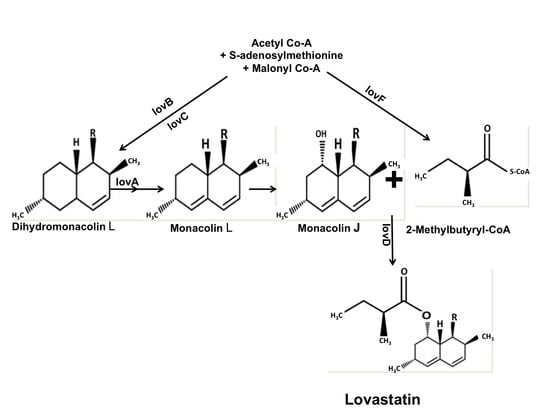
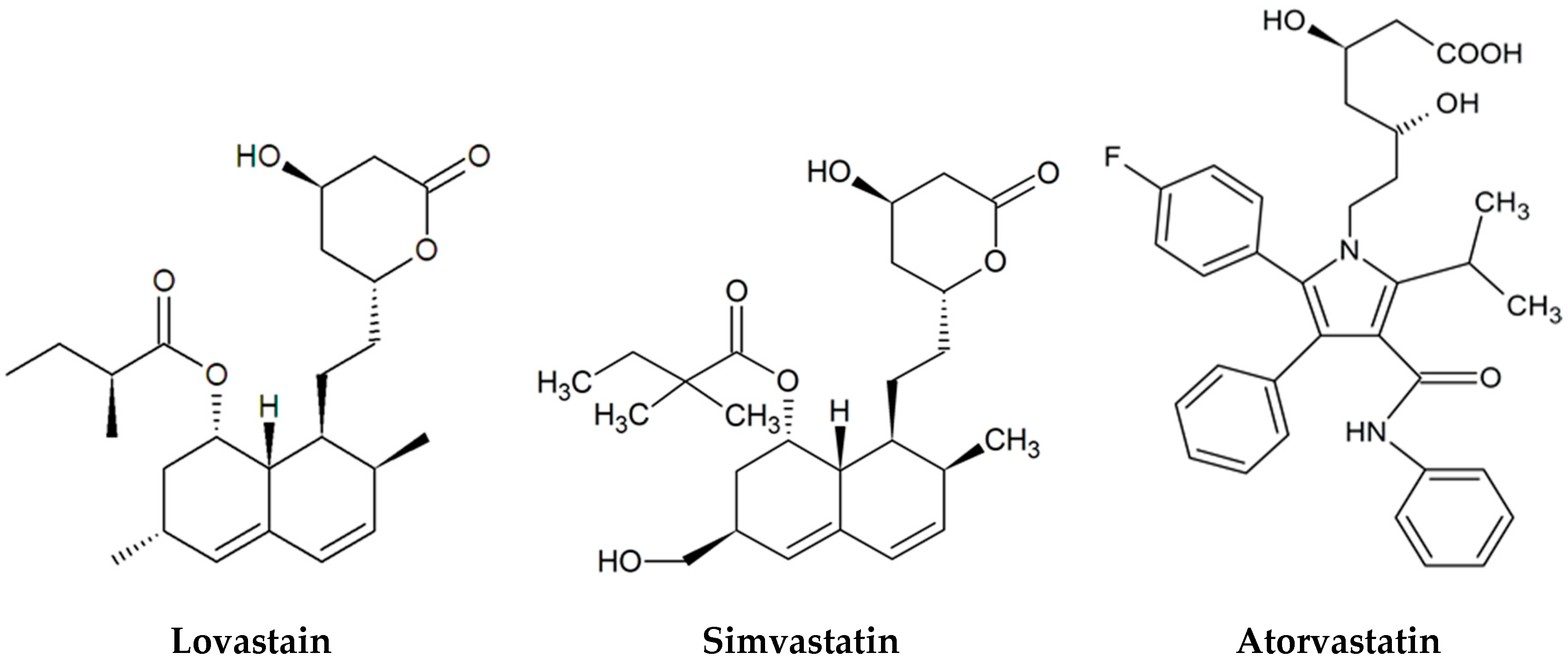
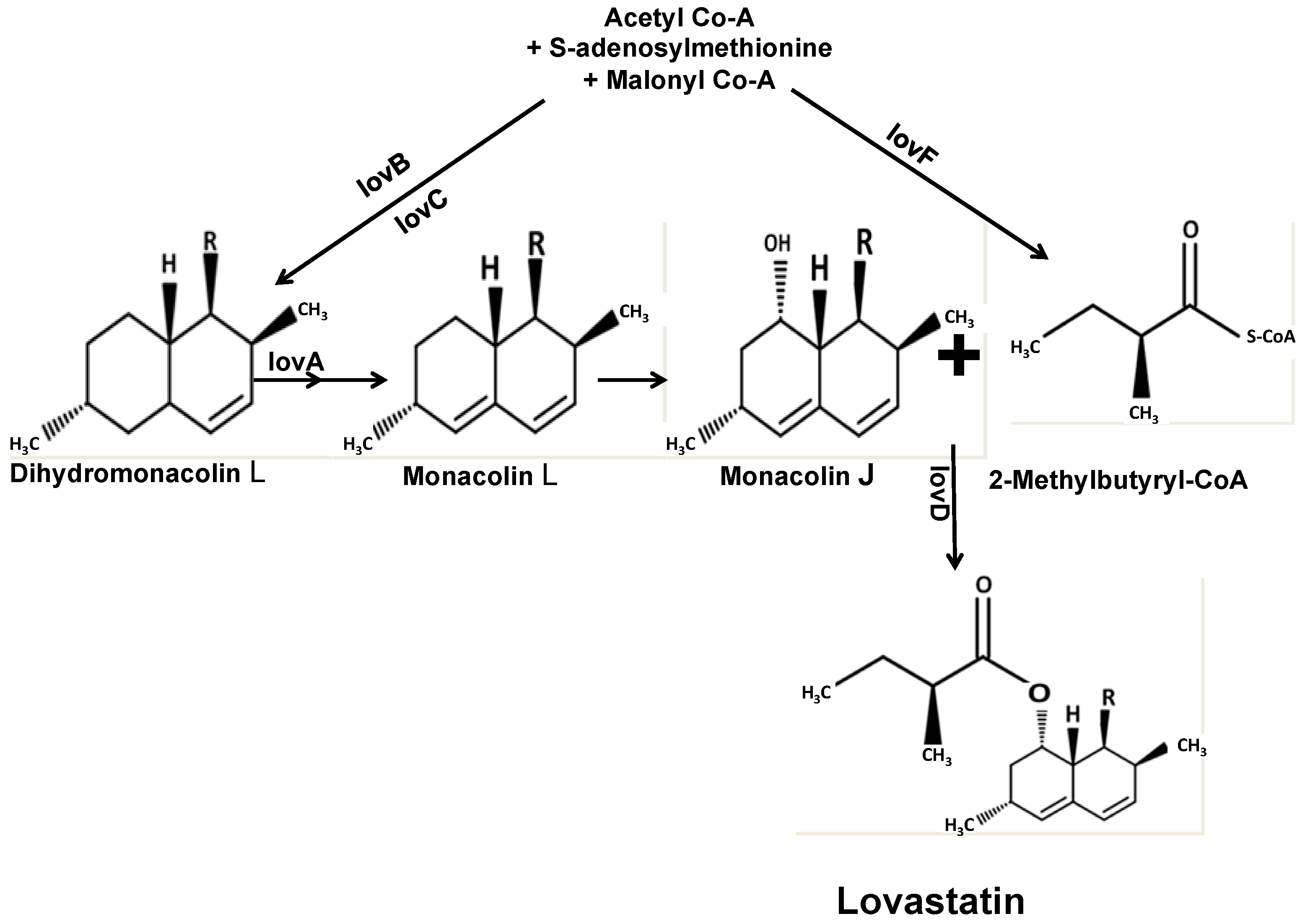
Exploitation of Aspergillus terreus for the Production of Natural Statins
Abstract
:1. Introduction
2. Discovery of Statins
3. Exploitation of A. terreus for Statin Production
3.1. Effect of Nutrients on Production of Statins
3.2. Feedback Inhibition Regulation Strategy
3.3. Effect of Other Additives
3.4. Mutagenesis for Strain Improvement
3.5. Systems Biology and Application of A. terreus Genome Knowledge
3.6. Statistical Designing
- Response surface methodology (RSM)
- Central composite design (CCD)
- Box−Behnken design (BBD)
- Plackett−Burman (PB)
- Taguchi design
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| gds | grams of dry support |
| SSF | solid state fermentation |
| CMC | carboxymethylcellulose |
| LDL | low density lipoprotein |
| EMS | ethyl methanesulfonate |
| NTG | N-Methyl-N’-nitro-N-nitrosoguanidine |
References
- Endo, A. The origin of the statins. Int. Congress Ser. 2004, 1262, 3–8. [Google Scholar] [CrossRef]
- Istvan, E. Statin inhibition of HMG-CoA reductase: A 3-dimensional view. Atheroscler. Suppl. 2003, 4, 3–8. [Google Scholar] [CrossRef]
- Bizukojc, M.; Ledakowicz, S. A macrokinetic modelling of the biosynthesis of lovastatin by Aspergillus terreus. J. Biotechnol. 2007, 130, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, A.; Subhagar, S.; Aravindan, R.; Viruthagiri, T. Microbial production and biomedical applications of lovastatin. Indian J. Pharm. Sci. 2008, 70, 701–709. [Google Scholar] [PubMed]
- Endo, A. A historical perspective on the discovery of statins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.C.; Huffman, M.; Ebrahim, S. Statin therapy for primary prevention of cardiovascular disease. JAMA 2013, 310, 2451–2452. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Lu, G.P.; Cai, N.S.; Wu, Z.G.; Li, Y.H.; Chen, H.; Zhu, J.Q.; Jin, X.J.; Wouters, B.C.; Zhao, J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: A randomized controlled trial. Arch. Intern. Med. 2003, 163, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.W., II; Shen, G.X. Statins as cellular antithrombotics. Pathophysiol. Haemost. Thromb. 1999, 29, 166–169. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Tangney, C.C. Antiatherothrombotic properties of statins: Implications for cardiovascular event reduction. JAMA 1998, 279, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.J.; Gotto, A.M., Jr.; Basson, C.T. The evolving role of statins in the management of atherosclerosis. J. Am. College Cardiol. 2000, 35, 1–10. [Google Scholar] [CrossRef]
- Chong, P.H.; Seeger, J.D.; Franklin, C. Clinically relevant differences between the statins: Implications for therapeutic selection. Am. J. Med. 2001, 111, 390–400. [Google Scholar] [CrossRef]
- De Sutter, J.; Tavernier, R.; De Buyzere, M.; Jordaens, L.; De Backer, G. Lipid lowering drugs and recurrences of life-threatening ventricular arrhythmias in high-risk patients. J. Am. College Cardiol. 2000, 36, 766–772. [Google Scholar] [CrossRef]
- Meier, C.R.; Schlienger, R.G.; Kraenzlin, M.E.; Schlegel, B.; Jick, H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA 2000, 283, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Glorioso, N.; Troffa, C.; Filigheddu, F.; Dettori, F.; Soro, A.; Parpaglia, P.P.; Collatina, S.; Pahor, M. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension 1999, 34, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Stossel, T.P. The discovery of statins. Cell 2008, 134, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Kuroda, M.; Tsujita, Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogensis produced by Penicillium citrinum. J. Antibiot. 1976, 29, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar] [PubMed]
- Endo, A. Monacloin K, a new hypochlesterolemic agent produced by a monascus species. J. Antibiot. 1979, 32, 853–854. [Google Scholar] [CrossRef]
- Sayyad, S.A.; Panda, B.P.; Javed, S.; Ali, M. Optimization of nutrient parameters for lovastatin production by Monascus purpureus MTCC 369 under submerged fermentation using response surface methodology. Appl. Microbiol. Biotechnol. 2006, 73, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Lee, C.-L.; Pan, T.-M. Improvement of monacolin K, γ-aminobutyric acid and citrinin production ratio as a function of environmental conditions of Monascus purpureus NTU 601. J. Ind. Microbiol. Biotechnol. 2003, 30, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Porcel, E.R.; López, J.L.C.; Ferrón, M.A.V.; Pérez, J.A.S.; Sánchez, J.L.G.; Chisti, Y. Effects of the sporulation conditions on the lovastatin production by Aspergillus terreus. Bioprocess Biosyst. Eng. 2006, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hajjaj, H.; Niederberger, P.; Duboc, P. Lovastatin biosynthesis by Aspergillus terreus in a chemically defined medium. Appl. Environ. Microbiol. 2001, 67, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Valera, H.R.; Gomes, J.; Lakshmi, S.; Gururaja, R.; Suryanarayan, S.; Kumar, D. Lovastatin production by solid state fermentation using Aspergillus flavipes. Enzyme Microb. Technol. 2005, 37, 521–526. [Google Scholar] [CrossRef]
- Samiee, S.M.; Moazami, N.; Haghighi, S.; Aziz Mohseni, F.; Mirdamadi, S.; Bakhtiari, M.R. Screening of lovastatin production by filamentous fungi. Iran. Biomed. J. 2003, 7, 29–33. [Google Scholar]
- MEVACOR™ Daily Tablets (Nonprescription lovastatin 20 mg). Available online: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4331b1-02-Merck.pdf (accessed on 1 January 2016).
- Barrios-González, J.; Miranda, R.U. Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol. 2009, 85, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Caetano, E.P.; de Oliveira, J.S.; Castelo-Branco, D.d.S.C.M.; Souza, E.R.Y.; de Alencar, L.P.; Cordeiro, R.d.A.; Bandeira, T.d.J.P.G.; Sidrim, J.J.C.; Rocha, M.F.G. Simvastatin inhibits planktonic cells and biofilms of Candida and Cryptococcus species. Braz. J. Infect. Dis. 2015, 19, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Kontoyiannis, D.P.; Wan, Z.; Li, R.; Liu, W. Antifungal activity of statins against Aspergillus species. Med. Mycol. 2007, 45, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Wikhe, K.; Westermeyer, C.; Macreadie, I.G. Biological consequences of statins in Candida species and possible implications for human health. Biochem. Soc. Trans. 2007, 35, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Macreadie, I.G.; Johnson, G.; Schlosser, T.; Macreadie, P.I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 2006, 262, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.T.; Parks, L.W. Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 1990, 34, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar] [PubMed]
- Galgóczy, L.; Nyilasi, I.; Papp, T.; Vágvölgyi, C. Are statins applicable for the prevention and treatment of zygomycosis? Clin. Infect. Dis. 2009, 49, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Lyons, C.N.; Holleman, S.; Oliver, B.G.; White, T.C. Antifungal activity of fluconazole in combination with lovastatin and their effects on gene expression in the ergosterol and prenylation pathways in Candida albicans. Med. Mycol. 2003, 41, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.E.; Figueroa, L.I.C.; Fariña, J.I. Synergistic antifungal activity of statin–azole associations as witnessed by Saccharomyces cerevisiae- and Candida utilis-bioassays and ergosterol quantification. Rev. Iberoam. Micol. 2013, 30, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.; Delgado, O.; Sampietro, D.; Catalan, C.; Figueroa, L.; Farina, J. Antifungal activity and the potential correlation with statin-producing ability: An optimized screening applied to filamentous fungi from las yungas subtropical rainforest. Res. J. Microbiol. 2010, 5, 833–848. [Google Scholar] [CrossRef] [Green Version]
- Babu, R.H.; Rupa, A.; Radha, S.; Prasad, N.B.L.; Narasimha, G. Screening of lovastatin producing fungi by yeast growth inhibition assay method. J. Pharmacy Res. 2011, 4, 2967–2968. [Google Scholar]
- Upendra, R.; Khandelwal, P.; Amiri, Z.; Shwetha, L.; Ausim, M. Screening and molecular characterization of natural fungal isolates producing lovastatin. Microb. Biochem. Technol. 2013, 5, 25–30. [Google Scholar]
- Lisec, B.; Radež, I.; Žilnik, L.F. Solvent extraction of lovastatin from a fermentation broth. Sep. Purif. Technol. 2012, 96, 187–193. [Google Scholar] [CrossRef]
- Hajko, P.; Vesel, T.; Radez, I.; Pokorny, M. Process for the isolation of lovastatin. Google Patents PCT/ISI1994/000010, 1994. [Google Scholar]
- Yang, D.-J.; Hwang, L.S. Study on the conversion of three natural statins from lactone forms to their corresponding hydroxy acid forms and their determination in Pu-Erh tea. J. Chromatogr. A 2006, 1119, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, Y.; Li, Y.; Wang, Y. Conversion investigation for lovastatin and its derivatives by HPLC. J. Chromatogr. Sci. 2010, 48, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Kumar, P.M.; Sarnaik, H.M.; Sadhukhan, A.K. A rapid technique for screening of lovastatin-producing strains of Aspergillus terreus by agar plug and Neurospora crassa bioassay. J. Microbiol. Methods 2000, 40, 99–104. [Google Scholar] [CrossRef]
- Bizukojc, M.; Pecyna, M. Lovastatin and (+)-geodin formation by Aspergillus terreus ATCC 20542 in a batch culture with the simultaneous use of lactose and glycerol as carbon sources. Eng. Life Sci. 2011, 11, 272–282. [Google Scholar] [CrossRef]
- Lai, L.-S.T.; Hung, C.-S.; Lo, C.-C. Effects of lactose and glucose on production of itaconic acid and lovastatin by Aspergillus terreus ATCC 20542. J. Biosci. Bioeng. 2007, 104, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E.; Chisti, Y. Production of lovastatin by Aspergillus terreus: Effects of the C:N ratio and the principal nutrients on growth and metabolite production. Enzym. Microb. Technol. 2003, 33, 270–277. [Google Scholar] [CrossRef]
- Lai, L.-S.T.; Tsai, T.-H.; Wang, T.C.; Cheng, T.-Y. The influence of culturing environments on lovastatin production by Aspergillus terreus in submerged cultures. Enzym. Microb. Technol. 2005, 36, 737–748. [Google Scholar] [CrossRef]
- Gupta, K.; Mishra, P.K.; Srivastava, P. A correlative evaluation of morphology and rheology of Aspergillus terreus during lovastatin fermentation. Biotechnol. Bioprocess Eng. 2007, 12, 140–146. [Google Scholar] [CrossRef]
- Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; Rodríguez Porcel, E.M.; Chisti, Y. Pellet morphology, culture rheology and lovastatin production in cultures of Aspergillus terreus. J. Biotechnol. 2005, 116, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Pansuriya, R.C.; Singhal, R.S. Response surface methodology for optimization of production of lovastatin by solid state fermentation. Braz. J. Microbiol. 2010, 41, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, V.; Janani, B.; Angayarkanni, J. Optimization of process parameters for lovastatin production from red gram bran by solid state fermentation. Int. J. Sci. Res. 2012, 3, 1413–1418. [Google Scholar]
- Osman, M.E.; Khattab, O.H.; Zaghlol, G.M.; El-Hameed, R.M.A. Optimization of some physical and chemical factors for lovastatin productivity by local strain of Aspergillus terreus. Aust. J. Basic Appl. Sci. 2011, 5, 718–732. [Google Scholar]
- Pawlak, M.; Bizukojc, M. Feeding profile is not the sole factor influencing lovastatin production by Aspergillus terreus ATCC20542 in a continuous fed-batch stirred tank bioreactor. Biochem. Eng. J. 2013, 81, 80–89. [Google Scholar] [CrossRef]
- Bizukojc, M.; Pawlak, M.; Boruta, T.; Gonciarz, J. Effect of pH on biosynthesis of lovastatin and other secondary metabolites by Aspergillus terreus ATCC 20542. J. Biotechnol. 2012, 162, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W.; Li, M.; Song, H.-P.; Feng, J.-L.; Tai, X.-S. Induction of a high-yield lovastatin mutant of Aspergillus terreus by 12c6+ heavy-ion beam irradiation and the influence of culture conditions on lovastatin production under submerged fermentation. Appl. Biochem. Biotechnol. 2011, 165, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.P.; Javed, S.; Ali, M. Optimization of fermentation parameters for higher lovastatin production in red mold rice through co-culture of monascus purpureus and monascus ruber. Food Bioprocess Technol. 2010, 3, 373–378. [Google Scholar] [CrossRef]
- Syed, M.B.; Rajasimman, M. Fermentative production and optimization of mevastatin in submerged fermentation using Aspergillus terreus. Biotechnol. Rep. 2015, 6, 124–128. [Google Scholar] [CrossRef]
- Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P. Lovastatin production by Aspergillus terreus using agro-biomass as substrate in solid state fermentation. J. Biomed. Biotechnol. 2012, 2012, 196264. [Google Scholar] [CrossRef] [PubMed]
- Gulyamova, T.; Ruzieva, D.; Masmetova, S.; Sattarova, R.; Lobanova, K.; Abdulmyanova, L.; Rasulova, G. Lovastatin production by Aspergillus terreus in solid state and submerged fermentations. Int. J. Eng. Sci. Technol. 2013, 5, 19–24. [Google Scholar] [CrossRef]
- Patil, R.H.; Krishnan, P.; Maheshwari, V.L. Production of lovastatin by wild strains of Aspergillus terreus. Natl. Prod. Commun. 2011, 6, 183–186. [Google Scholar] [PubMed]
- Baños, J.G.; Tomasini, A.; Szakács, G.; Barrios-González, J. High lovastatin production by Aspergillus terreus in solid-state fermentation on polyurethane foam: An artificial inert support. J. Biosci. Bioeng. 2009, 108, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.-l.; Xu, Z.-n.; Cen, P.-l. Lovastatin production by Aspergillus terreus in solid-state fermentation. J. Zhejiang Univ. Sci. A 2007, 8, 1521–1526. [Google Scholar] [CrossRef]
- Sorrentino, F.; Roy, I.; Keshavarz, T. Impact of linoleic acid supplementation on lovastatin production in Aspergillus terreus cultures. Appl. Microbiol. Biotechnol. 2010, 88, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, X.; Zhao, Y.; Cao, X. Enhancement of lovastatin production by supplementing polyketide antibiotics to the submerged culture of Aspergillus terreus. Appl. Biochem. Biotechnol. 2010, 160, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, X.; Zhao, Y.; Cao, X. Effects of divalent metal cations on lovastatin biosynthesis from Aspergillus terreus in chemically defined medium. World J. Microbiol. Biotechnol. 2009, 25, 1235–1241. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, X.; Cao, X. Effects of carbon sources on fungal morphology and lovastatin biosynthesis by submerged cultivation of Aspergillus terreus. Asia-Pacific J. Chem. Eng. 2009, 4, 672–677. [Google Scholar] [CrossRef]
- Porcel, E.M.R.; López, J.L.C.; Pérez, J.A.S.; Chisti, Y. Lovastatin production by Aspergillus terreus in a two-staged feeding operation. J. Chem. Technol. Biotechnol. 2008, 83, 1236–1243. [Google Scholar] [CrossRef]
- Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E.; Chisti, Y. Fermentation optimization for the production of lovastatin by Aspergillus terreus: Use of response surface methodology. J. Chem. Technol. Biotechnol. 2004, 79, 1119–1126. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, A.; Saini, H.; Chadha, B. Screening and selection of lovastatin hyper-producing mutants of Aspergillus terreus using cyclic mutagenesis. Acta Microbiol. Immunol. Hung. 2009, 56, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Luthra, U.; Singh, N.; Tripathi, A.; Vora, S.; Bhosle, V. Media optimization for lovastatin production by statistical approach using Aspergillus terreus by submerged fermentation. J. Med. Sci. Clin. Res. 2015, 3, 4520–4528. [Google Scholar]
- Nasmetova, S.; Ruzieva, D.; Rasulova, G.; Sattarova, R.; Gulyamova, T. Effect of the principal nutrients on simvastatin production by wild strain Aspergillus terreus 20 in submerged fermentation. Int. J. Curr. Microbiol. App. Sci 2015, 4, 894–898. [Google Scholar]
- Abd Rahim, M.H.; Hasan, H.; Montoya, A.; Abbas, A. Lovastatin and (+)-geodin production by Aspergillus terreus from crude glycerol. Eng. Life Sci. 2015, 15, 220–228. [Google Scholar] [CrossRef]
- Kumar, M.S.; Jana, S.K.; Senthil, V.; Shashanka, V.; Kumar, S.V.; Sadhukhan, A.K. Repeated fed-batch process for improving lovastatin production. Process Biochem. 2000, 36, 363–368. [Google Scholar] [CrossRef]
- Manzoni, M.; Rollini, M.; Bergomi, S.; Cavazzoni, V. Production and purification of statins from Aspergillus terreus strains. Biotechnol. Tech. 1998, 12, 529–532. [Google Scholar] [CrossRef]
- Gulyamova, T.; Nasmetova, S.; Ruzieva, D.; Ziyavitdinov, J.; Sattarova, R.; Rasulova, G. Composition of statins produced by indigenous strain of Aspergillus terreus. Int. J. Eng. Sci. Technol. 2014, 6, 71–76. [Google Scholar] [CrossRef]
- Raina, S.; De Vizio, D.; Palonen, E.K.; Odell, M.; Brandt, A.M.; Soini, J.T.; Keshavarz, T. Is quorum sensing involved in lovastatin production in the filamentous fungus Aspergillus terreus? Process Biochem. 2012, 47, 843–852. [Google Scholar] [CrossRef]
- Bizukojc, M.; Pawlowska, B.; Ledakowicz, S. Supplementation of the cultivation media with b-group vitamins enhances lovastatin biosynthesis by Aspergillus terreus. J. Biotechnol. 2007, 127, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Bonnarme, P.; Gillet, B.; Sepulchre, A.M.; Role, C.; Beloeil, J.C.; Ducrocq, C. Itaconate biosynthesis in Aspergillus terreus. J. Bacteriol. 1995, 177, 3573–3578. [Google Scholar] [PubMed]
- Barrios-Gonzalez, J.; Fernandez, F.; Tomasini, A. Microbial secondary metabolites production and strain improvement. Indian J. Biotechnol. 2003, 2, 322–333. [Google Scholar]
- Vilches Ferrón, M.A.; Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; Chisti, Y. Rapid screening of Aspergillus terreus mutants for overproduction of lovastatin. World J. Microbiol. Biotechnol. 2005, 21, 123–125. [Google Scholar] [CrossRef]
- Sreedevi, K.; VenkateswaraRao, J.; Lakshmi, N.; Fareedullah, M.d. Strain improvement of Aspergillus terreus for the enhanced production of lovastatin, a HMG-COA reductase inhibitor. J. Microbiol. Biotech 2011, 1, 96–100. [Google Scholar]
- Mukhtar, H.; Ijaz, S.S.; Ikram-ul-Haq. Upstream and downstream processing of lovastatin by Aspergillus terreus. Cell Biochem. Biophys. 2014, 70, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, X.; Cao, X.; Liu, J.; Qin, B. Production of lovastatin by a self-resistant mutant of Aspergillus terreus. Ann. Microbiol. 2011, 61, 615–621. [Google Scholar] [CrossRef]
- Vinci, V.A.; Hoerner, T.D.; Coffman, A.D.; Schimmel, T.G.; Dabora, R.L.; Kirpekar, A.C.; Ruby, C.L.; Stieber, R.W. Mutants of a lovastatin-hyperproducing Aspergillus terreus deficient in the production of sulochrin. J. Ind. Microbiol. 1991, 8, 113–119. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.M. Cloning and bioinformatic analysis of lovastatin biosynthesis regulatory gene lovE. Chin. Med. J. 2009, 122, 1800–1805. [Google Scholar] [PubMed]
- Barrios-González, J.; Baños, J.G.; Covarrubias, A.A.; Garay-Arroyo, A. Lovastatin biosynthetic genes of Aspergillus terreus are expressed differentially in solid-state and in liquid submerged fermentation. Appl. Microbiol. Biotechnol. 2008, 79, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.D.; Vederas, J.C. Biosynthesis of lovastatin and related metabolites formed by fungal iterative PKS enzymes. Biopolymers 2010, 93, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Auclair, K.; Kendrew, S.G.; Park, C.; Vederas, J.C.; Richard Hutchinson, C. Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science 1999, 284, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, L.; Ray Davis, C.; Roach, C.; Kim Nguyen, D.; Aldrich, T.; McAda, P.C.; Reeves, C.D. Lovastatin biosynthesis in Aspergillus terreus: Characterization of blocked mutants, enzyme activities and a multifunctional polyketide synthase gene. Chem. Biol. 1999, 6, 429–439. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Kennedy, J.; Park, C.; Kendrew, S.; Auclair, K.; Vederas, J. Aspects of the biosynthesis of non-aromatic fungal polyketides by iterative polyketide synthases. Antonie van Leeuwenhoek 2000, 78, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Askenazi, M.; Driggers, E.M.; Holtzman, D.A.; Norman, T.C.; Iverson, S.; Zimmer, D.P.; Boers, M.-E.; Blomquist, P.R.; Martinez, E.J.; Monreal, A.W.; et al. Integrating transcriptional and metabolite profiles to direct the engineering of lovastatin-producing fungal strains. Nat. Biotech. 2003, 21, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Pasma, S.A.; Daik, R.; Maskat, M.Y.; Hassan, O. Application of box-behnken design in optimization of glucose production from oil palm empty fruit bunch cellulose. Int. J. Polymer Sci. 2013, 2013, 8. [Google Scholar] [CrossRef]
- Rahman, S.H.A.; Choudhury, J.P.; Ahmad, A.L.; Kamaruddin, A.H. Optimization studies on acid hydrolysis of oil palm empty fruit bunch fiber for production of xylose. Bioresour. Technol. 2007, 98, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.-S.T.; Pan, C.-C.; Tzeng, B.-K. The influence of medium design on lovastatin production and pellet formation with a high-producing mutant of Aspergillus terreus in submerged cultures. Process Biochem. 2003, 38, 1317–1326. [Google Scholar] [CrossRef]
- Syed, M.B.; Rajendran, A.; Seraman, S.; Thangavelu, V. Valorization of agricultural residues for compactin production by Aspergillus terreus MTCC 279 in mixed substrate solid state fermentation. Waste Biomass Valoriz. 2013, 5, 715–724. [Google Scholar] [CrossRef]
- Goswami, S.; Bhunia, B.; Mandal, T. Optimization of media components for lovastatin production from Aspergillus terreus (JX081272) using Taguchi methodology. J. Bioprocess Eng. Biorefinery 2013, 2, 46–53. [Google Scholar] [CrossRef]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of box-behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod. Process. 2009, 87, 139–144. [Google Scholar] [CrossRef]
- Vohra, A.; Satyanarayana, T. Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Process Biochem. 2002, 37, 999–1004. [Google Scholar] [CrossRef]
- Muthukumar, M.; Mohan, D.; Rajendran, M. Optimization of mix proportions of mineral aggregates using box behnken design of experiments. Cement Concr. Compos. 2003, 25, 751–758. [Google Scholar] [CrossRef]
- Oh, S.; Rheem, S.; Sim, J.; Kim, S.; Baek, Y. Optimizing conditions for the growth of Lactobacillus casei YIT 9018 in tryptone-yeast extract-glucose medium by using response surface methodology. Appl. Environ. Microbiol. 1995, 61, 3809–3814. [Google Scholar] [PubMed]
- Gomes, A.M.P.; Malcata, F.X. Development of probiotic cheese manufactured from goat milk: Response surface analysis via technological manipulation. J. Dairy Sci. 1998, 81, 1492–1507. [Google Scholar] [CrossRef]
| A. terreus Strain | Solid Substrate | Yield | References |
|---|---|---|---|
| MTCC 279 | Green peas, Millet, Ragi | 389.34 mg/gds | [59] |
| ATCC 74135 | Rice straw | 0.261 mg/g | [60] |
| 4 | Wheat bran | 9.7 mg/g | [61] |
| 20 | Oat bran | 9.5 mg/g | [61] |
| PM3 | Wheat bran | 12.5 mg/g | [62] |
| UV 1718 | Wheat bran | 3.723 mg/g | [52] |
| * | Lactose, Soybean meal | 19.95 mg/g | [63] |
| ATCC 20542 | Rice powder, Glucose | 2.9 mg/g | [64] |
| A. terreus Strain | Carbon Source | Nitrogen Source | Yield (mg/L) | References |
|---|---|---|---|---|
| ATCC 20542 | Lactose, Glycerol | Yeast extract | 161.8 | [46] |
| Z15-7 | Glycerol | Corn meal, Sodium nitrate | 916.7 | [57] |
| MUCL 38669 | Lactose, Glucose | Peptonized milk, Yeast extract | 212.5 | [65] |
| LA414 | Soluble starch | Yeast extract | 952.7 | [66] |
| LA414 | Soluble starch | Sodium glutamate | 523.9 | [67] |
| LA414 | Glycerol | Yeast extract | 937.5 | [68] |
| ATCC 20542 | Lactose | Soybean meal | 140 | [69] |
| NRRL 255 | Glucose, malt extract | Milk powder, Soybean meal | 920 | [50] |
| ATCC 20542 | Lactose | Soybean meal | 186.5 | [23] |
| ATCC 20542 | Lactose | Soybean meal | 80 | [70] |
| ATCC 20542 | Lactose | Soybean meal | 250 | [51] |
| GD13 | Lactose | Soybean meal | 1242 | [71] |
| * | Glucose | Soybean meal | 110.78 | [59] |
| ATCC 20542 | Lactose | Yeast extract | 83.8 | [55] |
| * | Dextrose | Soy flour | 100 | [72] |
| 20 | Lactose | Yeast extract | 120 | [73] |
| ATCC 20542 | Crude glycerol | Yeast extract | 300 | [74] |
| A. terreus Strain | Additive (Concentration) | Yield (mg/L) | Reference |
|---|---|---|---|
| ATCC 20542 | Polyketide Antibiotics (50 mg/L) | 952.7 | [66] |
| ATCC 20542 | Itaconic acid (0.5 g/L) | 953.3 | [47] |
| PM3 | CMC (1%) | 240 | [62] |
| MUCL 38669 | Linoleic acid (320 μM) | 212.5 | [65] |
| MUCL 38669 | Butyrolactone I (100 nM) | 3100 | [78] |
| ATCC 20542 | B-group vitamins (0.5–5 mg/L) | Unknown | [79] |
| ATCC 20542 | Divalent metal cations (5 mM) | 524 | [67] |
| A. terreus Strain | Mode of Mutation | A. terreus Strain after Mutation | Improved Yield (mg/L) | Fold Increase | References |
|---|---|---|---|---|---|
| GD 13 | UV | EM 19 | 1424 | 7.5× | [71] |
| 20452 | EMS | E354 | 60.3 | 4× | [82] |
| NRRL 265 | UV | UV-4 | 977.1 | 3.5× | [84] |
| MTCC 10831 | UV + EMS | SPUV002 | 663 | 1.8× | [83] |
| ATCC 20452 | UV | LA414 | 883.2 | 3× | [85] |
| CA99 | Heavy-ion beams | Z15-7 | 916.7 | 4× | [57] |
| AH6 | UV | CB4 | 58 | 1.16× | [86] |
| 20451 | EMS+UV+NTG | DRCC 122 | 2200 | 1.73× | [75] |
| DRCC 86 | EMS+UV | LS-3031 | 40 | 1.38× | [45] |
| A. terreus Strain | Statistical Models | Statins | Yield | Reference |
|---|---|---|---|---|
| ATCC 20542 | BBD | Lovastatin | 186.5 mg/L | [23] |
| Strain not given | PB, CCD | Mevastatin | 170.4 mg/L | [59] |
| ATCC 20542 | PB, FD, RSM | Lovastatin | 100 mg/L | [96] |
| MTCC 279 | CCD | Compactin | 389 mg/gds | [97] |
| MTCC 279 | CCD | Lovastatin | 1467 mg/gds | [97] |
| JX081272 | Taguchi Design | Lovastatin | 255 mg/L | [98] |
| UV 1718 | RSM, CCD | Lovastatin | 372 mg/g | [52] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhan, M.; Faryal, R.; Macreadie, I. Exploitation of Aspergillus terreus for the Production of Natural Statins. J. Fungi 2016, 2, 13. https://doi.org/10.3390/jof2020013
Subhan M, Faryal R, Macreadie I. Exploitation of Aspergillus terreus for the Production of Natural Statins. Journal of Fungi. 2016; 2(2):13. https://doi.org/10.3390/jof2020013
Chicago/Turabian StyleSubhan, Mishal, Rani Faryal, and Ian Macreadie. 2016. "Exploitation of Aspergillus terreus for the Production of Natural Statins" Journal of Fungi 2, no. 2: 13. https://doi.org/10.3390/jof2020013
APA StyleSubhan, M., Faryal, R., & Macreadie, I. (2016). Exploitation of Aspergillus terreus for the Production of Natural Statins. Journal of Fungi, 2(2), 13. https://doi.org/10.3390/jof2020013