The Fungus HL-29: A Promising Weed Pathogen with Bioherbicidal Potential and Crop Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Purification of Fungal Strains
2.3. Strain Identification and Phylogenetic Analysis
2.3.1. Morphological Identification
2.3.2. Molecular Biology and Phylogenetic Analysis
2.4. Pathogenicity Assay on Detached Leaves
2.5. Pathogenicity Assay in Pot Culture
2.6. Crop Safety Evaluation
2.7. Scanning Electron Microscopy (SEM) Sample Preparation and Observation
2.8. Data Analysis
3. Results and Analysis
3.1. Results of Strain Identification and Phylogenetic Analysis
3.1.1. Morphological Identification
3.1.2. Molecular Identification and Phylogenetic Analysis
3.2. Pathogenicity on Detached Leaves
3.3. Pathogenicity in Pot Culture
3.4. Safety Evaluation Results for Crops
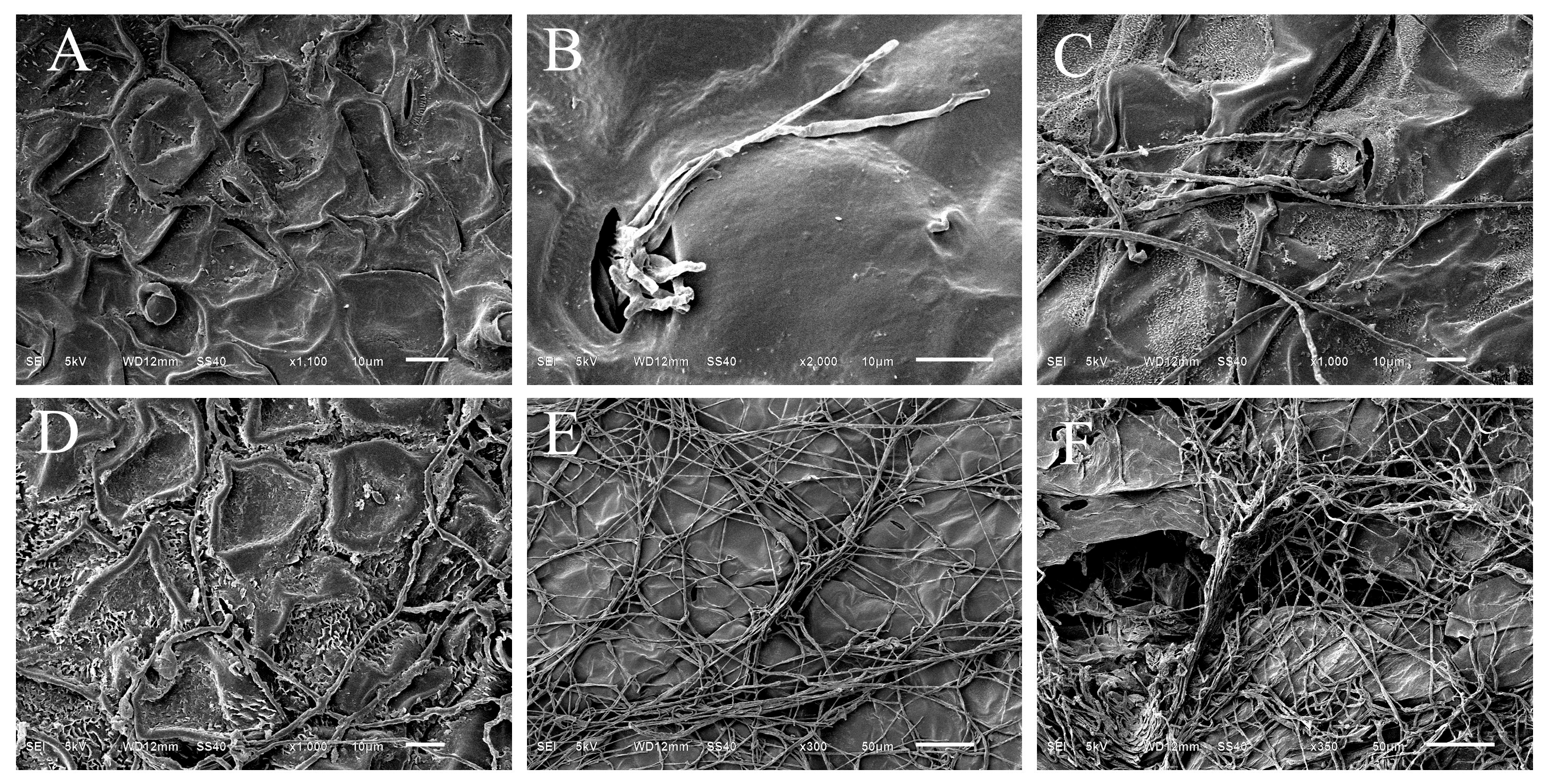
3.5. Scanning Electron Microscopy Observation of Ultrastructural Features During Infection of C. album by HL-29
4. Discussion and Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santosh Kumar, P.; Santa, M.; Ravi, N. Herbicidal weed management practices: History and future prospects of nanotechnology in an eco-friendly crop production system. Heliyon 2024, 10, e26527. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tang, Y.; Luo, F. Weed25: A deep learning dataset for weed identification. Front. Plant Sci. 2022, 13, 1053329. [Google Scholar] [CrossRef] [PubMed]
- Bourdôt, G.W.; Casonato, S.G. Broad host-range pathogens as bioherbicides: Managing nontarget plant disease risk. Pest Manag. Sci. 2024, 80, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Liphadzi, K.B.; Reinhardt, C.F. Using companion plants to assist establishment on former agricultural lands. S. Afr. J. Bot. 2006, 72, 403–408. [Google Scholar] [CrossRef]
- Lyu, Y.; Liu, Y.; Guo, Y.; Sang, J.; Tian, J.; Chen, L. Review of green development of Chinese industrial parks. Energy Strategy Rev. 2022, 42, 100867. [Google Scholar] [CrossRef]
- Kenney, D.S. Devine®—The way it was developed—An industrialist’s view. Weed Sci. 1986, 34, 15–16. [Google Scholar] [CrossRef]
- Nagano, H.; Yamashita, H.; Hashimoto, J. Discovery of a novel herbicide, MgAG, through genome mining of Streptomyces sp. J. Antibiot. 2020, 73, 467–475. [Google Scholar]
- Zhu, H.; Ma, Y.; Guo, Q. Biological weed control using Trichoderma polysporum strain HZ-3. Crop Prot. 2020, 134, 105161. [Google Scholar] [CrossRef]
- Bo, A.B.; Kim, J.D.; Kim, Y.S. Isolation, identification and characterization of Streptomyces metabolites as a potential bioherbicide. PLoS ONE 2019, 14, e0222933. [Google Scholar] [CrossRef] [PubMed]
- Pantović, J.G.; Sečanski, M.; Gordanić, S.; Todosijević, L.Š. Weed biological control with fungi-based bioherbicides. Acta Agric. Serbica 2023, 28, 55. [Google Scholar] [CrossRef]
- Hu, R.; Liao, S.; Huang, C.; Huang, Y.; Yang, T. An Inducible Fusaric Acid Tripartite Efflux Pump Contributes to the Fusaric Acid Resistance in Stenotrophomonas maltophilia. PLoS ONE 2012, 7, e51053. [Google Scholar] [CrossRef]
- Tan, W.Z.; Li, Q.J.; Qing, L. Biological control of alligatorweed (Alternanthera philoxeroides) with a Fusarium sp. BioControl 2002, 47, 463–479. [Google Scholar] [CrossRef]
- Rana, A.; Sahgal, M.; Johri, B.N. Fusarium oxysporum: Genomics, diversity and plant–host interaction. In Developments in Fungal Biology and Applied Mycology; Springer: Singapore, 2017; pp. 159–199. [Google Scholar]
- Cheng, H. Herbicidal activity and crop safety of Alternaria alternata DT-XRKA and Fusarium avenaceum DT-QKBD004A. Sci. Rep. 2025, 15, 9933. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Liu, J.; Peng, Y.; Li, Z.; Wang, M.; Kan, B. High Carriage of Extended-Spectrum, Beta Lactamase-Producing, and Colistin-Resistant Enterobacteriaceae in Tibetan Outpatients with Diarrhea. Antibiotics 2022, 11, 508. [Google Scholar] [CrossRef]
- Zhu, H.X. Scanning Electron Microscopy Observation of Trichoderma polysporum HZ-31 Strain Infecting Wild Oats. Qinghai Sci. Technol. Agric. For. 2023, 88–92. [Google Scholar]
- Pearson, K.A.; Taylor, A.F.S.; Fuchs, R.M.E.; Woodward, S. Characterisation and pathogenicity of Fusarium taxa isolated from ragwort (Jacobaea vulgaris) roots. Fungal Ecol. 2016, 20, 186–192. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Hami, A.; Rasool, R.S.; Khan, N.A.; Mansoor, S.; Mir, M.A.; Ahmed, N.; Masoodi, K.Z. Morpho-molecular identification and first report of Fusarium equiseti in causing chilli wilt from Kashmir (Northern Himalayas). Sci. Rep. 2021, 11, 3610. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annual Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Yadeta, K.A.; Thomma, B.P.H.J. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Geiser, D.M.; Aoki, T.; Bacon, C.W.; Baker, S.E.; Bhattacharyya, M.K.; Brandt, M.E.; Brown, D.W.; Burgess, L.W.; Chulze, S.; Coleman, J.J. One fungus, one name: Defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 2013, 103, 400–408. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Fan, F.; Qiu, D.; Jiang, L. The transcription cofactor FgSwi6 plays a role in growth and development, carbendazim sensitivity, cellulose utilization, lithium tolerance, deoxynivalenol production and virulence in the filamentous fungus Fusarium graminearum. Fungal Genet. Biol. 2013, 58, 42–52. [Google Scholar] [CrossRef]
- Bi, L.; Xu, H.; Li, T.; Zhou, Z.; Li, Z.; Wang, J.; Duan, Y.; Zhou, M. Antifungal activity of quinofumelin against Fusarium graminearum and its inhibitory effect on DON biosynthesis. Toxins 2021, 12, 348. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited FAO estimate of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Walker, C.; Maciel, C.G.; Milanesi, P.M.; Muniz, M.F.B.; Mezzomo, R.; Pollet, C.S. Caracterização morfológica, molecular patogenicidade Fusarium acuminatum Fusarium verticillioides a Cordia americana. Ciência Florest. 2016, 26, 463–473. [Google Scholar] [CrossRef]
- Charudattan, R. Use of Plant Pathogens as Bioherbicides: The Science, the Technology, and the Future. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014; pp. 345–364. [Google Scholar]
- Boyette, C.D.; Templeton, G.E.; Smith, R.J., Jr. Control of winged waterprimrose (Jussiaea decurrens) and northern jointvetch (Aeschynomene virginica) with fungal pathogens. Weed Sci. 1979, 27, 497–501. [Google Scholar] [CrossRef]
- Mesquita, E.; Hu, S.; Lima, T.; Golo, P.; Bidochka, M. Utilization of Metarhizium as an insect biocontrol agent and a plant bioinoculant with special reference to Brazil. Front. Fungal Biol. 2023, 4, 1276287. [Google Scholar] [CrossRef]







| Primer | Nucleotide Sequence |
|---|---|
| ITS1 | 5′-TCCGTAGGTGAACCTGCGG-3′ |
| ITS4-R | 5′-TCCTCCGCTTATTGATATGC-3′ |
| EFI-F | 5′- CATCGAGAAGTTCGAGAAGG-3′ |
| EFI-R | 5′-TACTTGAAGGAACCCTTACC-3′ |
| RPB2-5F2 | 5′-GGGGWGAYCAGAAGAAGGC-3’ |
| RPB2-7cR | 5’-CCCATRGCTTGYTTRCCCAT-3’ |
| Test Crops | Disease |
|---|---|
| T. aestivum | LS |
| P. sativum | NS |
| H. vulgare | LS |
| V. faba | NS |
| B. napus | NS |
| C. sativus | NS |
| Z. mays | NS |
| S. melongena | NS |
| S. lycopersicum | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yang, L.; Peng, C.; Zhu, H.; Ma, Y. The Fungus HL-29: A Promising Weed Pathogen with Bioherbicidal Potential and Crop Safety. J. Fungi 2026, 12, 17. https://doi.org/10.3390/jof12010017
Yang L, Peng C, Zhu H, Ma Y. The Fungus HL-29: A Promising Weed Pathogen with Bioherbicidal Potential and Crop Safety. Journal of Fungi. 2026; 12(1):17. https://doi.org/10.3390/jof12010017
Chicago/Turabian StyleYang, Lan, Chao Peng, Haixia Zhu, and Yongqiang Ma. 2026. "The Fungus HL-29: A Promising Weed Pathogen with Bioherbicidal Potential and Crop Safety" Journal of Fungi 12, no. 1: 17. https://doi.org/10.3390/jof12010017
APA StyleYang, L., Peng, C., Zhu, H., & Ma, Y. (2026). The Fungus HL-29: A Promising Weed Pathogen with Bioherbicidal Potential and Crop Safety. Journal of Fungi, 12(1), 17. https://doi.org/10.3390/jof12010017






