Phase Separation-Regulated Fungal Growth, Sexual Development, Adaptation and Synthetic Biology Applications
Abstract
1. Introduction
2. LLPS in Fungal Photomorphogenesis, Hyphae Growth and Pathogenesis
2.1. Photomorphogenesis
2.2. Hyphal Growth and Pathogenesis
3. LLPS in Cellular Processes in Response to Stresses
3.1. RNA Processing
3.2. Translation
3.3. Chromatin Organization
3.4. Other Cellular Processes
4. Identification of Intrinsically Disordered Proteins/Regions
5. LLPS in Synthetic Biology
5.1. Engineering Strategies of Protein Condensates
5.2. Applications of LLPS in Synthetic Biology
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| LLPS | liquid–liquid phase separation |
| RRM | RNA recognition motif |
| P-bodies | processing bodies |
| IDR | intrinsically disordered region |
| MLO | membraneless organelles |
| PTM | post-translational modification |
| CC | coiled coil |
| LC | low complexity |
| U(L)CST | upper(lower) critical solution temperature |
| SUMO | Small Ubiquitin-like Modifier |
| RBPs | RNA binding proteins |
| H3K27me3 | trimethylation of lysine 27 in histone H3 |
| Cdc19 | yeast pyruvate kinase |
| FRQ | clock gene frequency |
| FRH | FRQ-interacting RNA helicase |
| WCC | White Collar Complex |
| WC-1 | White Collar protein-1 |
| VVD | VIVID protein |
| HMM | hidden Markov-like model |
| PSSMs | position-specific scoring matrices |
| PDB | protein data bank |
| ncRNA | non-coding RNA |
| kAAP | k-spaced amino acid pairs |
| Fol | Fusarium oxysporum f. sp. lycopersici |
| UA | ursolic acid |
| RNPGs | ribonucleoprotein granules |
| Pum2 | Pumilio2 homology domain |
| EMP | Embden–Meyerhoff–Paranas |
| PY | phosphotyrosine |
| PLD | prion-like domain |
| PSPs | phase-separating proteins |
| SG | stress granule |
| LCR | low complex region |
| cmRNPs | circadian ribonucleoprotein granules |
| LARKS | low-complexity aromatic-rich kinked segments |
| FUS | fused in sarcoma protein |
| ELP | elastin-like polypeptides |
| RLR | resilin-like polypeptides |
| SH3 | SRC-homology 3 domains |
| eIF2α | eukaryotic translation initiation factor 2α |
| SlISP | iron-sulfur protein |
| Pex | peroxisomal proteins |
| PPAT | PRPP amidotransferase |
| BP1 | Bromo-adjacent homology-plant homeodomain domain containing protein 1 |
| IDR2 | intrinsically disordered region 2 of BP1 |
| CK1 | Casein Kinase 1 |
| Pab1 | Poly(A)-binding protein1 |
| TPOT | Tree-based Pipeline Optimization Tool |
| GBDT | gradient boosting decision tree |
| LM | language model |
| CLRC | Cryptic Loci Regulator complex, |
| TEV | tobacco etch virus |
| ManNAc | N-acetylmannosamine |
| ncAAs | noncanonical amino acids |
| ELRs | eastin-like recombinames |
| IDPPs | intrinsically disordered protein polymers |
| NOG | non-oxidative glycolysis |
| AKAPs | A kinase-anchoring proteins |
References
- Guan, H.; Wang, H.; Cai, X.; Wang, J.; Chai, Z.; Wang, J.; Wang, H.; Zhang, M.; Wu, Z.; Zhu, J.; et al. Liquid-liquid phase separation of membrane-less condensates: From biogenesis to function. Front. Cell Dev. Biol. 2025, 13, 1600430. [Google Scholar] [CrossRef]
- Marzoll, D.; Serrano, F.E.; Shostak, A.; Schunke, C.; Diernfellner, A.C.R.; Brunner, M. Casein kinase 1 and disordered clock proteins form functionally equivalent, phospho-based circadian modules in fungi and mammals. Proc. Natl. Acad. Sci. USA 2022, 119, e2118286119. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dunlap, J.C. Domains Required for FRQ-WCC Interaction within the Core Circadian Clock of Neurospora. bioRxiv 2023. [Google Scholar] [CrossRef]
- Iserman, C.; Desroches Altamirano, C.; Jegers, C.; Friedrich, U.; Zarin, T.; Fritsch, A.W.; Mittasch, M.; Domingues, A.; Hersemann, L.; Jahnel, M.; et al. Condensation of Ded1p Promotes a Translational Switch from Housekeeping to Stress Protein Production. Cell 2020, 181, 818–831.e19. [Google Scholar] [CrossRef]
- Stormo, B.M.; McLaughlin, G.A.; Frederick, L.K.; Jalihal, A.P.; Cole, S.J.; Seim, I.; Dietrich, F.S.; Gladfelter, A.S. Biomolecular condensates in fungi are tuned to function at specific temperatures. bioRxiv 2023. [Google Scholar] [CrossRef]
- Huang, H.T.; Maruyama, J.; Kitamoto, K. Aspergillus oryzae AoSO is a novel component of stress granules upon heat stress in filamentous fungi. PLoS ONE 2013, 8, e72209. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; Kedersha, N.; Anderson, P. Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb. Perspect. Biol. 2019, 11, a032813. [Google Scholar]
- Sivananthan, S.; Gosse, J.T.; Huard, S.; Baetz, K. Pab1 acetylation at K131 decreases stress granule formation in Saccharomyces cerevisiae. J. Biol. Chem. 2023, 299, 102834. [Google Scholar] [CrossRef]
- Marshall, A.C.; Cummins, J.; Kobelke, S.; Zhu, T.; Widagdo, J.; Anggono, V.; Hyman, A.; Fox, A.H.; Bond, C.S.; Lee, M. Different Low-complexity Regions of SFPQ Play Distinct Roles in the Formation of Biomolecular Condensates. J. Mol. Biol. 2023, 435, 168364. [Google Scholar] [CrossRef]
- Shen, C.H.; Komi, Y.; Nakagawa, Y.; Kamatari, Y.O.; Nomura, T.; Kimura, H.; Shida, T.; Burke, J.; Tamai, S.; Ishida, Y.; et al. Exposed Hsp70-binding site impacts yeast Sup35 prion disaggregation and propagation. Proc. Natl. Acad. Sci. USA 2024, 121, e2318162121. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ki, M.R.; Chung, D.Y.; Pack, S.P. Biomolecule-Based Coacervation: Mechanisms, Applications, and Future Perspectives in Biomedical and Biotechnological Fields. Biomolecules 2025, 15, 861. [Google Scholar] [CrossRef]
- Hou, S.; Hu, J.; Yu, Z.; Li, D.; Liu, C.; Zhang, Y. Machine learning predictor PSPire screens for phase-separating proteins lacking intrinsically disordered regions. Nat. Commun. 2024, 15, 2147. [Google Scholar] [CrossRef] [PubMed]
- van Mierlo, G.; Jansen, J.R.G.; Wang, J.; Poser, I.; van Heeringen, S.J.; Vermeulen, M. Predicting protein condensate formation using machine learning. Cell Rep. 2021, 34, 108705. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, H.; Meng, X.; Zuo, H.; Qi, M.; Guo, L.; Gao, C.; Song, W.; Wu, J.; Chen, X.; et al. Engineered Artificial Membraneless Organelles in Saccharomyces cerevisiae To Enhance Chemical Production. Angew. Chem. Int. Ed. Engl. 2023, 62, e202215778. [Google Scholar] [CrossRef]
- Cohrs, K.C.; Schumacher, J. The Two Cryptochrome/Photolyase Family Proteins Fulfill Distinct Roles in DNA Photorepair and Regulation of Conidiation in the Gray Mold Fungus Botrytis cinerea. Appl. Environ. Microbiol. 2017, 83, e00812-17. [Google Scholar] [CrossRef]
- Froehlich, A.C.; Liu, Y.; Loros, J.J.; Dunlap, J.C. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 2002, 297, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Tariq, D.; Maurici, N.; Bartholomai, B.M.; Chandrasekaran, S.; Dunlap, J.C.; Bah, A.; Crane, B.R. Phosphorylation, disorder, and phase separation govern the behavior of Frequency in the fungal circadian clock. eLife 2024, 12, RP90259. [Google Scholar] [CrossRef]
- Marzoll, D.; Serrano, F.E.; Diernfellner, A.C.R.; Brunner, M. Neurospora casein kinase 1a recruits the circadian clock protein FRQ via the C-terminal lobe of its kinase domain. FEBS Lett. 2022, 596, 1881–1891. [Google Scholar] [CrossRef]
- Ding, Z.; Lamb, T.M.; Boukhris, A.; Porter, R.; Bell-Pedersen, D. Circadian Clock Control of Translation Initiation Factor eIF2α Activity Requires eIF2γ-Dependent Recruitment of Rhythmic PPP-1 Phosphatase in Neurospora crassa. mBio 2021, 12, e00871-21. [Google Scholar] [CrossRef]
- Karki, S.; Castillo, K.; Ding, Z.; Kerr, O.; Lamb, T.M.; Wu, C.; Sachs, M.S.; Bell-Pedersen, D. Circadian clock control of eIF2α phosphorylation is necessary for rhythmic translation initiation. Proc. Natl. Acad. Sci. USA 2020, 117, 10935–10945. [Google Scholar] [CrossRef]
- Wang, B.; Kettenbach, A.N.; Gerber, S.A.; Loros, J.J.; Dunlap, J.C. Neurospora WC-1 recruits SWI/SNF to remodel frequency and initiate a circadian cycle. PLoS Genet. 2014, 10, e1004599. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yang, Y.; Wang, Y.; Dong, C. CmVVD is involved in fruiting body development and carotenoid production and the transcriptional linkage among three blue-light receptors in edible fungus Cordyceps militaris. Environ. Microbiol. 2020, 22, 466–482. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Liu, Q.; Niu, Y.; Hu, Q.; Deng, H.; Cha, J.; Wang, Y.; Liu, Y.; He, Q. Role for Protein Kinase A in the Neurospora Circadian Clock by Regulating White Collar-Independent frequency Transcription through Phosphorylation of RCM-1. Mol. Cell. Biol. 2015, 35, 2088–2102. [Google Scholar] [CrossRef]
- Kim, R.J.A.; Fan, D.; He, J.; Kim, K.; Du, J.; Chen, M. Photobody formation spatially segregates two opposing phytochrome B signaling actions of PIF5 degradation and stabilization. Nat. Commun. 2024, 15, 3519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhong, Z.; Gu, L.; Zhang, X.; Wei, J.; Ye, C.; Lin, G.; Qu, G.; Xiang, X.; Wen, C.; et al. Light-induced LLPS of the CRY2/SPA1/FIO1 complex regulating mRNA methylation and chlorophyll homeostasis in Arabidopsis. Nat. Plants 2023, 9, 2042–2058. [Google Scholar] [CrossRef] [PubMed]
- Pardi, S.A.; Nusinow, D.A. Out of the Dark and Into the Light: A New View of Phytochrome Photobodies. Front. Plant Sci. 2021, 12, 732947. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Zhang, J.; Zhang, L.; Yang, Z.; Yang, L.; Yao, N.; Xiao, Y.; Li, T.; Li, Y.; Zhang, G.; et al. Arabidopsis cryptochrome 2 forms photobodies with TCP22 under blue light and regulates the circadian clock. Nat. Commun. 2022, 13, 2631. [Google Scholar] [CrossRef]
- Holmes, K.J.; Klass, D.M.; Guiney, E.L.; Cyert, M.S. Whi3, an S. cerevisiae RNA-binding protein, is a component of stress granules that regulates levels of its target mRNAs. PLoS ONE 2013, 8, e84060. [Google Scholar] [CrossRef]
- Stormo, B.M.; McLaughlin, G.A.; Jalihal, A.P.; Frederick, L.K.; Cole, S.J.; Seim, I.; Dietrich, F.S.; Chilkoti, A.; Gladfelter, A.S. Intrinsically disordered sequences can tune fungal growth and the cell cycle for specific temperatures. Curr. Biol. 2024, 34, 3722–3734.e3727. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, J.; Han, X.; Tursic-Wunder, A.; Toh, J.D.W.; Hong, W.; Gao, Y.G.; Miao, Y. Polarisome scaffolder Spa2-mediated macromolecular condensation of Aip5 for actin polymerization. Nat. Commun. 2019, 10, 5078. [Google Scholar] [CrossRef]
- Ma, Q.; Surya, W.; He, D.; Yang, H.; Han, X.; Nai, M.H.; Lim, C.T.; Torres, J.; Miao, Y. Spa2 remodels ADP-actin via molecular condensation under glucose starvation. Nat. Commun. 2024, 15, 4491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, W.; Sun, S.; Chng, C.P.; Xie, Y.; Zhu, K.; He, D.; Liang, Q.; Ma, Z.; Wu, X.; et al. Bacterial XopR subverts RIN4 complex-mediated plant immunity via plasma membrane-associated percolation. Dev. Cell 2025, 60, 2081–2096.e10. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Ma, Q.; Han, L.; Tang, D.; Miao, Y. The phase-separating Magnaporthe oryzae MoSpa2 complex organizes actin nucleation centers for plant infection. Plant Cell 2025, 37, koaf097. [Google Scholar] [CrossRef]
- Chen, K.; Cao, X. Biomolecular condensates: Phasing in regulated host-pathogen interactions. Trends Immunol. 2025, 46, 29–45. [Google Scholar] [CrossRef]
- Srinivas, C.; Nirmala Devi, D.; Narasimha Murthy, K.; Mohan, C.D.; Lakshmeesha, T.R.; Singh, B.; Kalagatur, N.K.; Niranjana, S.R.; Hashem, A.; Alqarawi, A.A.; et al. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity—A review. Saudi J. Biol. Sci. 2019, 26, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Torun, A.; Tugral, H.; Banerjee, S. Crosstalk Between Phase-Separated Membraneless Condensates and Membrane-Bound Organelles in Cellular Function and Disease. Adv. Exp. Med. Biol. 2025, 1483, 141–169. [Google Scholar]
- Riback, J.A.; Katanski, C.D.; Kear-Scott, J.L.; Pilipenko, E.V.; Rojek, A.E.; Sosnick, T.R.; Drummond, D.A. Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell 2017, 168, 1028–1040.e1019. [Google Scholar] [CrossRef]
- Kroschwald, S.; Munder, M.C.; Maharana, S.; Franzmann, T.M.; Richter, D.; Ruer, M.; Hyman, A.A.; Alberti, S. Different Material States of Pub1 Condensates Define Distinct Modes of Stress Adaptation and Recovery. Cell Rep. 2018, 23, 3327–3339. [Google Scholar] [CrossRef]
- Lindstrom, M.; Chen, L.; Jiang, S.; Zhang, D.; Gao, Y.; Zheng, J.; Hao, X.; Yang, X.; Kabbinale, A.; Thoma, J.; et al. Lsm7 phase-separated condensates trigger stress granule formation. Nat. Commun. 2022, 13, 3701. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Na, Z.; Slavoff, S.A. P-Bodies: Composition, Properties, and Functions. Biochemistry 2018, 57, 2424–2431. [Google Scholar] [CrossRef]
- Elbaum-Garfinkle, S.; Kim, Y.; Szczepaniak, K.; Chen, C.C.; Eckmann, C.R.; Myong, S.; Brangwynne, C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189–7194. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, S.; Lu, W.; Luo, T.; Shi, R.; Li, J.; Shen, H. Liquid-liquid phase separation of DDX3X: Mechanisms, pathological implications, and therapeutic potential. Int. J. Biol. Macromol. 2025, 317, 144835. [Google Scholar]
- Valiente-Echeverria, F.; Melnychuk, L.; Vyboh, K.; Ajamian, L.; Gallouzi, I.E.; Bernard, N.; Mouland, A.J. eEF2 and Ras-GAP SH3 domain-binding protein (G3BP1) modulate stress granule assembly during HIV-1 infection. Nat. Commun. 2014, 5, 4819. [Google Scholar] [CrossRef]
- Fernandez-Gomez, A.; Velasco, B.R.; Izquierdo, J.M. Dynamics of T-Cell Intracellular Antigen 1-Dependent Stress Granules in Proteostasis and Welander Distal Myopathy under Oxidative Stress. Cells 2022, 11, 884. [Google Scholar] [CrossRef]
- Mariani, D.; Setti, A.; Castagnetti, F.; Vitiello, E.; Stufera Mecarelli, L.; Di Timoteo, G.; Giuliani, A.; D’Angelo, A.; Santini, T.; Perego, E.; et al. ALS-associated FUS mutation reshapes the RNA and protein composition of stress granules. Nucleic Acids Res. 2024, 52, 13269–13289. [Google Scholar] [CrossRef] [PubMed]
- Nissan, T.; Rajyaguru, P.; She, M.; Song, H.; Parker, R. Decapping activators in Saccharomyces cerevisiae act by multiple mechanisms. Mol. Cell 2010, 39, 773–783. [Google Scholar] [CrossRef]
- Tishinov, K.; Spang, A. The mRNA decapping complex is buffered by nuclear localization. J. Cell Sci. 2021, 134, jcs259156. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. The role of disordered protein regions in the assembly of decapping complexes and RNP granules. Genes Dev. 2013, 27, 2628–2641. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, J. Accumulation of P-bodies in Candida albicans under different stress and filamentous growth conditions. Fungal Genet. Biol. 2011, 48, 1116–1123. [Google Scholar] [CrossRef]
- Wei, M.T.; Chang, Y.C.; Shimobayashi, S.F.; Shin, Y.; Strom, A.R.; Brangwynne, C.P. Nucleated transcriptional condensates amplify gene expression. Nat. Cell Biol. 2020, 22, 1187–1196. [Google Scholar] [CrossRef]
- Lombardi, S.; Zilocchi, M.; Nicsanu, R.; Barabino, S.M.L. Emerging connections: Poly(ADP-ribose), FET proteins and RNA in the regulation of DNA damage condensates. DNA Repair 2025, 150, 103846. [Google Scholar]
- Grimes, B.; Jacob, W.; Liberman, A.R.; Kim, N.; Zhao, X.; Masison, D.C.; Greene, L.E. The Properties and Domain Requirements for Phase Separation of the Sup35 Prion Protein In Vivo. Biomolecules 2023, 13, 1370. [Google Scholar] [CrossRef]
- Franzmann, T.M.; Jahnel, M.; Pozniakovsky, A.; Mahamid, J.; Holehouse, A.S.; Nuske, E.; Richter, D.; Baumeister, W.; Grill, S.W.; Pappu, R.V.; et al. Phase separation of a yeast prion protein promotes cellular fitness. Science 2018, 359, eaao5654. [Google Scholar] [CrossRef]
- Gotor, N.L.; Armaos, A.; Calloni, G.; Torrent Burgas, M.; Vabulas, R.M.; De Groot, N.S.; Tartaglia, G.G. RNA-binding and prion domains: The Yin and Yang of phase separation. Nucleic Acids Res. 2020, 48, 9491–9504. [Google Scholar] [CrossRef]
- Jung, J.H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef]
- Kim, Y.R.; Joo, J.; Lee, H.J.; Kim, C.; Park, J.C.; Yu, Y.S.; Kim, C.R.; Lee, D.H.; Cha, J.; Kwon, H.; et al. Prion-like domain mediated phase separation of ARID1A promotes oncogenic potential of Ewing’s sarcoma. Nat. Commun. 2024, 15, 6569. [Google Scholar] [CrossRef]
- Larson, A.G.; Elnatan, D.; Keenen, M.M.; Trnka, M.J.; Johnston, J.B.; Burlingame, A.L.; Agard, D.A.; Redding, S.; Narlikar, G.J. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 2017, 547, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.D.; Liu, P.Z.; Banigan, E.J.; Almassalha, L.M.; Backman, V.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol. Biol. Cell 2018, 29, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Roche, B.; Bhattacharjee, S.; Todeschini, L.; Chang, A.Y.; Hammell, C.; Verdel, A.; Martienssen, R.A. Clr4(SUV39H1) ubiquitination and non-coding RNA mediate transcriptional silencing of heterochromatin via Swi6 phase separation. Nat. Commun. 2024, 15, 9384. [Google Scholar] [CrossRef] [PubMed]
- Bensaha, S.; Lewandowska, D.; Muzzopappa, F.; Hutin, S.; Tully, M.D.; Anfossi, M.; Cammas, F.M.; Normand, C.; Erdel, F. HP1 loses its chromatin clustering and phase separation function across evolution. Nat. Commun. 2025, 16, 6375. [Google Scholar] [CrossRef]
- Gerace, E.L.; Halic, M.; Moazed, D. The methyltransferase activity of Clr4Suv39h triggers RNAi independently of histone H3K9 methylation. Mol. Cell 2010, 39, 360–372. [Google Scholar] [CrossRef]
- Zhang, Q.; McKenzie, N.J.; Warneford-Thomson, R.; Gail, E.H.; Flanigan, S.F.; Owen, B.M.; Lauman, R.; Levina, V.; Garcia, B.A.; Schittenhelm, R.B.; et al. RNA exploits an exposed regulatory site to inhibit the enzymatic activity of PRC2. Nat. Struct. Mol. Biol. 2019, 26, 237–247. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Zhan, X.; Yang, F.; Wu, M.; Zhang, X. Structure of the yeast Swi/Snf complex in a nucleosome free state. Nat. Commun. 2020, 11, 3398. [Google Scholar]
- Patil, A.; Strom, A.R.; Paulo, J.A.; Collings, C.K.; Ruff, K.M.; Shinn, M.K.; Sankar, A.; Cervantes, K.S.; Wauer, T.; St Laurent, J.D.; et al. A disordered region controls cBAF activity via condensation and partner recruitment. Cell 2023, 186, 4936–4955.e4926. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Bacellar, I.O.L.; Castellanos-Girouard, X.; Wahba, H.M.; Zhang, Z.; Omichinski, J.G.; Kisley, L.; Michnick, S.W. Peroxisome biogenesis initiated by protein phase separation. Nature 2023, 617, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, S.; Xu, P.; Yue, S.; Zhuang, M. PEX14 condensates recruit receptor and cargo pairs for peroxisomal protein import. Nat. Struct. Mol. Biol. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Handa, T.; Kundu, D.; Dubey, V.K. Perspectives on evolutionary and functional importance of intrinsically disordered proteins. Int. J. Biol. Macromol. 2023, 224, 243–255. [Google Scholar]
- Tang, G.; Xia, H.; Huang, Y.; Guo, Y.; Chen, Y.; Ma, Z.; Liu, W. Liquid-liquid phase separation of H3K27me3 reader BP1 regulates transcriptional repression. Genome Biol. 2024, 25, 67. [Google Scholar]
- Takaine, M.; Morita, R.; Yoshinari, Y.; Nishimura, T. Phase separation of the PRPP amidotransferase into dynamic condensates promotes de novo purine synthesis in yeast. PLoS Biol. 2025, 23, e3003111. [Google Scholar]
- Grignaschi, E.; Cereghetti, G.; Grigolato, F.; Kopp, M.R.G.; Caimi, S.; Faltova, L.; Saad, S.; Peter, M.; Arosio, P. A hydrophobic low-complexity region regulates aggregation of the yeast pyruvate kinase Cdc19 into amyloid-like aggregates in vitro. J. Biol. Chem. 2018, 293, 11424–11432. [Google Scholar] [CrossRef]
- Ginell, G.M.; Emenecker, R.J.; Lotthammer, J.M.; Keeley, A.T.; Plassmeyer, S.P.; Razo, N.; Usher, E.T.; Pelham, J.F.; Holehouse, A.S. Sequence-based prediction of intermolecular interactions driven by disordered regions. Science 2025, 388, eadq8381. [Google Scholar] [CrossRef]
- Lin, Y.; Currie, S.L.; Rosen, M.K. Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs. J. Biol. Chem. 2017, 292, 19110–19120. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699.e616. [Google Scholar] [CrossRef]
- Lancaster, A.K.; Nutter-Upham, A.; Lindquist, S.; King, O.D. PLAAC: A web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 2014, 30, 2501–2502. [Google Scholar] [CrossRef]
- Iglesias, V.; de Groot, N.S.; Ventura, S. Computational analysis of candidate prion-like proteins in bacteria and their role. Front. Microbiol. 2015, 6, 1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Yan, Q.; Liao, S.; Tang, W.; Xu, P.; Gao, Y.; Li, Q.; Dou, Z.; Yang, W.; et al. LLPSDB v2.0: An updated database of proteins undergoing liquid-liquid phase separation in vitro. Bioinformatics 2022, 38, 2010–2014. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Dou, Z.; Yang, W.; Huang, B.; Lou, J.; Zhang, Z. Protein Databases Related to Liquid-Liquid Phase Separation. Int. J. Mol. Sci. 2020, 21, 6796. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Gao, W.; Miao, Y. From Pan-Life Phase Insights to PhaseHub: Analyzing Condensate Complexity. bioRxiv 2025. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Ren, S.; Wang, Z.; Jin, X.; Lu, X.; Zhang, Y.; Min, X.; Ge, S.; Zhang, J.; et al. MambaPhase: Deep learning for liquid-liquid phase separation protein classification. Brief. Bioinform. 2025, 26, bbaf230. [Google Scholar] [CrossRef]
- Chu, X.; Sun, T.; Li, Q.; Xu, Y.; Zhang, Z.; Lai, L.; Pei, J. Prediction of liquid-liquid phase separating proteins using machine learning. BMC Bioinform. 2022, 23, 72. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, C.; Wang, L.; Yu, C.; Chen, T.; Shen, B.; Hou, Y.; Li, P.; Li, T. Screening membraneless organelle participants with machine-learning models that integrate multimodal features. Proc. Natl. Acad. Sci. USA 2022, 119, e2115369119. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Peng, N.; Xie, Y.; Kumar, N.; Gao, W.; Miao, Y. MolPhase, an advanced prediction algorithm for protein phase separation. EMBO J. 2024, 43, 1898–1918. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Tang, X.; Yu, Y.; Nilsen, R.; Kim, R.; Griffith, J.; Arnold, J.; Schuttler, H.B. Systems biology of the clock in Neurospora crassa. PLoS ONE 2008, 3, e3105. [Google Scholar] [CrossRef]
- Talora, C.; Franchi, L.; Linden, H.; Ballario, P.; Macino, G. Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 1999, 18, 4961–4968. [Google Scholar] [CrossRef]
- Gollapalli, S.; Sooram, B.; Sugandh, H.; Saudagar, P. The landscape of intrinsically disordered proteins in Leishmania parasite: Implications for drug discovery. Int. J. Biol. Macromol. 2024, 283, 137290. [Google Scholar] [CrossRef] [PubMed]
- Fernando, K.S.; Jahanmir, G.; Unarta, I.C.; Chau, Y. Multiscale Computational Framework for the Liquid-Liquid Phase Separation of Intrinsically Disordered Proteins. Langmuir 2024, 40, 7607–7619. [Google Scholar] [CrossRef]
- Milligan, J.J.; Strader, R.L.; Chakrabarty, A.; Ney, M.; Sirohi, P.; Su, J.C.; Varanko, A.K.; Shmidov, Y.; Tsolova, K.; Fontes, C.M.; et al. Controlling Release Kinetics of an Adjuvant from a Depot Improves the Efficacy of Local Immunotherapy in Metastatic Cancer. Adv. Sci. 2025, e03591. [Google Scholar] [CrossRef]
- Singh, A. Inducing phase separation using artificial disordered proteins. Nat. Methods 2020, 17, 955. [Google Scholar] [CrossRef]
- Quiroz, F.G.; Chilkoti, A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 2015, 14, 1164–1171. [Google Scholar] [CrossRef]
- de Haas, R.J.; Ganar, K.A.; Deshpande, S.; de Vries, R. pH-Responsive Elastin-Like Polypeptide Designer Condensates. ACS Appl. Mater. Interfaces 2023, 15, 45336–45344. [Google Scholar] [CrossRef]
- Dai, Y.; Farag, M.; Lee, D.; Zeng, X.; Kim, K.; Son, H.I.; Guo, X.; Su, J.; Peterson, N.; Mohammed, J.; et al. Programmable synthetic biomolecular condensates for cellular control. Nat. Chem. Biol. 2023, 19, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.M.; Suek, N.; Wilson, M.Z.; Dine, E.; Pannucci, N.L.; Gitai, Z.; Avalos, J.L.; Toettcher, J.E. Light-based control of metabolic flux through assembly of synthetic organelles. Nat. Chem. Biol. 2019, 15, 589–597. [Google Scholar] [CrossRef]
- Bock, A.S.; Murthy, A.C.; Tang, W.S.; Jovic, N.; Shewmaker, F.; Mittal, J.; Fawzi, N.L. N-terminal acetylation modestly enhances phase separation and reduces aggregation of the low-complexity domain of RNA-binding protein fused in sarcoma. Protein Sci. 2021, 30, 1337–1349. [Google Scholar] [CrossRef]
- Lizatovic, R.; Aurelius, O.; Stenstrom, O.; Drakenberg, T.; Akke, M.; Logan, D.T.; Andre, I. A De Novo Designed Coiled-Coil Peptide with a Reversible pH-Induced Oligomerization Switch. Structure 2016, 24, 946–955. [Google Scholar] [CrossRef]
- Burton, A.J.; Thomas, F.; Agnew, C.; Hudson, K.L.; Halford, S.E.; Brady, R.L.; Woolfson, D.N. Accessibility, reactivity, and selectivity of side chains within a channel of de novo peptide assembly. J. Am. Chem. Soc. 2013, 135, 12524–12527. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Wang, R.; Qiu, J.; She, Z.; Qu, J.; Xia, J. Modulating Liquid-Liquid Phase Separation of Nck Adaptor Protein against Enteropathogenic Escherichia coli Infection. ACS Cent. Sci. 2023, 9, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Gruenheid, S.; DeVinney, R.; Bladt, F.; Goosney, D.; Gelkop, S.; Gish, G.D.; Pawson, T.; Finlay, B.B. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 2001, 3, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Lascorz, J.; Codina-Fabra, J.; Reverter, D.; Torres-Rosell, J. SUMO-SIM interactions: From structure to biological functions. Semin. Cell Dev. Biol. 2022, 132, 193–202. [Google Scholar] [CrossRef]
- Yu, W.; Jin, K.; Wang, D.; Wang, N.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Chen, J.; et al. De novo engineering of programmable and multi-functional biomolecular condensates for controlled biosynthesis. Nat. Commun. 2024, 15, 7989. [Google Scholar] [CrossRef]
- Kang, W.; Ma, T.; Liu, M.; Qu, J.; Liu, Z.; Zhang, H.; Shi, B.; Fu, S.; Ma, J.; Lai, L.T.F.; et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux. Nat. Commun. 2019, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Chen, J.; Liu, L.; Lv, X. Light-induced programmable solid-liquid phase transition of biomolecular condensates for improved biosynthesis. Trends Biotechnol. 2025, 43, 1403–1424. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Yao, B.; Zhou, J.; Wang, Y.; Ding, Q. Engineering a Silk Protein-Mediated Customizable Compartment for Modular Metabolic Synthesis. ACS Synth. Biol. 2024, 13, 4180–4190. [Google Scholar] [CrossRef] [PubMed]
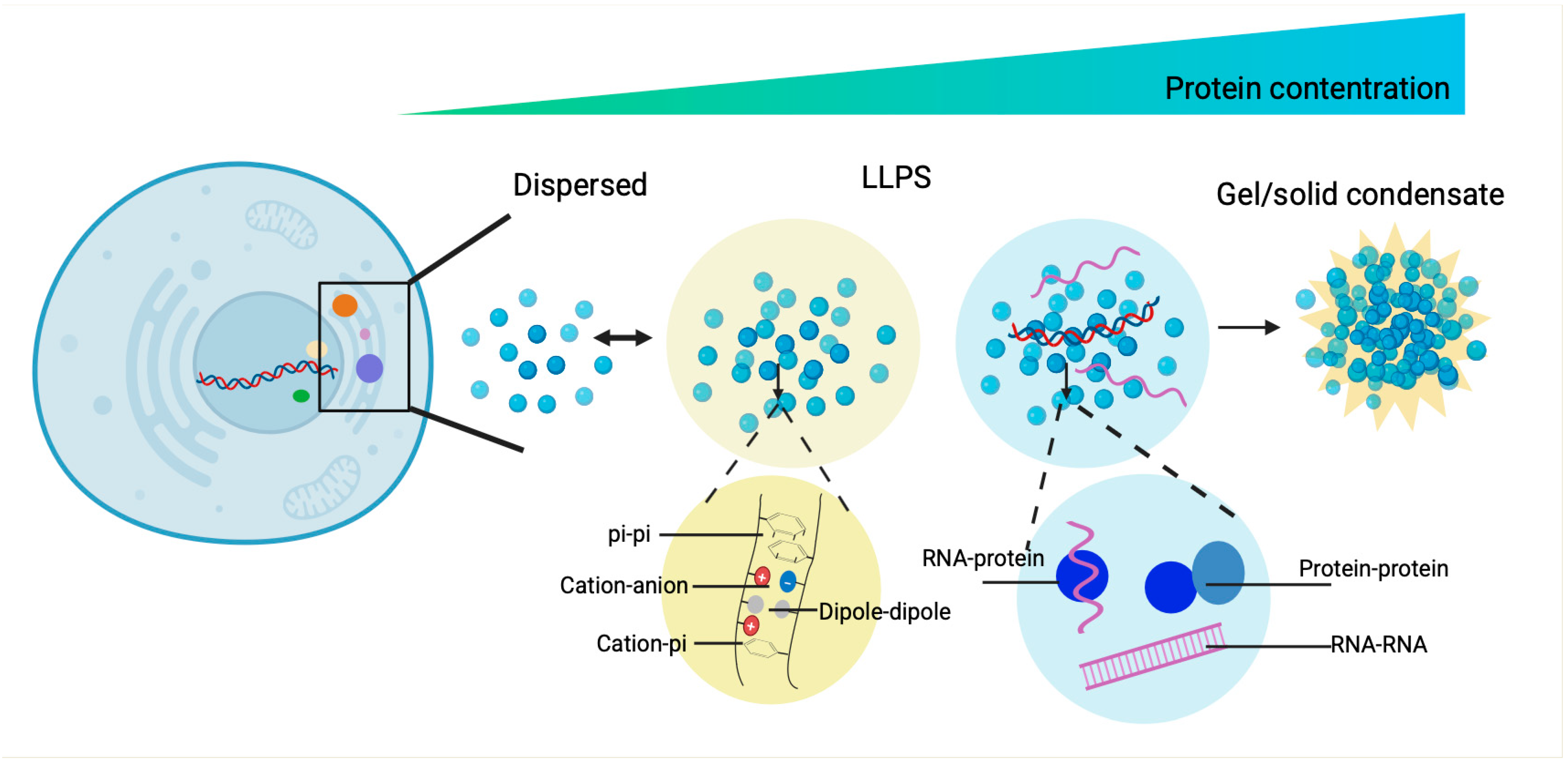
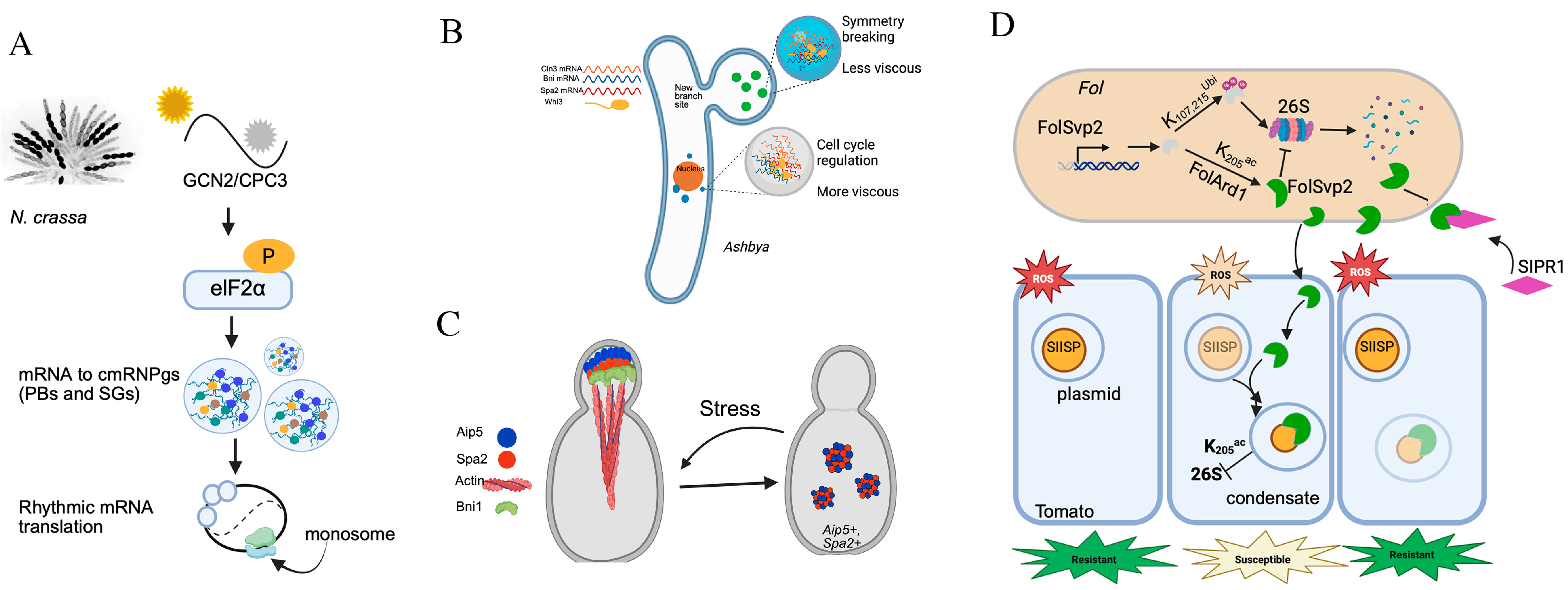


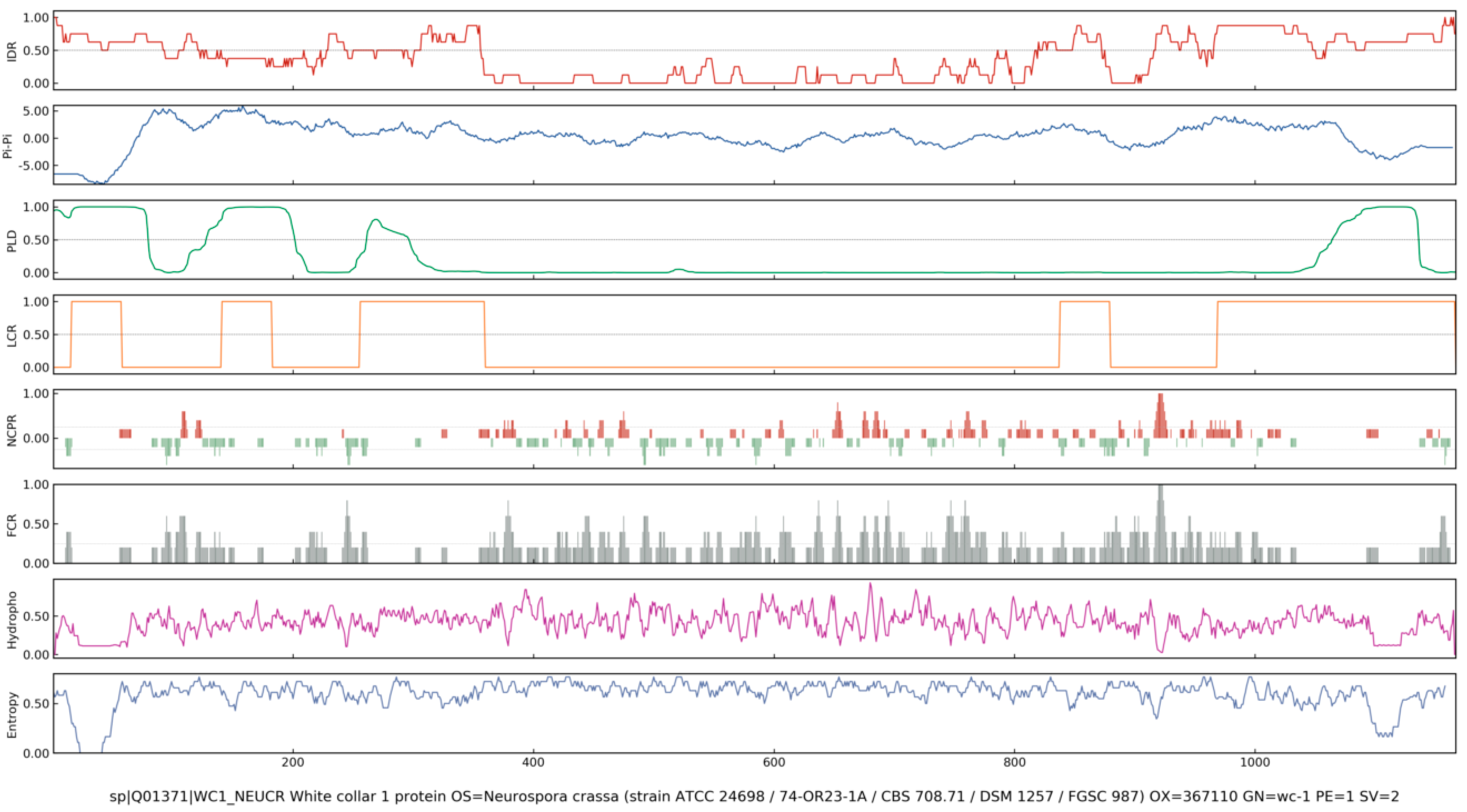
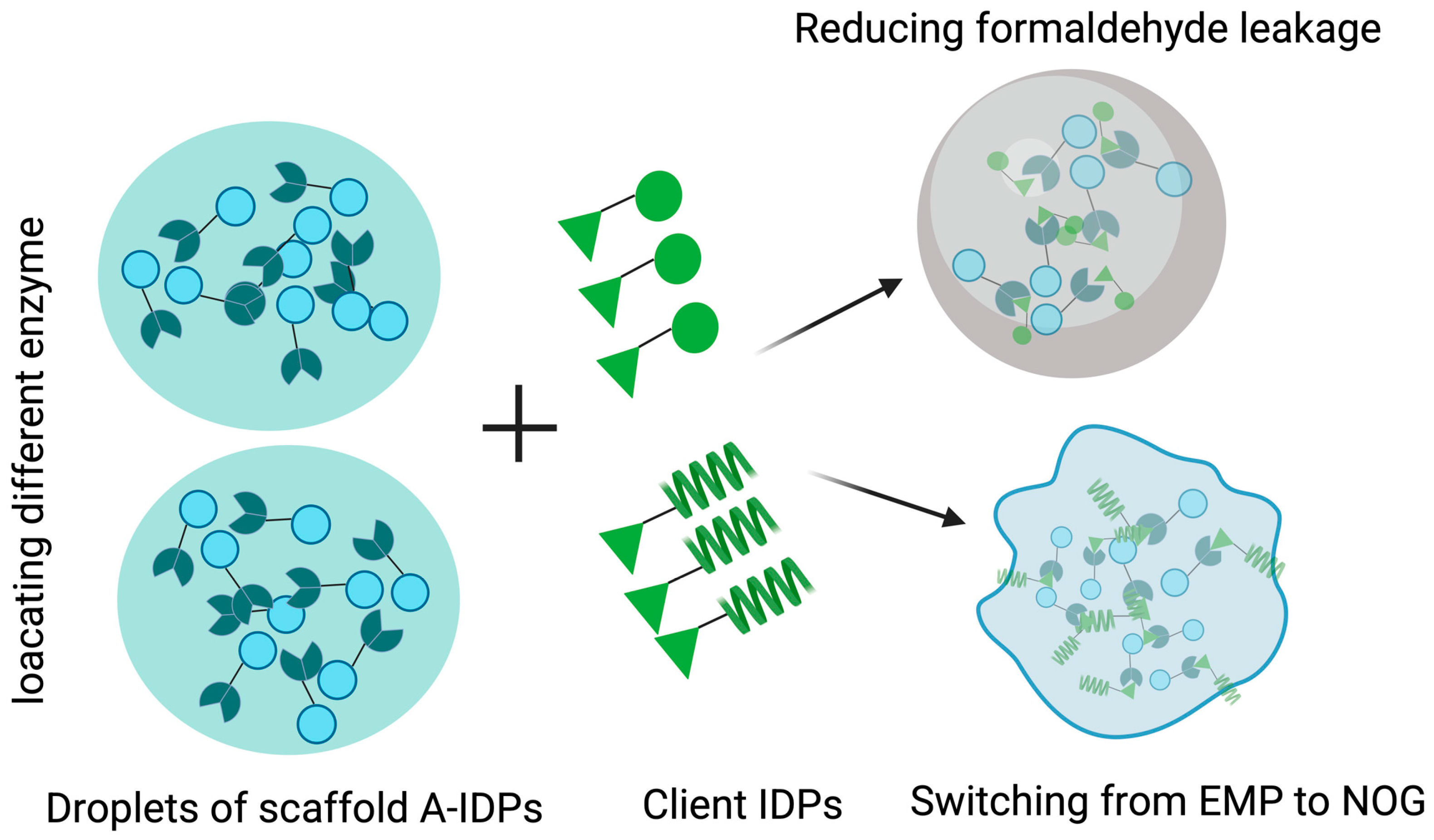
| Assembly Proteins | Species | LLPS Region | Biological Mechanism | Stress | Refs. |
|---|---|---|---|---|---|
| FRQ/FRH/CK1 | N. crassa | IDR | modulates CK1 activity, coordinates repression of WCC, FRQ phosphorylation enhances conformational flexibility and alters oligomeric state | light | [2,3] |
| eIF2α | N. crassa | - | PTM-mediated circadian rhythms and stress tolerance | light | [20,21] |
| MoSpa2 | M. oryzae | N-terminal IDRs | polarized actin cable assembly | pathogenesis | [34] |
| FolSvp2 | F. oxysporum | - | translocates SlISP from plastids into effector condensates in planta, attenuates host ROS production to facilitates the fungal invasion | pathogenesis | [36] |
| Whi3/SPA2/BNI1 RNAs | S. cerevisiae | QRR | maintains tip growth and initiates lateral branching | heat stress | [29,30] |
| Whi3/CLN3 | S. cerevisiae Ashbya | QRR | temperature adaptation | cold stress | [29,30] |
| Aip5/Bni134/Spa2 | S. cerevisiae, and filamentous fungi | N-terminal domain | protects actin assembly | pH or energy depletion response | [31] |
| Scd6/Dcp1/2/Pat1/Edc3 | S. pombe | HLMs | mRNA storage and decay | - | [47,48] |
| Lsm7 foci | S. cerevisiae | IDR | mRNA storage and decay | conditions of energy and nutrient limitation | [40] |
| Sup35 | S. cerevisiae | PrLDs | rescuing essential Sup35 translation factor from stress-induced damage | pH stress response | [11,53] |
| Snf5p | S. cerevisiae | - | transcription and chromatin remodeling | pH stress | [64] |
| Pub1 | S. cerevisiae | RRMs drive self-assembly while IDRs modify condensate properties | helps cells recover from heat shock | heat shock/pH stress | [39] |
| Pab1 | S. cerevisiae | LCR | - | heat stress | [38] |
| Ded1p | S. pombe | IDR | enhances survival | heat stress | [4] |
| BP1 | N. crassa F. graminearum. | DR2 | regulates BP1–PRC2 interaction and H3K27me3 recognition to repress secondary metabolism-related genes expression, particularly those involved in deoxynivalenol mycotoxin biosynthesis | - | [69] |
| Pex5/Pex13/Pex14 | S. cerevisiae | IDR | forms minimal transport machinery on nuclear pore and peroxisome membrane | - | [66,67] |
| Ubc4-CLRC | S. cerevisiae | IDR | regulates centromeric transcription; chromodomain function via ubiquitination | - | [60] |
| HP1α/Swi6 | S. pombe | multivalent interactions of the N-terminus and hinge region | promotes H3K9me2 deposition, modulates nuclear stiffness | - | [58] |
| Cdc19 | S. cerevisiae | IDR | protects Cdc19 from stress-induced degradation and inactivates enzymes | glucose starvation and heat shock stress response | [71] |
| PPAT | S. cerevisiae | - | purine synthesis | purine-depleted environment | [70] |
| Motif. | Sequence | Nanostructure/Assembly | Refs. |
|---|---|---|---|
| IDPs | an octapeptide repeats of (G-R-G-D-S-P-Y-S) | macroscopic PS | [86,89] |
| ELR, LCST like IDPPs | (VPGXG)n, (IPGXG)n, (VPAXG)n, (VPAPVG)n X can be any amino acid except L-proline (P). | coacervate/spherical micelles/micellar aggregates | [90,91] |
| RLR, UCST like IDPPs | (GRGDSPYS)20 (RDGSPSS-GRGDYPYS)10 (GGRPSDSXGAPGGGN)n X must be an aromatic amino acid such as tryptophan (W) or phenylalanine (F), in the case of tyrosine (Y) | nanofibrillar assemblies | [92] |
| FUS LC domain | (G/S-Y-G/S)27 | a gel-like state, β amyloid-like polymers, fibrils | [94] |
| LARKS | 58 NFGAFS63 | amyloid-like protofilaments, hydrogels | [51] |
| CC | heptad repeat, usually containing hydrophobic amino acids at the first and fourth position of the repeat. | fiber-forming | [95,96] |
| Nck | SH3 domains | - | [97,98] |
| SUMO-SIM | an extended β-strand-like conformation | - | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Zhang, D.; Zhu, Z. Phase Separation-Regulated Fungal Growth, Sexual Development, Adaptation and Synthetic Biology Applications. J. Fungi 2025, 11, 680. https://doi.org/10.3390/jof11090680
Tong X, Zhang D, Zhu Z. Phase Separation-Regulated Fungal Growth, Sexual Development, Adaptation and Synthetic Biology Applications. Journal of Fungi. 2025; 11(9):680. https://doi.org/10.3390/jof11090680
Chicago/Turabian StyleTong, Xinxin, Daixi Zhang, and Zhenhong Zhu. 2025. "Phase Separation-Regulated Fungal Growth, Sexual Development, Adaptation and Synthetic Biology Applications" Journal of Fungi 11, no. 9: 680. https://doi.org/10.3390/jof11090680
APA StyleTong, X., Zhang, D., & Zhu, Z. (2025). Phase Separation-Regulated Fungal Growth, Sexual Development, Adaptation and Synthetic Biology Applications. Journal of Fungi, 11(9), 680. https://doi.org/10.3390/jof11090680






