Three New Species and Three New Records of Arthoniaceae (Ascomycota, Arthoniales) from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological and Chemical Analyses
2.2. DNA Extraction and PCR Sequencing
2.3. Phylogenetic Analyses
3. Results
3.1. Phylogenetic Results
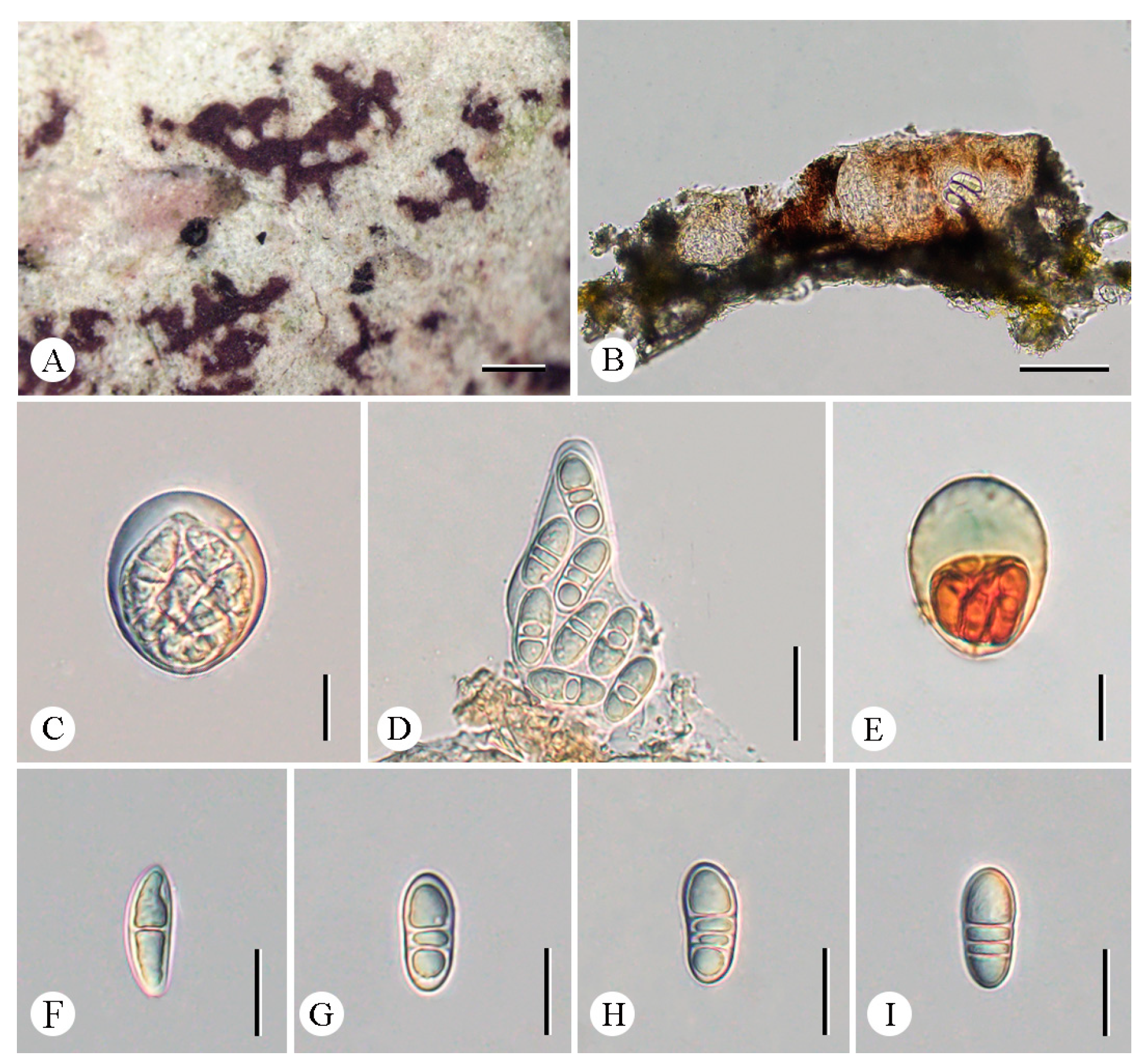
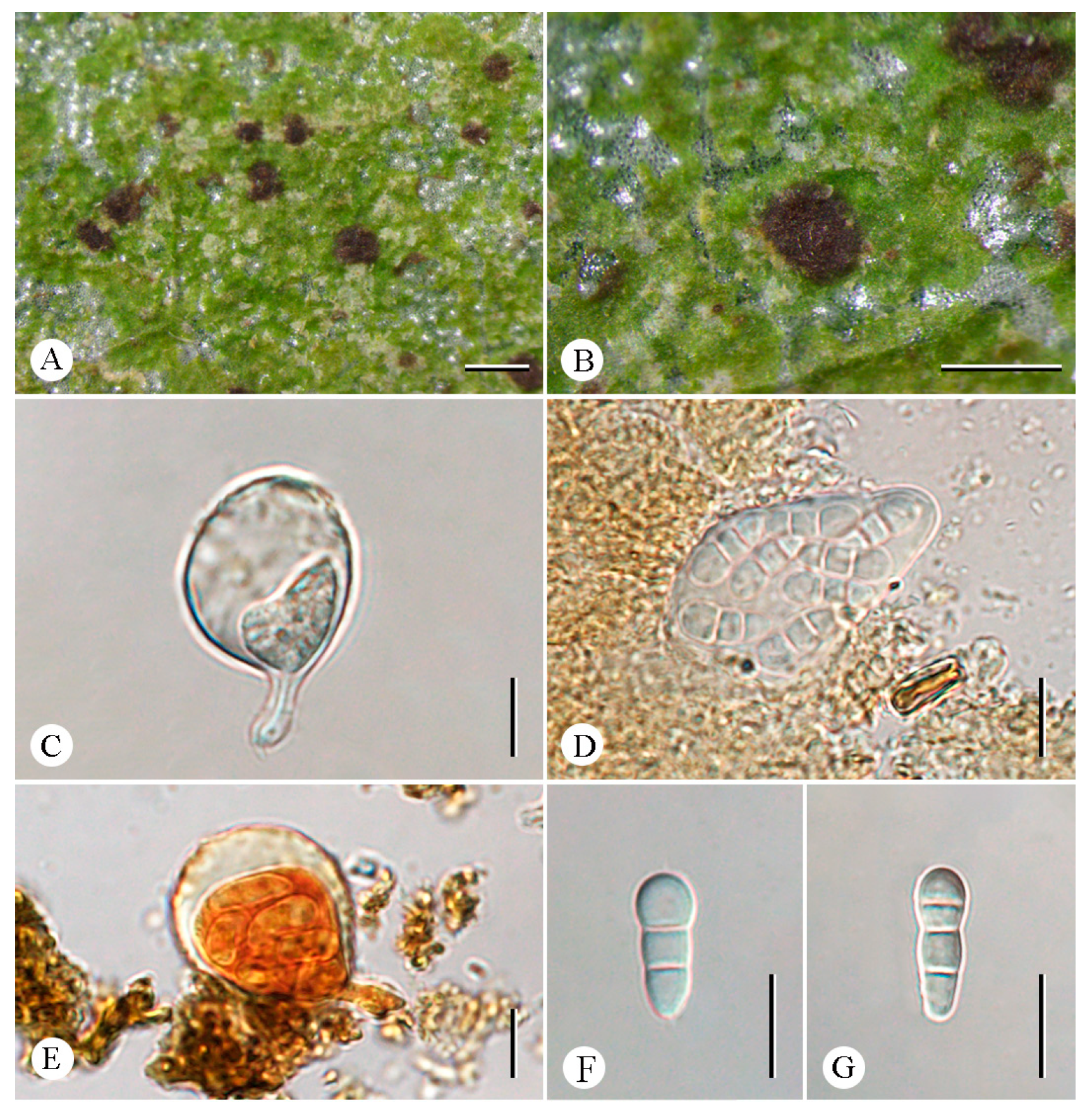
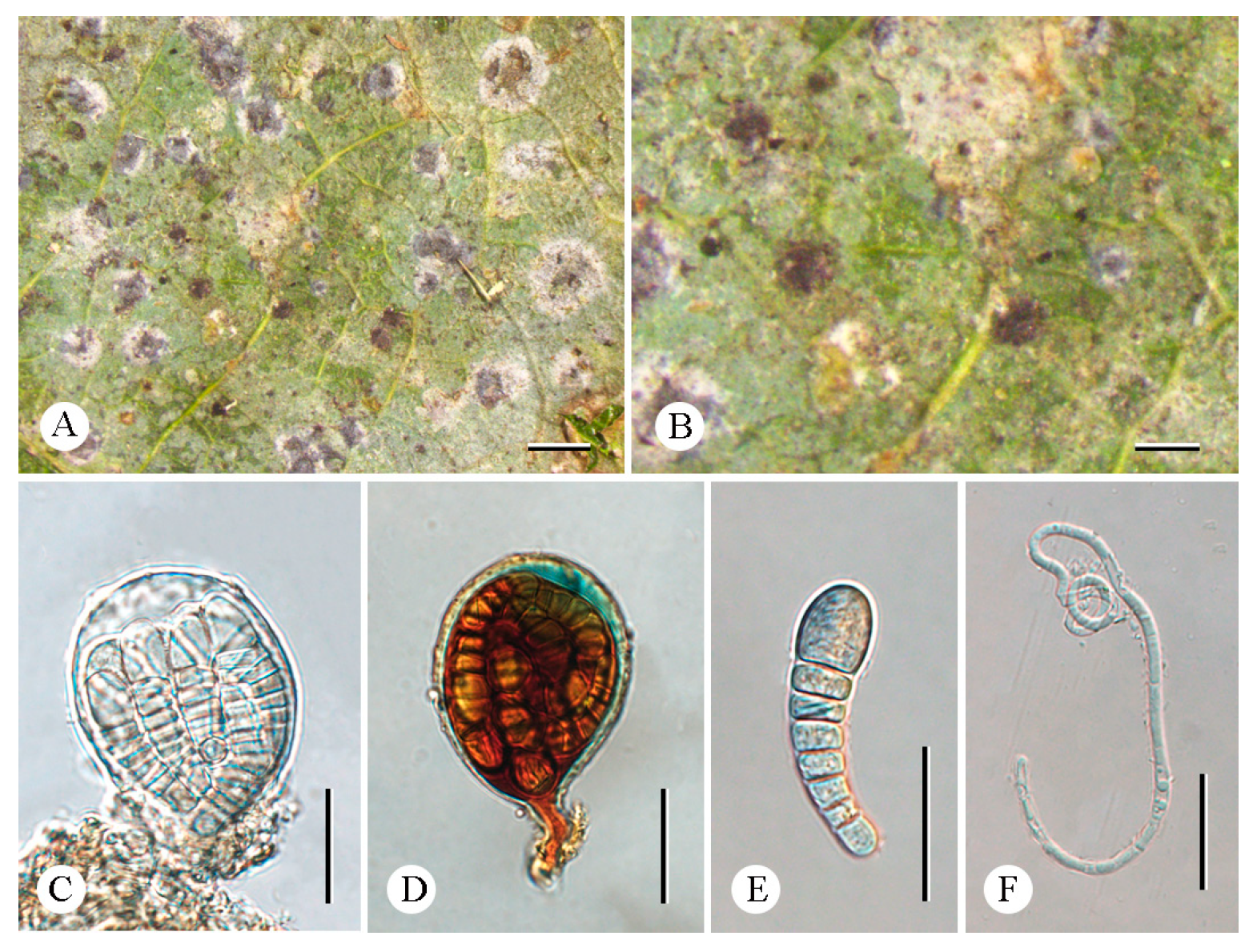
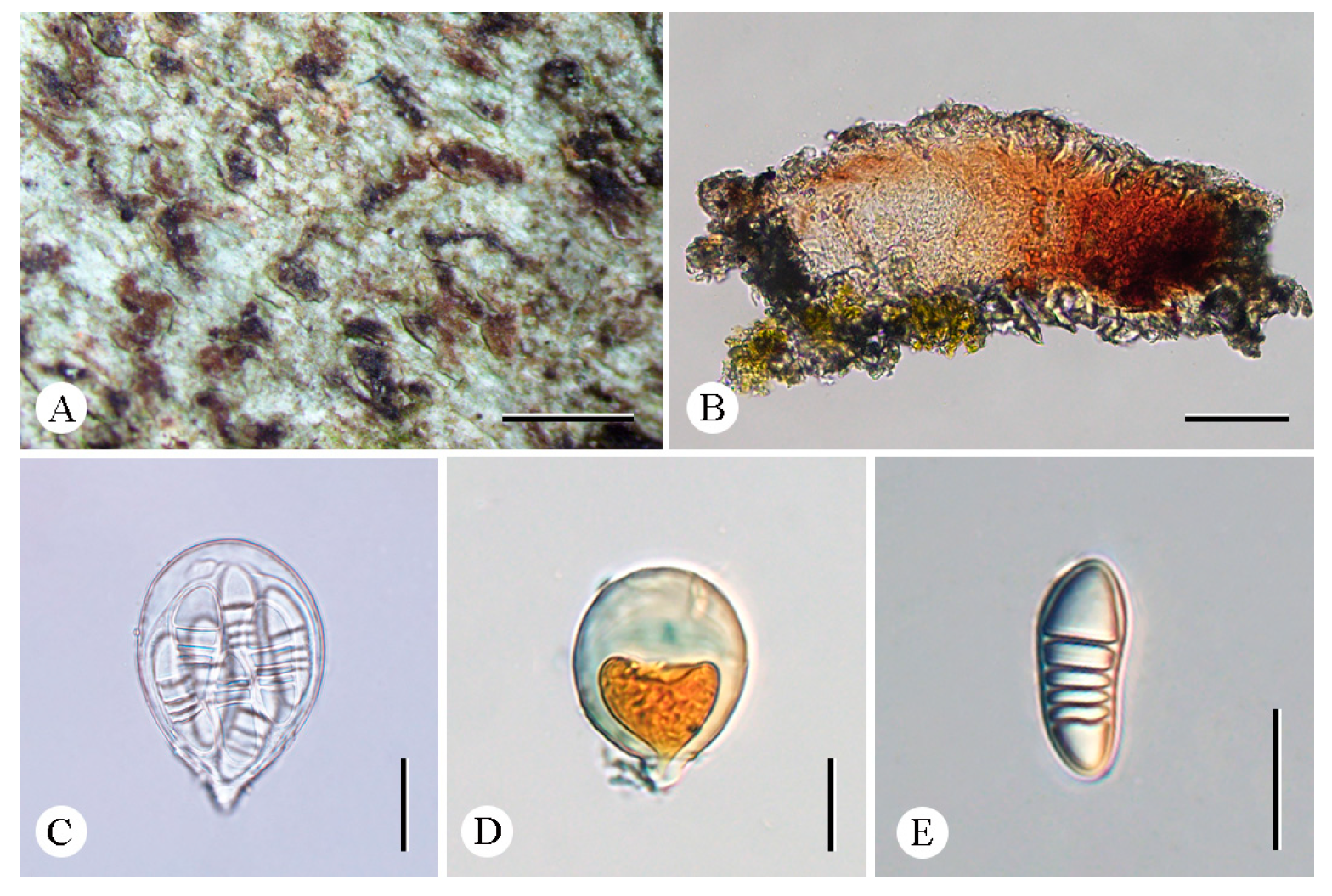
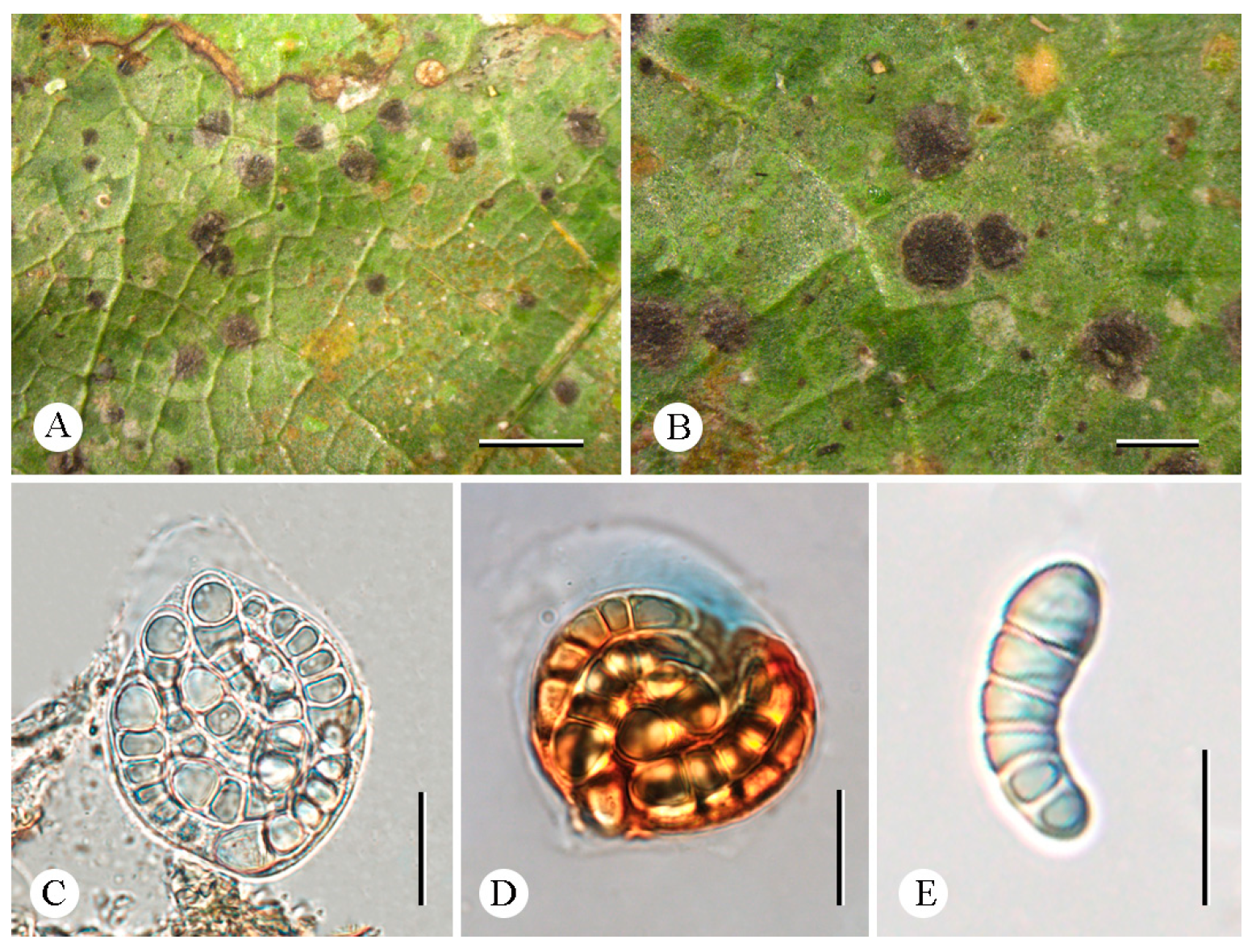
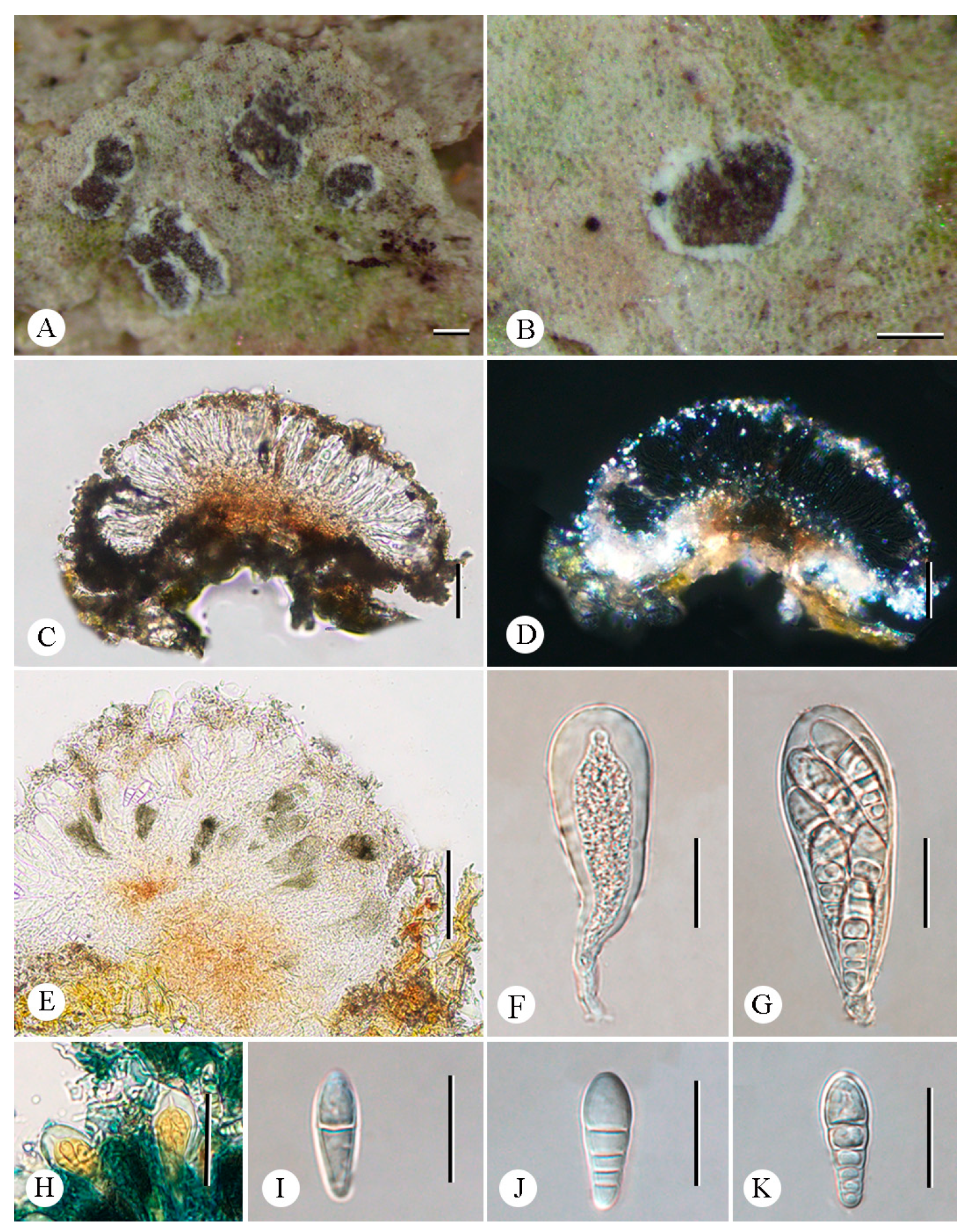
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichenbach, H.T.L. Der Deutsche Botaniker; Her barienbuch, D., Ed.; Arnoldische Buchandlung: Leipzig, Germany; Dresden, Germany, 1841; Volume 1, p. 240. [Google Scholar]
- Tehler, A. A new approach to the phylogeny of Euascomycetes with a cladistic outline of Arthoniales focussing on Roccellaceae. Can. J. Bot. 1990, 68, 2458–2492. [Google Scholar] [CrossRef]
- Grube, M. Classification and Phylogeny in the Arthoniales (Lichenized ascomycetes). Bryologist 1998, 101, 377–391. [Google Scholar] [CrossRef]
- Frisch, A.; Thor, G.; Moon, K.H.; Ohmura, Y. Arthonia incarnata (Arthoniaceae), a rare and poorly known old-growth forest lichen new to Asia. Nord. J. Bot. 2017, 35, 587–594. [Google Scholar] [CrossRef]
- Thiyagaraja, V.; Lücking, R.; Ertz, D.; Wanasinghe, D.N.; Karunarathna, S.C.; Camporesi, E.; Hyde, K.D. Evolution of non-lichenized, saprotrophic species of Arthonia (Ascomycota, Arthoniales) and resurrection of Naevia, with notes on Mycoporum. Fungal Divers. 2020, 102, 205–224. [Google Scholar] [CrossRef]
- Frisch, A.; Thor, G.; Ertz, D.; Grube, M. The Arthonialean challenge: Restructuring Arthoniaceae. Taxon 2014, 63, 727–744. [Google Scholar] [CrossRef]
- Aptroot, A.; Cáceres, M.E.D.S.; Santos, L.A.D. The taxonomy of sterile Arthoniaceae from Brazil: White crusts on overhanging tropical trees can be named. Lichenologist 2024, 56, 1–13. [Google Scholar] [CrossRef]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 2017, 119, 361. [Google Scholar] [CrossRef]
- Sundin, R.; Thor, G.; Frisch, A. A literature review of Arthonia s. lat. Bibl. Lichenol. 2012, 108, 257–290. [Google Scholar]
- Grube, M.; Matzer, M.; Hafellner, J. A Preliminary Account of the Lichenicolous Arthonia Species with Reddish, K+ Reactive Pigments. Lichenologist 1995, 27, 25–42. [Google Scholar] [CrossRef]
- Cannon, P.; Ertz, D.; Frisch, A.; Aptroot, A.; Chambers, S.; Coppins, B.; Sanderson, N.; Simkin, J.; Wolseley, P. Arthoniales: Arthoniaceae, including the genera Arthonia, Arthothelium, Briancoppinsia, Bryostigma, Coniocarpon, Diarthonis, Inoderma, Naevia, Pachnolepia, Reichlingia, Snippocia, Sporodophoron, Synarthonia and Tylophoron. Revis. Br. Ir. Lichens 2020, 1, 3–48. [Google Scholar]
- Sydow, H. Beitrag zur Kenntnis der Pilzflora der Philippinen-Inseln. Ann. Mycol. 1917, 15, 165–268. [Google Scholar]
- Sérusiaux, E. Reinstatement of the lichenized genus Eremothecella Sydow. Syst. Ascomycetum 1992, 11, 39–47. [Google Scholar]
- Lücking, R. Foliicolous Lichenized Fungi; Flora Neotropica Monograph: New York, NY, USA, 2008; p. 866. [Google Scholar]
- Santesson, R. Foliicolous Lichens 1. A Revision of the Taxonomy of the Obligately Foliicolous Lichenized Fungi; Symbolae Botanicae Upsalienses: Uppsala, Sweden, 1952; Volume 12, p. 590. [Google Scholar]
- Jagadeesh Ram, T.A.M.; Sinha, G.P. New species and first records of Eremothecella (Arthoniales) from the Andaman and Nicobar Islands, India. Lichenologist 2019, 51, 507–513. [Google Scholar] [CrossRef]
- Van Den Broeck, D.; Frisch, A.; Razafindrahaja, T.; Van De Vijver, B.; Ertz, D. Phylogenetic position of Synarthonia (lichenized Ascomycota, Arthoniaceae), with the description of six new species. Plant Ecol. Evol. 2018, 151, 327–351. [Google Scholar] [CrossRef]
- Joseph, S.; Sinha, G.P. Contributions to the genus Synarthonia (lichenized Ascomycota, Arthoniaceae). Lichenologist 2015, 47, 123–130. [Google Scholar] [CrossRef]
- Ertz, D.; Aptroot, A.; Sanderson, N.; Coppins, B.; Van Den Broeck, D.; Diederich, P. A new species of Synarthonia from Luxembourg, and a new combination in the genus Reichlingia (Arthoniaceae). Lichenologist 2020, 52, 261–266. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, S.; Jia, Z. Mazosia weii sp. nov. (Roccellaceae) from China, a new species supported by molecular data. Bryologist 2021, 124, 335–342. [Google Scholar] [CrossRef]
- Cui, C.; Dou, M.; Jiang, S.; Jia, Z. The Lichen Genus Letrouitia (Brigantiaeaceae, Ascomycota) in China. Diversity 2024, 16, 254. [Google Scholar] [CrossRef]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2001; p. 101. [Google Scholar]
- Zoller, S.; Scheidegger, C.; Sperisen, C. Pcr Primers for the Amplification of Mitochondrial Small Subunit Ribosomal DNA of Lichen-forming Ascomycetes. Lichenologist 1999, 31, 511–516. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Jia, Z.; Zhao, X. A New Species and Two New Records of the Lichen Genus Fissurina from China. Diversity 2023, 15, 959. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Fée, A.L.A. Essai sur les Cryptogames des Écorces Exotiques Officinales; Firmin Didot père et fils: Paris, France, 1825; p. 180. [Google Scholar]
- Nylander, W. Synopsis du genre Arthonia. Mémoires Société Impériale Sci. Nat. Cherbg. 1856, 4, 85–104. [Google Scholar]
- Willey, H. A Synopsis of the Genus Arthonia; Willey: New Bedford, MA, USA, 1890; p. 62. [Google Scholar]
- Seavey, F.; Seavey, J. Caloplaca lecanorae (Teloschistaceae), a new lichenicolous lichen and several additions to the North American lichenized mycota from Everglades National Park. Bryologist 2012, 115, 322–328. [Google Scholar] [CrossRef]
- Vainio, E.A. Lichenes insularum Philippinarum, III. Ann. Acad. Sci. Fenn. Ser. A 1921, 15, 1–368. [Google Scholar]
- Wang, W.C.; Wei, J.C. Arthonia, Byssoloma, Calenia, Chroodiscus, Coenogonium, Eremothecella, and Semigyalecta spp. new to China. Mycotaxon 2018, 133, 487–497. [Google Scholar] [CrossRef]
- Lücking, R.; Streimann, H.; Elix, J.A. Further records of foliicolous lichens and lichenicolous fungi from Australasia, with an updated checklist for continental Australia. Lichenologist 2001, 33, 195–210. [Google Scholar] [CrossRef]
- Esslinger, T.L. A Cumulative Checklist for the Lichen-Forming, Lichenicolous and Allied Fungi of the Continental United States and Canada, Version 21. Opusc. Philolichenum 2016, 15, 136–390. [Google Scholar] [CrossRef]
- Diederich, P.; Common, R.S.; Braun, U.; Heuchert, B.; Millanes, A.; Suija, A.; Ertz, D. Lichenicolous fungi from Florida growing on Graphidales. Plant Fungal Syst. 2019, 64, 249–282. [Google Scholar] [CrossRef]
- Stirton, J. New and rare lichens from India and the Himalayas. Proc. Philos. Soc. Glasg. 1879, 11, 306–322. [Google Scholar]
- Nakanishi, M. Notes on lichen species of Graphis of the Yaeyama Islands, Japan. Hikobia Suppl. 1981, 1, 211–220. [Google Scholar]
- Müller, J. Lichenes. In Primitiae Florae Costaricensis; Durand, T.H., Pittier, H., Eds.; Bulletin de la Société Royale de Botanique de Belgique: Brussels, Belgium, 1891; Volume 30, pp. 49–97. [Google Scholar]
- Dodge, C.W. Some lichens of tropical Africa. Ann. Mo. Bot. Gard. 1953, 40, 271–401. [Google Scholar] [CrossRef]
- Jiang, S.H.; Lücking, R.; Xavier-Leite, A.B.; Cáceres, M.E.S.; Aptroot, A.; Portilla, C.V.; Wei, J.C. Reallocation of foliicolous species of the genus Strigula into six genera (lichenized Ascomycota, Dothideomycetes, Strigulaceae). Fungal Divers. 2020, 102, 257–291. [Google Scholar] [CrossRef]
- Lebreton, E.; Ertz, D.; Lücking, R.; Aptroot, A.; Carriconde, F.; Ah-Peng, C.; Huang, J.-P.; Chen, K.-H.; Stenger, P.-L.; Cáceres, M.E.D.S.; et al. Global phylogeny of the family Gomphillaceae (Ascomycota, Graphidales) sheds light on the origin, diversification and endemism in foliicolous lineages. IMA Fungus 2025, 16, e144194. [Google Scholar] [CrossRef]


| Species | Specimen Voucher | GenBank Accession Number | |
|---|---|---|---|
| mtSSU | RPB2 | ||
| Arthonia atra | L10170 | KY983978 | KY983985 |
| Arthonia calcarea | Thor 11/6a | KJ850974 | KJ851105 |
| Arthonia calcarea | Ertz 7545 | EU704063 | EU704027 |
| Arthonia didyma | Ertz 7587 | EU704047 | EU704010 |
| Arthonia didyma | Frisch 11/Se41 | - | KJ851106 |
| Arthonia granithophila | Frisch 10/Se74 | KJ850981 | KJ851107 |
| Arthonia graphidicola | Frisch 10/Jp102 | KJ850980 | - |
| Arthonia ilicina | McCune 31067 | KJ850982 | - |
| Arthonia incarnata | Frisch 10/Jp94 | KY983972 | KY983980 |
| Arthonia incarnata | Frisch 12/Jp289 | KY983973 | KY983981 |
| Arthonia incarnata | Frisch 13/Jp215 | KY983974 | KY983982 |
| Arthonia maculiformis | Wedin 9393b | - | KF707658 |
| Arthonia pararubella | YN242036 | PV569153 | PV590068 |
| Arthonia pararubella | YN241783 | PV569152 | - |
| Arthonia physcidiicola | Frisch 11/Ug318 | KF707646 | KF707657 |
| Arthonia radiata | Frisch 10/Se29 | KJ850968 | KJ851108 |
| Arthonia radiata | Frisch 11/Se25 | KJ850969 | KJ851109 |
| Arthonia rubella | 49024 | MH308714 | - |
| Arthonia rubella | YN241343 | PV569139 | - |
| Arthonia rubella | YN241366 | PV569140 | - |
| Arthonia subfuscicola | Thor 11/1 | KJ850971 | KJ851110 |
| Arthonia subfuscicola | Frisch 11/Se15 | KJ850972 | KJ851111 |
| Arthonia thoriana | Sanderson 2176 | MG207687 | - |
| Arthonia thoriana | Sanderson 2174 | MG207685 | - |
| Arthonia yunnanensis | YN242413 | PV569142 | PV575682 |
| Arthonia yunnanensis | YN242411 | PV569141 | PV575683 |
| Arthonia atra | L10170 | KY983978 | KY983985 |
| Arthonia calcarea | Thor 11/6a | KJ850974 | KJ851105 |
| Arthonia calcarea | Ertz 7545 | EU704063 | EU704027 |
| Arthonia didyma | Ertz 7587 | EU704047 | EU704010 |
| Arthonia didyma | Frisch 11/Se41 | - | KJ851106 |
| Arthonia granithophila | Frisch 10/Se74 | KJ850981 | KJ851107 |
| Arthonia graphidicola | Frisch 10/Jp102 | KJ850980 | - |
| Arthonia ilicina | McCune 31067 | KJ850982 | - |
| Arthonia incarnata | Frisch 10/Jp94 | KY983972 | KY983980 |
| Arthonia incarnata | Frisch 12/Jp289 | KY983973 | KY983981 |
| Arthonia incarnata | Frisch 13/Jp215 | KY983974 | KY983982 |
| Arthonia maculiformis | Wedin 9393b | - | KF707658 |
| Arthonia pararubella | YN242036 | PV569153 | PV590068 |
| Arthonia pararubella | YN241783 | PV569152 | - |
| Arthonia physcidiicola | Frisch 11/Ug318 | KF707646 | KF707657 |
| Arthonia radiata | Frisch 10/Se29 | KJ850968 | KJ851108 |
| Arthonia radiata | Frisch 11/Se25 | KJ850969 | KJ851109 |
| Arthonia rubella | 49024 | MH308714 | - |
| Arthonia rubella | YN241343 | PV569139 | - |
| Arthonia rubella | YN241366 | PV569140 | - |
| Arthonia subfuscicola | Thor 11/1 | KJ850971 | KJ851110 |
| Arthonia subfuscicola | Frisch 11/Se15 | KJ850972 | KJ851111 |
| Arthonia thoriana | Sanderson 2176 | MG207687 | - |
| Arthonia thoriana | Sanderson 2174 | MG207685 | - |
| Arthonia yunnanensis | YN242413 | PV569142 | PV575682 |
| Arthonia yunnanensis | YN242411 | PV569141 | PV575683 |
| Arthothelium galapagoense | Ertz 11790 | - | HQ454657 |
| Arthothelium galapagoense | Ertz 11654 | - | HQ454658 |
| Arthothelium norvegicum | McCune 31061 | - | KJ851114 |
| Arthothelium orbilliferum | TRH-L-15449 | KY983977 | - |
| Arthothelium punctatum | KoLRI 044205 | MF616614 | - |
| Arthothelium punctatum | KoLRI 044206 | MF616615 | - |
| Arthothelium ruanum | KoLRI 038018 | MF616609 | MF616619 |
| Arthothelium ruanum | KoLRI 038257 | MF616610 | MF616620 |
| Arthothelium ruanum | KoLRI 038261 | MF616611 | MF616621 |
| Arthothelium ruanum | KoLRI 038275 | MF616612 | - |
| Arthothelium ruanum | KoLRI 038275 | MF616613 | - |
| Arthothelium ruanum | Zimmerman 1117 | GU327683 | - |
| Arthothelium sp. | Joensson Guyana 10 | KJ850957 | KJ851094 |
| Arthothelium sp. | Joensson Guyana 8 | KJ850958 | KJ851095 |
| Arthothelium spectabile | TNS:Frisch12Jp179a | KP870144 | KP870160 |
| Briancoppinsia cytospora | Diederich 16849 | JF830771 | - |
| Briancoppinsia cytospora | Ertz 15244 | JF830772 | - |
| Bryostigma apatetica | Svensson 2017 | KJ850992 | KJ851125 |
| Bryostigma apatetica | Svensson 1939 | KJ850993 | KJ851126 |
| Bryostigma apotheciorum | Frisch 11/Se23 | KJ850970 | KJ851148 |
| Bryostigma biatoricola | Thor 24350 | KJ850990 | KJ851149 |
| Bryostigma lapidicola | Westberg Frisch 11/Se47 | KJ850997 | KJ851119 |
| Bryostigma lobariicola | Frisch 10/Jp124 | KJ851002 | KJ851127 |
| Bryostigma lobariicola | Frisch 10/Jp737 | KJ851001 | KJ851128 |
| Bryostigma molendoi | Frisch 11/Se36 | KJ851000 | KJ851117 |
| Bryostigma molendoi | Frisch 11/Se22 | MH177777 | MH177770 |
| Bryostigma muscigenum | Thor 26206 | KJ850991 | KJ851124 |
| Bryostigma neglectula | Frisch 10/Se91 | KJ850989 | KJ851118 |
| Bryostigma parietinaria | Frisch 17/No25 | MH177778 | MH177771 |
| Bryostigma peltigerina | Westberg Frisch 11/Se46 | KJ850998 | KJ851122 |
| Bryostigma phaeophysciae | Ertz 11/2 | - | KJ851112 |
| Bryostigma sp. | Svensson 2324 | KJ851019 | KJ851123 |
| Bryostigma sp. | SK L06 | MH510022 | MH510019 |
| Bryostigma sp. | Svensson 2148 | KJ850995 | KJ851120 |
| Bryostigma sp. | SK L04 | MH510023 | MH510020 |
| Bryostigma stereocaulina | Westberg Frisch 11/Se48 | KJ850999 | - |
| Bryostigma toensbergii | Frisch N4-2-Pa4-1 | MH177775 | - |
| Bryostigma toensbergii | Frisch N4-2-Pa6-1 | MH177776 | - |
| Coniocarpon aff. cinnabarinum | Frisch 11/Ug3 | KJ850978 | KJ851102 |
| Coniocarpon cinnabarinum | Johnsen111003 | KJ850976 | KJ851103 |
| Coniocarpon cinnabarinum | Frisch11Ug296 | KP870158 | KP870170 |
| Coniocarpon fallax | LD:L10075 | KJ850979 | KJ851101 |
| Coniocarpon rubrocinctum | Nelsen 4010 | GU327684 | - |
| Crypthonia aff. vandenboomii | Frisch 11/Ug21 | KJ850960 | KJ851085 |
| Crypthonia palaeotropica | Frisch 11/Ug457 | KJ850961 | KJ851084 |
| Crypthonia palaeotropica | Frisch11Ug26B | KP870145 | KP870161 |
| Cryptophaea phaeospora | Van den Broeck 5809 | KX077541 | - |
| Cryptophaea phaeospora | Van den Broeck 5964 | KX077540 | - |
| Cryptothecia assimilis | Lumbsch 198151 | GU327688 | - |
| Cryptothecia austrocoreana | KoLRI No. 041892 | MF769374 | - |
| Cryptothecia austrocoreana | KoLRI No. 044721 | MF769375 | - |
| Cryptothecia punctosorediata | F:Nelsen 4038 | JX046450 | - |
| Cryptothecia sp. | Frisch 11/Ug39 | KJ850954 | KJ851086 |
| Cryptothecia sp. | Frisch 11/Ug18 | KJ850955 | KJ851092 |
| Cryptothecia sp. | Frisch 11/Ug194 | KJ850956 | KJ851093 |
| Cryptothecia subnidulans | v.d.Boom 40613 | KJ850952 | KJ851087 |
| Cryptothecia subnidulans | Joensson Guyana 6a | KJ850953 | KJ851088 |
| Diarthonis spadicea | Thor 26200 | - | KJ851116 |
| Diarthonis spadicea | Frisch 11/Se31 | - | KJ851115 |
| Eremothecella pruinocarpa | YN242390-1 | PV569148 | PV575678 |
| Eremothecella pruinocarpa | YN242384 | PV569149 | PV575679 |
| Eremothecella pruinocarpa | YN242386-2 | PV569150 | PV575680 |
| Eremothecella pruinocarpa | YN242387-2 | PV569151 | PV575681 |
| Eremothecella calamicola | YN242386-1 | PV569146 | - |
| Eremothecella calamicola | YN242387-1 | PV569147 | - |
| Eremothecella calamicola | YN242388 | PV569144 | - |
| Eremothecella calamicola | YN242390-2 | PV569145 | PV575677 |
| Glomerulophoron mauritiae | BR Ertz19164 | KP870153 | KP870167 |
| Herpothallon inopinatum | Rudolphi 12 | KJ850964 | KJ851099 |
| Herpothallon kigeziense | Frisch 11/Ug26 | KF707644 | KF707654 |
| Herpothallon rubrocinctum | Rudolphi 5 | KF707643 | KF707655 |
| Herpothallon rubrocinctum | Nelsen 4006 | GU327693 | - |
| Herpothallon sp. | Frisch 11/Ug401 | KF707645 | KF707653 |
| Inoderma afromontanum | Frisch 11/Ug164 | KJ850963 | KJ851090 |
| Inoderma byssaceum | Thor 25952 | KJ850962 | KJ851089 |
| Inoderma byssaceum | Lif 186 | - | KJ851091 |
| Inoderma nipponicum | TNS:Frisch12Jp227 | KP870146 | KP870162 |
| Inoderma nipponicum | TNS:Frisch13Jp1 | KP870147 | KP870163 |
| Inoderma sorediatum | Kukwa 15631 & Lubek | MG207690 | - |
| Inoderma subabietinum | Ertz16885 | KP870150 | KP870164 |
| Leprantha cinereopruinosa | Kukwa 17127 & Lubek | MG207692 | - |
| lnoderma sorediatum | Kukwa 15630 & Lubek | MG207689 | - |
| Melarthonis piceae | Thor 25995 | KJ851016 | - |
| Myriostigma candidum | Ertz 9260 | EU704052 | EU704015 |
| Myriostigma candidum | Frisch 11/Ug125 | KJ850959 | KJ851096 |
| Myriostigma miniatum | Silva T2A29 | KP843606 | - |
| Naevia aff punctiformis | Thor 24702 | KJ850975 | - |
| Naevia dispersa | UPSC 2583 | AY571383 | - |
| Naevia pinastri | MFLU 17-3497 | MN842780 | - |
| Naevia punctiformis | Thor 26158 | KJ850973 | KJ851113 |
| Pachnolepia pruinata | Tehler 9139 | - | HQ454650 |
| Pachnolepia pruinata | Frisch 11/Se34 | KJ850967 | KJ851098 |
| Reichlingia leopoldii | Ertz 13293 | JF830773 | HQ454722 |
| Reichlingia leopoldii | Ertz 13294 | JF830774 | HQ454723 |
| Reichlingia syncesioides | Frisch 11/Ug14 | KF707651 | KF707656 |
| Reichlingia zwackhii | Thor 11/3 | KF707652 | KF707662 |
| Reichlingia zwackhii | Ertz 10928 | - | HQ454655 |
| Snippocia nivea | Ertz 17437 | MG207695 | - |
| Snippocia nivea | Sanderson 2180 | MG207694 | - |
| Snippocia nivea | Sanderson 2179 | MG207693 | - |
| Sporodophoron cretaceum | Thor27720 | KP870159 | - |
| Sporodophoron gossypinum | TNS:Frisch12Jp186 | KP870154 | KP870168 |
| Sporodophoron gossypinum | TNS:Frisch12Jp197 | KP870156 | - |
| Sporodophoron primorskiense | TNS:Y.Ohmura10607 | LC086299 | - |
| Sporodophoron primorskiense | TNS:Ohmura10509 | KP870157 | KP870169 |
| Stirtonia neotropica | Cáceres & Aptroot 11112 | KP843611 | - |
| Stirtonia sp. | Frisch 11/Ug325 | KJ850965 | - |
| Synarthonia albopruinosa | VDB 6086 | MH251873 | - |
| Synarthonia aurantiacopruinosa | VDB 5764 | MH251874 | - |
| Synarthonia fuscata | VDB 6101 | MH251875 | - |
| Synarthonia inconspicua | VDB 7013B | MH251881 | - |
| Synarthonia inconspicua | ERTZ 19739A | MH251879 | - |
| Synarthonia inconspicua | YN242231 | PV569143 | - |
| Synarthonia josephiana | ERTZ 19739B | MH251876 | - |
| Synarthonia muriformis | Frisch 11/Ug41 | KJ851025 | - |
| Synarthonia muriformis | ERTZ 19344 | MH251877 | - |
| Synarthonia ochracea | VDB 6653 | MH251884 | - |
| Synarthonia pilosella | ERTZ 7808 | MH251883 | - |
| Tylophoron galapagoense | Bungartz 8749 | JF830776 | - |
| Tylophoron galapagoense | Bungartz 8750 | JF830777 | - |
| Tylophoron hibernicum | Diederich 16335 | JF830779 | - |
| Tylophoron hibernicum | Frisch 11/Ug220 | KJ850966 | KJ851097 |
| Tylophoron moderatum | Ertz 14504 | JF830780 | - |
| Tylophoron stalactiticum | Ertz 10880 | JF830781 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Hao, C.; Jiang, S.; Jia, Z. Three New Species and Three New Records of Arthoniaceae (Ascomycota, Arthoniales) from China. J. Fungi 2026, 12, 42. https://doi.org/10.3390/jof12010042
Hao C, Jiang S, Jia Z. Three New Species and Three New Records of Arthoniaceae (Ascomycota, Arthoniales) from China. Journal of Fungi. 2026; 12(1):42. https://doi.org/10.3390/jof12010042
Chicago/Turabian StyleHao, Chengyue, Shuhua Jiang, and Zefeng Jia. 2026. "Three New Species and Three New Records of Arthoniaceae (Ascomycota, Arthoniales) from China" Journal of Fungi 12, no. 1: 42. https://doi.org/10.3390/jof12010042
APA StyleHao, C., Jiang, S., & Jia, Z. (2026). Three New Species and Three New Records of Arthoniaceae (Ascomycota, Arthoniales) from China. Journal of Fungi, 12(1), 42. https://doi.org/10.3390/jof12010042






