatus [14]. This demonstrated that fungi must have a conserved stress response pathway that induces chitin synthesis upon depletion of cell wall glucan.
In fact, up to three signaling pathways were identified to upregulate chitin synthesis in response to echinocandin treatment. First, Walker et al. found that in C. albicans the cell wall integrity (CWI), the high osmolarity glycerol (HOG), and the calcineurin signaling pathways mutually stimulate the chitin synthases Chs2 and Chs8 to overcome echinocandin-induced stress [59]. Similar results were obtained for A. fumigatus. We have shown that disruption of the Wsc1 signaling branch of the CWI pathway increases the susceptibility of A. fumigatus to echinocandins [61]. However, direct evidence that links the susceptibility of such CWI signaling mutants with chitin synthesis is still lacking. The increase in total chitin synthase activity in the presence of caspofungin has been shown to be calcineurin-dependent [57]. This was revealed by analyses of A. fumigatus deletion mutants of the calcineurin A catalytic subunit CnaA and the downstream calcineurin-responsive zinc finger transcription factor CrzA. Interestingly, cnaA and crzA deletion mutants showed increased caspofungin susceptibility and no paradoxical growth [57]. Later, Lamoth et al. demonstrated that heat shock protein 90 (Hsp90) is involved in A. fumigatus calcineurin-dependent stress response to echinocandins. Similar to the cnaA and crzA mutants, inhibition of Hsp90 function results in increased caspofungin susceptibility and abolishes paradoxical growth [62,63].
Based on these results, it was tempting to speculate that the echinocandin-induced increase in cell wall chitin mediates paradoxical growth. However, we have recently shown that the chitin levels of paradoxically growing Aspergillus hyphae return to normal when compared to echinocandin-inhibited but non-paradoxically growing hyphae [34]. Moreover, we found that the paradoxical effect depends on expression of the β-GS and that paradoxically growing hyphae expose β-1,3-glucan again [34]. Importantly, the paradoxically growing hyphae show a rather normal morphology and emerge from an initially growth-inhibited, β-1,3-glucan-depleted, chitin-rich and hyperbranched mycelium that shows frequent hyphal lysis phenomena [14,34] (Figure 1). Interestingly, it takes approximately two to three days before any paradoxical growth can be observed [34]. These results were also confirmed by Moreno-Velásquez et al. [64]. Besides the switch in morphology, they showed that exposure to growth-inhibitory echinocandin concentrations causes a mislocalization of the β-GS to vacuoles. As soon as paradoxical growth begins, the β-GS showed renewed localization at the hyphal tips [64]. In summary, these data demonstrate that the paradoxical effect of echinocandins in the first line relies on the reconstitution of the β-GS activity.
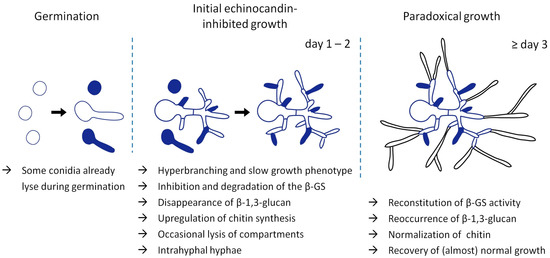
Figure 1.
Manifestation of paradoxical growth of A. fumigatus exposed to echinocandins. A limited number of conidia do not survive germination in the presence of echinocandin antifungals. Surviving microcolonies exposed to high echinocandin concentrations show a slow growth phenotype characterized by hyperbranching, occasional lysis of hyphal compartments (colored compartments), translocation of the β-GS to vacuoles, disappearance of cell wall β-1,3-glucan and compensatory increase of cell wall chitin (blue cell walls). Regenerative intrahyphal growth initiated from the septa occurs in some lysed compartments. After approximately 2–3 days, paradoxically growing hyphae emerge from the β-1,3-glucan-depleted and growth-inhibited microcolonies. These paradoxically growing hyphae are characterized by fast growth, normal morphology, renewed localization of the β-GS to the hyphal tips, reconstitution of β-1,3-glucan synthesis, and normalization of the cell wall chitin levels.
Nevertheless, the initial increase in cell wall chitin that is regularly observed in echinocandin-inhibited non-paradoxically growing fungi could represent an essential precondition. It has been shown for multiple fungal species that inhibition of chitin synthesis strengthens the fungicidal activity of echinocandins [23,65,66]. Consequently, the initial increase in cell wall chitin facilitates survival of the organisms upon inhibition of β-1,3-glucan synthesis. Survival is, of course, a prerequisite for the subsequent reconstitution of the β-GS activity and manifestation of the paradoxical effect (Figure 1). Still, the mechanism by which the fungus reconstitutes β-1,3-glucan synthesis remains unresolved.
The interesting observation of Moreno-Velásquez et al. that the β-GS localizes to vacuoles under growth inhibitory caspofungin concentrations suggests that echinocandins could change the enzyme’s conformation, thereby committing inhibited β-GS to degradation. Renewed localization to the hyphal tips and reconstitution of β-1,3-glucan synthesis during paradoxical growth could then indicate a conserved mechanism to stabilize or protect newly synthesized β-GS. It is, however, surprising that the manifestation of such a salvage mechanism appears to significantly depend on the echinocandin derivative, occurs only in certain strains and species, and requires days to manifest. To this end, many open questions remain about this mysterious echinocandin-specific phenomenon and should be addressed in future studies.
Author Contributions
Johannes Wagener and Veronika Loiko wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnson, M.E.; Edlind, T.D. Topological and mutational analysis of Saccharomyces cerevisiae Fks1. Eukaryot. Cell 2012, 11, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, A.; Drake, R.; Ng, K.; Diaquin, M.; Latgé, J.P. Characterization of the 1,3-β-glucan synthase of Aspergillus fumigatus. J. Gen. Microbiol. 1993, 139, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.J.; Brandt, K.; Capobianco, J.; Goldman, R. Characterization of (1,3)-β-glucan synthase in Candida albicans: Microsomal assay from the yeast or mycelial morphological forms and a permeabilized whole-cell assay. Microbiology 1994, 140, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Simenel, C.; Garnaud, C.; Clavaud, C.; Tada, R.; Barbin, L.; Mouyna, I.; Heddergott, C.; Popolo, L.; Ohya, Y.; et al. The Dual Activity Responsible for the Elongation and Branching of β-(1,3)-Glucan in the Fungal Cell Wall. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Free, S.J. Fungal cell wall organization and biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar] [PubMed]
- Thompson, J.R.; Douglas, C.M.; Li, W.; Jue, C.K.; Pramanik, B.; Yuan, X.; Rude, T.H.; Toffaletti, D.L.; Perfect, J.R.; Kurtz, M. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J. Bacteriol. 1999, 181, 444–453. [Google Scholar] [PubMed]
- Mazur, P.; Morin, N.; Baginsky, W.; el-Sherbeini, M.; Clemas, J.A.; Nielsen, J.B.; Foor, F. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol. 1995, 15, 5671–5681. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Alastruey-Izquierdo, A.; Healey, K.R.; Johnson, M.E.; Perlin, D.S.; Edlind, T.D. Fks1 and Fks2 are functionally redundant but differentially regulated in Candida glabrata: Implications for echinocandin resistance. Antimicrob. Agents Chemother. 2012, 56, 6304–6309. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.M.; D’Ippolito, J.A.; Shei, G.J.; Meinz, M.; Onishi, J.; Marrinan, J.A.; Li, W.; Abruzzo, G.K.; Flattery, A.; Bartizal, K.; et al. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 1997, 41, 2471–2479. [Google Scholar] [PubMed]
- Wiederhold, N.P. Echinocandin Resistance in Candida Species: A Review of Recent Developments. Curr. Infect. Dis. Rep. 2016, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Park, S.; Perlin, D.S. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: Implications for interpretive breakpoints. Antimicrob. Agents Chemother. 2009, 53, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: Implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.-A.; Slavin, M.A.; Sorrell, T.C. Echinocandin antifungal drugs in fungal infections: A comparison. Drugs 2011, 71, 11–41. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, K.; Samantaray, S.; Aimanianda, V.; Zhu, Z.; Prévost, M.-C.; Latgé, J.-P.; Ebel, F.; Wagener, J. Aspergillus fumigatus devoid of cell wall β-1,3-glucan is viable, massively sheds galactomannan and is killed by septum formation inhibitors. Mol. Microbiol. 2015, 95, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Mousset, S.; Buchheidt, D.; Heinz, W.; Ruhnke, M.; Cornely, O.A.; Egerer, G.; Krüger, W.; Link, H.; Neumann, S.; Ostermann, H.; et al. Treatment of invasive fungal infections in cancer patients-updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann. Hematol. 2014, 93, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.S.; Myles, C.; Pratt, K.J.; Washington, J.A. Cilofungin (LY121019), an antifungal agent with specific activity against Candida albicans and Candida tropicalis. Antimicrob. Agents Chemother. 1988, 32, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Espiritu, M.; Parmar, R. Paradoxical effect of caspofungin: Reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 2004, 48, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; White, T.C.; Perlin, D.S.; Selitrennikoff, C.P. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 2005, 51, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Antachopoulos, C.; Meletiadis, J.; Sein, T.; Roilides, E.; Walsh, T.J. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 2008, 52, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.E.; Albert, N.; Kontoyiannis, D.P. Paradoxical effect of Echinocandins across Candida species in vitro: Evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 2007, 51, 2257–2259. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, M.; Radecke, C.; Schulz, B.; Ruhnke, M. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Du, C.; Press, E.; Cheng, S.; Clancy, C.J. Paradoxical effect of caspofungin against Candida bloodstream isolates is mediated by multiple pathways but eliminated in human serum. Antimicrob. Agents Chemother. 2011, 55, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Sánchez-Carrillo, C.; Bouza, E.; Guinea, J. Frequency of the Paradoxical Effect Measured Using the EUCAST Procedure with Micafungin, Anidulafungin, and Caspofungin against Candida Species Isolates Causing Candidemia. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Walraven, C.J.; Bernardo, S.M.; Wiederhold, N.P.; Lee, S.A. Paradoxical antifungal activity and structural observations in biofilms formed by echinocandin-resistant Candida albicans clinical isolates. Med. Mycol. 2014, 52, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Fortwendel, J.R.; Juvvadi, P.R.; Perfect, B.Z.; Rogg, L.E.; Perfect, J.R.; Steinbach, W.J. Transcriptional regulation of chitin synthases by Calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents Chemother. 2010, 54, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Albert, N.D.; Kontoyiannis, D.P. Comparison of the dose-dependent activity and paradoxical effect of Caspofungin and Micafungin in a Neutropenic murine model of invasive pulmonary Aspergillosis. J. Antimicrob. Chemother. 2008, 61, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Elefanti, A.; Mouton, J.W.; Krompa, K.; Al-Saigh, R.; Verweij, P.E.; Zerva, L.; Meletiadis, J. Inhibitory and fungicidal effects of antifungal drugs against Aspergillus species in the presence of serum. Antimicrob. Agents Chemother. 2013, 57, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Nasar, A.; Ryan, L.; Frei, C.R.; Cota, J.M.; Wiederhold, N.P. Influence of Serum and Albumin on Echinocandin In Vitro Potency and Pharmacodynamics. Curr. Fungal Infect. Rep. 2013, 7, 89–95. [Google Scholar] [CrossRef]
- Melo, A.S.; Colombo, A.L.; Arthington-Skaggs, B.A. Paradoxical growth effect of Caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 2007, 51, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.G.; Carr, J.H.; Starling, C.E.F.; de Resende, M.A.; Donlan, R.M. Biofilm formation and effect of Caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob. Agents Chemother. 2009, 53, 4377–4384. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Clancy, C.J. Five-minute exposure to Caspofungin results in prolonged postantifungal effects and eliminates the paradoxical growth of Candida albicans. Antimicrob. Agents Chemother. 2011, 55, 3598–3602. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, B.; Henning, S.A.; Penzak, S.R.; Walsh, T.J. The postantifungal and paradoxical effects of Echinocandins against Candida spp. Future Microbiol. 2012, 7, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Loiko, V.; Wagener, J. The Paradoxical Effect of Echinocandins in Aspergillus fumigatus Relies on Recovery of the β-1,3-Glucan Synthase Fks1. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.S.; Current, W.L.; Goheen, M.P.; Boylan, C.J.; Lee, C.H.; Shaw, M.M.; Queener, S.F.; Smith, J.W. Semisynthetic echinocandins affect cell wall deposition of Pneumocystis carinii in vitro and in vivo. Antimicrob. Agents Chemother. 1996, 40, 1811–1816. [Google Scholar] [PubMed]
- Feldmesser, M.; Kress, Y.; Mednick, A.; Casadevall, A. The effect of the Echinocandin analogue Caspofungin on cell wall Glucan synthesis by Cryptococcus neoformans. J. Infect. Dis. 2000, 182, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Cheng, S.; Clancy, C.J.; Nguyen, M.H. 2013. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob. Agents Chemother. 2013, 57, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Paradoxical echinocandin activity: A limited in vitro phenomenon? Med. Mycol. 2009, 47 (Suppl. S1), S369–S375. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Andes, D.R. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb. Perspect. Med. 2014, 5, a019653. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Lamoth, F.; Juvvadi, P.R. Potential Microbiological Effects of Higher Dosing of Echinocandins. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S669–S677. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Rotstein, C.M.F.; Betts, R.F.; Nucci, M.; Talwar, D.; De Waele, J.J.; Vazquez, J.A.; Dupont, B.F.; Horn, D.L.; Ostrosky-Zeichner, L.; et al. Micafungin versus Caspofungin for treatment of Candidemia and other forms of invasive Candidiasis. Clin. Infect. Dis. 2007, 45, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Rodriguez, G.; Rolston, K.V.I.; O’Brien, S.; Khouri, I.F.; Shpall, E.J.; Keating, M.J.; Kantarjian, H.M.; Champlin, R.E.; Raad, I.I.; et al. High-dose caspofungin combination antifungal therapy in patients with hematologic malignancies and hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007, 39, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Rodriguez, G.; Zuniga, J.; Al Akhrass, F.; Pande, A. High-dose Caspofungin as a component of combination antifungal therapy in 91 patients with neoplastic diseases and hematopoietic stem cell transplantation: A critical review of short-term and long-term adverse events. J. Pharm. Pract. 2015, 28, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Betts, R.F.; Nucci, M.; Talwar, D.; Gareca, M.; Queiroz-Telles, F.; Bedimo, R.J.; Herbrecht, R.; Ruiz-Palacios, G.; Young, J.-A.H.; Baddley, J.W.; et al. Caspofungin High-Dose Study Group a Multicenter, double-blind trial of a high-dose Caspofungin treatment regimen versus a standard Caspofungin treatment regimen for adult patients with invasive Candidiasis. Clin. Infect. Dis. 2009, 48, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Nakamura, F.; Yoshimi, A.; Ichikawa, M.; Nannya, Y.; Kurokawa, M. Safety of high-dose Micafungin for patients with hematological diseases. Leuk. Lymphoma 2014, 55, 2572–2576. [Google Scholar] [CrossRef] [PubMed]
- Mariné, M.; Pastor, F.J.; Sahand, I.H.; Pontón, J.; Quindós, G.; Guarro, J. Paradoxical growth of Candida dubliniensis does not preclude in vivo response to Echinocandin therapy. Antimicrob. Agents Chemother. 2009, 53, 5297–5299. [Google Scholar] [CrossRef] [PubMed]
- Clemons, K.V.; Espiritu, M.; Parmar, R.; Stevens, D.A. Assessment of the paradoxical effect of Caspofungin in therapy of Candidiasis. Antimicrob. Agents Chemother. 2006, 50, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Bozza, S.; D’Angelo, C.; Casagrande, A.; Della Fazia, M.A.; Pitzurra, L.; Romani, L.; Aversa, F. Role of innate immune receptors in paradoxical Caspofungin activity in vivo in preclinical Aspergillosis. Antimicrob. Agents Chemother. 2012, 56, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- Petraitiene, R.; Petraitis, V.; Groll, A.H.; Sein, T.; Schaufele, R.L.; Francesconi, A.; Bacher, J.; Avila, N.A.; Walsh, T.J. Antifungal efficacy of Caspofungin (MK-0991) in experimental pulmonary Aspergillosis in persistently neutropenic rabbits: Pharmacokinetics, drug disposition, and relationship to Galactomannan antigenemia. Antimicrob. Agents Chemother. 2002, 46, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Kontoyiannis, D.P.; Chi, J.; Prince, R.A.; Tam, V.H.; Lewis, R.E. Pharmacodynamics of Caspofungin in a murine model of invasive pulmonary Aspergillosis: Evidence of concentration-dependent activity. J. Infect. Dis. 2004, 190, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Bayegan, S.; Majoros, L.; Kardos, G.; Kemény-Beke, A.; Miszti, C.; Kovacs, R.; Gesztelyi, R. In vivo studies with a Candida tropicalis isolate exhibiting paradoxical growth in vitro in the presence of high concentration of Caspofungin. J. Microbiol. 2010, 48, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Perez, W.B.; Jiménez-Ortigosa, C.; Hough, G.; Locke, J.B.; Ong, V.; Bartizal, K.; Perlin, D.S. CD101: A novel long-acting Echinocandin. Cell. Microbiol. 2016, 18, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N.; Castanheira, M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J. Antimicrob. Chemother. 2016, 71, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Ong, V.; Hough, G.; Schlosser, M.; Bartizal, K.; Balkovec, J.M.; James, K.D.; Krishnan, B.R. Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob. Agents Chemother. 2016, 60, 6872–6879. [Google Scholar] [CrossRef] [PubMed]
- Ong, V.; James, K.D.; Smith, S.; Krishnan, B.R. Pharmacokinetics of the Novel Echinocandin CD101 in Multiple Animal Species. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.; Cuenca-Estrella, M.; Zaragoza, O. Paradoxical growth of Candida albicans in the presence of Caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob. Agents Chemother. 2014, 58, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Fortwendel, J.R.; Juvvadi, P.R.; Pinchai, N.; Perfect, B.Z.; Alspaugh, J.A.; Perfect, J.R.; Steinbach, W.J. Differential effects of inhibiting chitin and 1,3-{β}-d-glucan synthesis in ras and Calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 2009, 53, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Ichinomiya, M.; Koshi, Y.; Horiuchi, H. Escape of Candida from Caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for β-1,6-glucan synthesis inhibition by Caspofungin. Antimicrob. Agents Chemother. 2006, 50, 3160–3161. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Munro, C.A.; de Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A.R. Stimulation of chitin synthesis rescues Candida albicans from Echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef] [PubMed]
- Bizerra, F.C.; Melo, A.S.A.; Katchburian, E.; Freymüller, E.; Straus, A.H.; Takahashi, H.K.; Colombo, A.L. Changes in cell wall synthesis and ultrastructure during paradoxical growth effect of caspofungin on four different Candida species. Antimicrob. Agents Chemother. 2011, 55, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, K.; Helmschrott, C.; Dirr, F.; Wagener, J. Deciphering cell wall integrity signalling in Aspergillus fumigatus: Identification and functional characterization of cell wall stress sensors and relevant Rho GTPases. Mol. Microbiol. 2012, 83, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Fortwendel, J.R.; Steinbach, W.J. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Gehrke, C.; Asfaw, Y.G.; Steinbach, W.J. Transcriptional activation of heat shock protein 90 mediated via a proximal promoter region as trigger of Caspofungin resistance in Aspergillus fumigatus. J. Infect. Dis. 2014, 209, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Velásquez, S.D.; Seidel, C.; Juvvadi, P.R.; Steinbach, W.J.; Read, N.D. Caspofungin-Mediated Growth Inhibition and Paradoxical Growth in Aspergillus fumigatus Involve Fungicidal Hyphal Tip Lysis Coupled with Regenerative Intrahyphal Growth and Dynamic Changes in β-1,3-Glucan Synthase Localization. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (Nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob. Agents Chemother. 2000, 44, 2547–2548. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.C.; Mavrogiorgos, N.; Tillem, E.; Hector, R.; Walsh, T.J. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between Nikkomycin Z and the Echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2001, 45, 3310–3321. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).