The Prognostic Value of (1→3)-β-D-Glucan in COVID-19 Patients with and Without Secondary Fungal Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Detection of (1→3)-β-D-Glucan
2.3. Statistical Analysis
3. Results
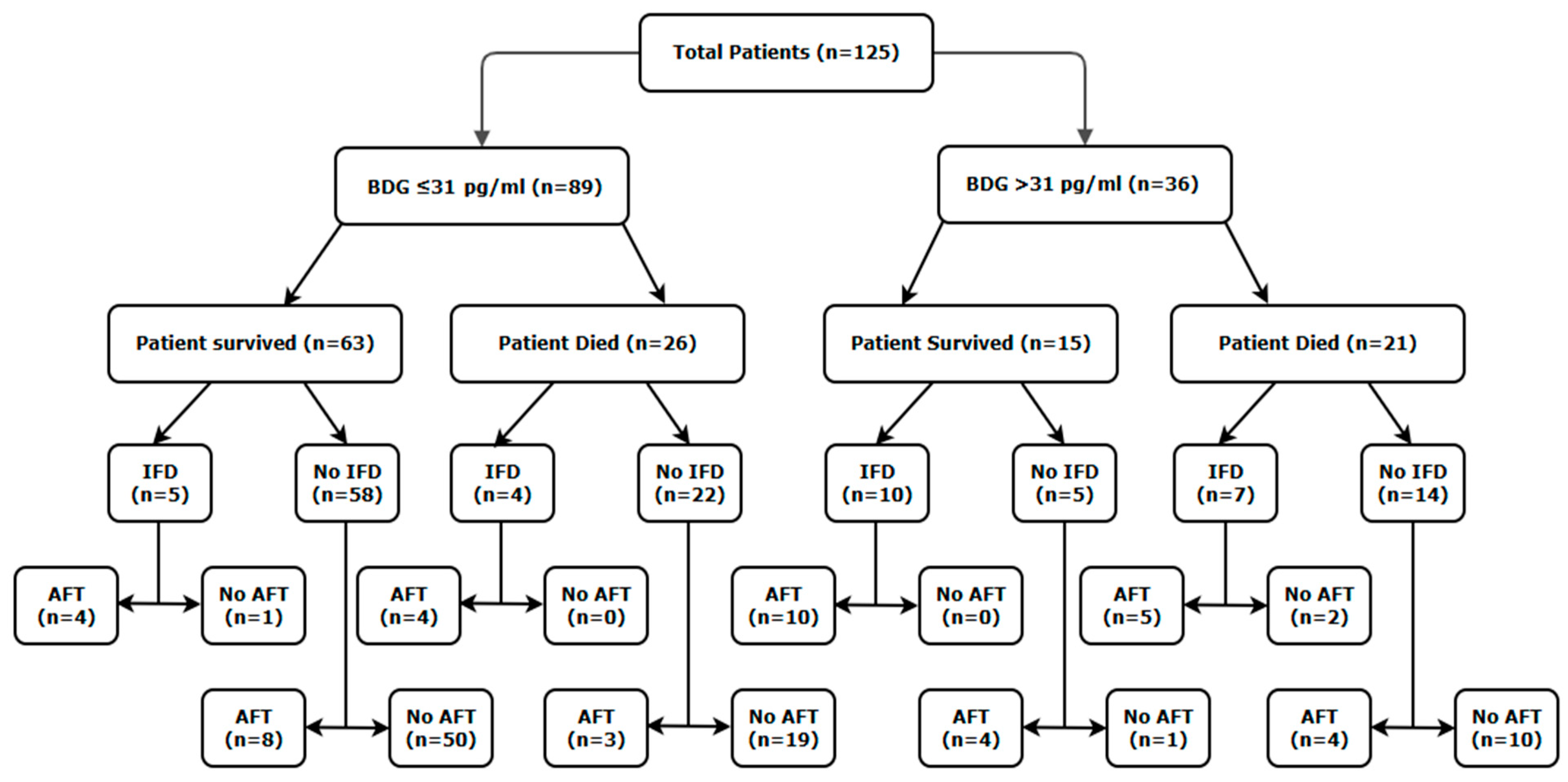
3.1. Population
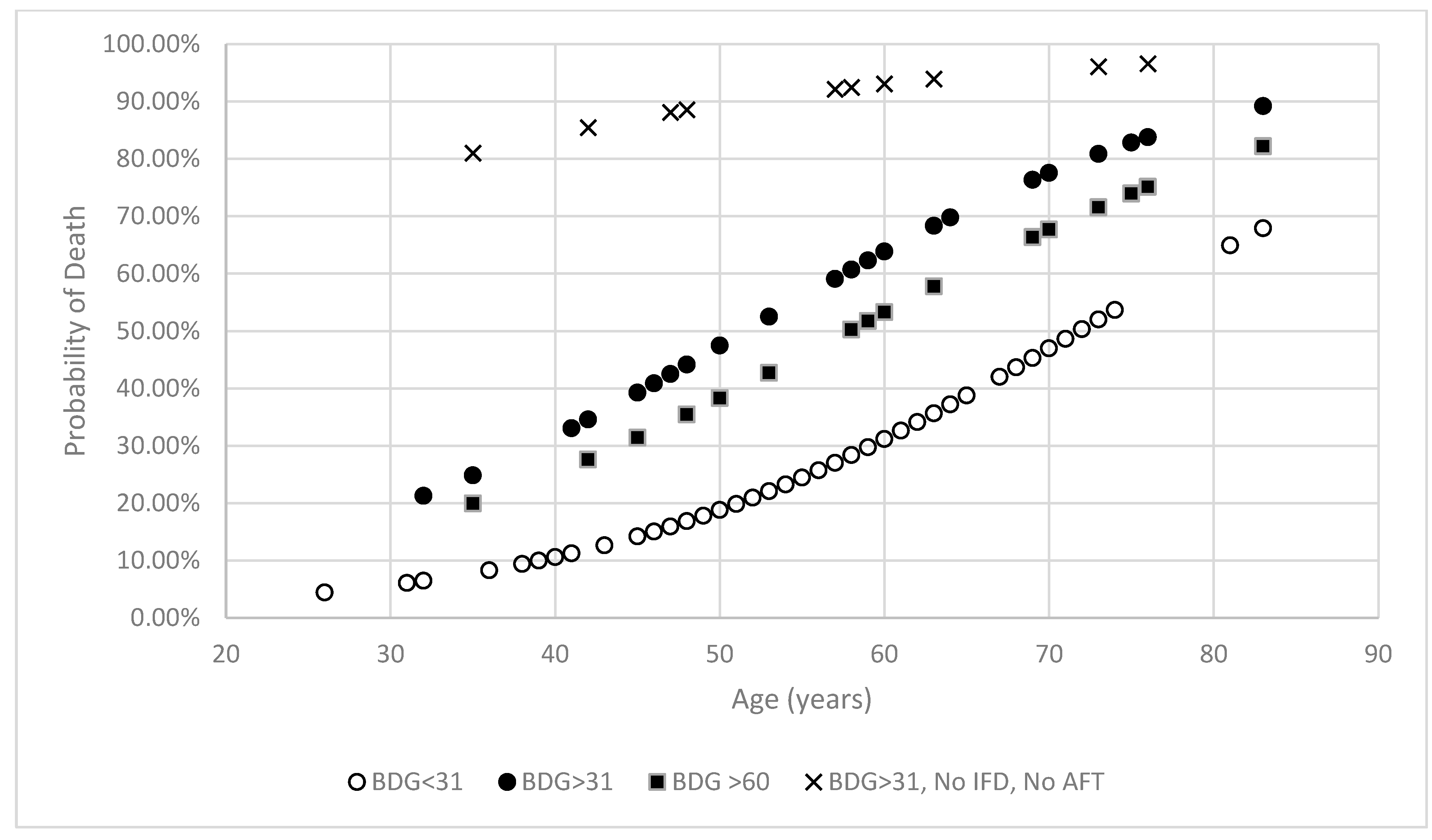
3.2. (1→3)-β-D-Glucan Concentration and Mortality
3.3. Antifungal Therapy and Mortality
3.4. Mortality Associated with Invasive Fungal Disease
3.5. Mortality Associated with Invasive Fungal Disease and the Use of Antifungal Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IFD | Invasive fungal disease |
| PCT | Procalcitonin |
| BDG | (1–3)-β-D-Glucan |
| AFT | Antifungal therapy |
| EORTC | European Organization for the Research and Treatment of Cancer |
| MSGERC | Mycoses Study Group Educational and Research Consortium |
| ECMM | European Confederation of Medical Mycology |
| ISHAM | International Society for Human and Animal Mycoses |
| CAPA | COVID-19-associated pulmonary aspergillosis |
| 95% CI | 95% confidence intervals |
| PAMPs | Pathogen-associated molecular pattern |
| LPS | Lipopolysaccharide |
| IC | Invasive candidiasis |
References
- Arnold, D.T.; Attwood, M.; Barratt, S.; Morley, A.; Elvers, K.T.; McKernon, J.; Donald, C.; Oates, A.; Noel, A.; MacGowan, A.; et al. Predicting outcomes of COVID-19 from admission biomarkers: A prospective UK cohort study. Emerg. Med. J. 2021, 38, 543–548. [Google Scholar] [CrossRef]
- Gold, J.A.W.; Adjei, S.; Gundlapalli, A.V.; Huang, Y.-L.A.; Chiller, T.; Benedict, K.; Toda, M. Increased Hospitalizations Involving Fungal Infections during COVID-19 Pandemic, United States, January 2020–December 2021. Emerg. Infect. Dis. 2023, 29, 1433–1437. [Google Scholar] [CrossRef]
- Mittal, R.; Chourasia, N.; Bharti, V.K.; Singh, S.; Sarkar, P.; Agrawal, A.; Ghosh, A.; Pal, R.; Kanwar, J.R.; Kotnis, A. Blood-based biomarkers for diagnosis, prognosis, and severity prediction of COVID-19: Opportunities and challenges. J. Fam. Med. Prim. Care 2022, 11, 4330–4341. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Esteves, P.; Bruzzi, P.; Mikulska, M.; Furfaro, E.; Mesini, A.; Tatarelli, P.; Grignolo, S.; Viscoli, C.; Colombo, A.L.; et al. Initial serum (1,3)-β-d-glucan as a predictor of mortality in proven candidaemia: Findings from a retrospective study in two teaching hospitals in Italy and Brazil. Clin. Microbiol. Infect. 2015, 21, 954.e9–954.e17. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Bhardwaj, A. β-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef]
- Yamamoto, T.; Oishi, K.; Suizu, J.; Murakawa, K.; Hisamoto, Y.; Fujii, T.; Harada, M.; Chikumoto, A.; Kikuchi, Y.; Hamada, K.; et al. False-positive Elevation of Beta-D-glucan and Aspergillus Galactomannan Levels Due to Mendelson’s Syndrome after Rice Aspiration. Intern. Med. 2022, 61, 2935–2939. [Google Scholar] [CrossRef] [PubMed]
- Carelli, S.; Posteraro, B.; Torelli, R.; De Carolis, E.; Vallecoccia, M.S.; Xhemalaj, R.; Cutuli, S.L.; Tanzarella, E.S.; Dell’Anna, A.M.; Lombardi, G.; et al. Prognostic value of serial (1,3)-β-d-glucan measurements in ICU patients with invasive candidiasis. Crit. Care 2024, 28, 236. [Google Scholar] [CrossRef]
- Hardison, S.E.; Brown, G.D. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 2012, 13, 817–822. [Google Scholar] [CrossRef]
- White, P.L.; Posso, R.; Parr, C.; Price, J.S.; Finkelman, M.; Barnes, R.A. The Presence of (1→3)-β-D-Glucan as Prognostic Marker in Patients After Major Abdominal Surgery. Clin. Infect. Dis. 2021, 73, e1415–e1422. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose COVID-19 Associated Invasive Fungal Disease in the ICU. SSRN J. 2020, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Carolis, E.D.; Marchionni, F.; Torelli, R.; Posteraro, P.; Pascale, G.D.; Carelli, S.; Sanguinetti, M.; Posteraro, B. Comparable Serum and Plasma 1,3-β-d-Glucan Values Obtained Using the Wako β-Glucan Test in Patients with Probable or Proven Fungal Diseases. J. Clin. Microbiol. 2019, 57, e00149-19. [Google Scholar] [CrossRef]
- De Pascale, G.; Posteraro, B.; D’Arrigo, S.; Spinazzola, G.; Gaspari, R.; Bello, G.; Montini, L.M.; Cutuli, S.L.; Grieco, D.L.; Di Gravio, V.; et al. (1,3)-β-d-Glucan-based empirical antifungal interruption in suspected invasive candidiasis: A randomized trial. Crit. Care 2020, 24, 550. [Google Scholar] [CrossRef]
- De Pascale, G.; Tumbarello, M. Fungal infections in the ICU: Advances in treatment and diagnosis. Curr. Opin. Crit. Care 2015, 21, 421–429. [Google Scholar] [CrossRef]
- Amornphimoltham, P.; Yuen, P.S.T.; Star, R.A.; Leelahavanichkul, A. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Dig. Dis. Sci. 2019, 64, 2416–2428. [Google Scholar] [CrossRef]
- Carbonell, R.; Urgelés, S.; Rodríguez, A.; Bodí, M.; Martín-Loeches, I.; Solé-Violán, J.; Díaz, E.; Gómez, J.; Trefler, S.; Vallverdú, M.; et al. Mortality comparison between the first and second/third waves among 3795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg. Health—Eur. 2021, 11, 100243. [Google Scholar] [CrossRef]
- Bakakos, A.; Koukaki, E.; Ampelioti, S.; Ioannidou, I.; Papaioannou, A.I.; Loverdos, K.; Koutsoukou, A.; Rovina, N. The Real Impact of Age on Mortality in Critically Ill COVID-19 Patients. J. Pers. Med. 2023, 13, 908. [Google Scholar] [CrossRef] [PubMed]
- Giron, L.B.; Peluso, M.J.; Ding, J.; Kenny, G.; Zilberstein, N.F.; Koshy, J.; Ying Hong, K.; Rasmussen, H.; Miller, G.E.; Bishehsari, F.; et al. Markers of fungal translocation are elevated during post-acute sequelae of SARS-CoV-2 and induce NF-κB signaling. JCI Insight 2022, 7, e160989. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Kitsios, G.D.; Kotok, D.; Yang, H.; Finkelman, M.A.; Zhang, Y.; Britton, N.; Li, X.; Levochkina, M.S.; Wagner, A.K.; Schaefer, C.; et al. Plasma 1,3-β-d-glucan levels predict adverse clinical outcomes in critical illness. JCI Insight 2021, 6, e141277. [Google Scholar] [CrossRef] [PubMed]
- Hurt, W.; Youngs, J.; Ball, J.; Edgeworth, J.; Hopkins, P.; Jenkins, D.R.; Leaver, S.; Mazzella, A.; Molloy, S.F.; Schelenz, S.; et al. COVID-19-associated pulmonary aspergillosis in mechanically ventilated patients: A prospective, multicentre UK study. Thorax 2024, 79, 75–82. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Highest BDG Concentration | p-Value | |
|---|---|---|---|
| ≤31 pg/mL (n = 89) | >31 pg/mL (n = 36) | ||
| M/F ratio | 2.6/1 | 1.8/1 | 0.3967 a |
| Age (median) | 57 | 58 | 0.8786 b |
| Co-morbidities c | HTN: 24 DM: 22 Respiratory: 21 Autoimmune: 9 Obesity: 8 Cancer: 8 Endocrine: 8 Neurological: 7 Cardiac: 6 GI: 4 Blood/vascular conditions: 3 Renal: 3 HM: 2 Other: 6 None: 18 | HTN: 12 DM: 12 Respiratory: 8 Autoimmune: 5 Obesity: 3 Cancer: 2 Endocrine: 1 Neurological: 2 Cardiac: 4 GI: 5 Blood/vascular conditions: 2 Renal: 5 HM: 3 Other: 4 None: 6 | 0.5165 a 0.3769 a 1.0000 a 0.5426 a 1.0000 a 0.7226 a 0.4447 a 1.0000 a 0.4718 a 0.1186 a 0.6252 a 0.0435 a 0.1432 a 0.4718 a 0.8035 a |
| IFD (Total = 26) (Organism (when available)—AFT used) | Total: 9 Fungaemia: 1 (Rhodotorula rubra—Caspofungin) CVC: 5 (All C. albicans—3× Fluconazole, 1× Caspofungin and 1× no AFT) Possible CAPA: 3 (All A. fumigatus—2× Voriconazole and 1× L-Amb) | Total: 17 Fungaemia: 1 (Unidentified yeast—fluconazole) Candidaemia: 4 (All C. albicans—2× Caspofungin, 1× L-Amb and 1× no AFT) CVC: 3 (1× Unidentified yeast, 1× C. albicans and 1× Candida spp.—2× Voriconazole and 1× fluconazole) Candida peritonitis: 2 (All C. albicans—1× Caspofungin and 1× fluconazole) Probable CAPA: 5 (4× A. fumigatus and 1× no culture—4× voriconazole and 1× L-Amb) Possible CAPA: 2 (1× A. fumigatus and 1× no culture—2× voriconazole) | N/A d |
| Documented bacteraemia (Total = 58) (%) | 40 (45) | 18 (50) | 0.6932 a |
| Antibacterials used (all patients, Total = 114) (n, (%)) | 78 (79) | 36 (100) | 0.0328 a |
| Antibacterials used (excluding 26 cases of IFD (n = 9 ≤ 31 pg/mL and n = 17 > 31 pg/mL, Total = 88)) (n (%)) | 69 (86) | 19 (100) | 0.1167 a |
| Population | Mortality Rate (n/N, (%)) | |||
|---|---|---|---|---|
| BDG Concentration | Difference in Mortality (95% CI) | Significance (p-Value) | ||
| ≤31 pg/mL | >31 pg/mL | |||
| Overall population (n = 125) a | 26/89 (29%) | 21/36 (58%) b | 29% (10.0 to 45.9) | 0.0039 |
| Patients on AFT (n = 42) c | 7/19 (37%) | 9/23 (39%) d | 2% (29.1 to −25.6) | 1.0000 |
| Patients not on AFT (n = 83) a | 19/70 (27%) | 12/13 (92%) e | 65% (37.1 to 76.2) | <0.0001 |
| Patients with IFD (n = 26) f | 4/9 (44%) | 7/17 (41%) g | 3% (31.0 to −38.2) | 1.0000 |
| Patients without IFD (n = 99) a | 22/80 (28%) | 14/19 (74%) h | 46% (21.3 to 63.0) | 0.0003 |
| Patients with IFD on AFT (n = 23) i | 4/8 (50%) | 5/15 (33%) j | 17% (21.2 to −50.4) | 0.6570 |
| Patients with IFD not on AFT (n = 3) k | 0/1 (0%) | 2/2 (100%) l | 100% (30.5 to −100) | 0.3333 |
| Patients without IFD on AFT (n = 19) a | 3/11 (27%) | 4/8 (50%) m | 23% (18.1 to −56.2) | 0.3765 |
| Patients without IFD not on AFT (n = 80) a | 19/69 (28%) | 10/11 (91%) n | 63% (32.5 to 75.2) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welagedara, U.; Price, J.; Posso, R.; Backx, M.; White, P.L. The Prognostic Value of (1→3)-β-D-Glucan in COVID-19 Patients with and Without Secondary Fungal Disease. J. Fungi 2025, 11, 656. https://doi.org/10.3390/jof11090656
Welagedara U, Price J, Posso R, Backx M, White PL. The Prognostic Value of (1→3)-β-D-Glucan in COVID-19 Patients with and Without Secondary Fungal Disease. Journal of Fungi. 2025; 11(9):656. https://doi.org/10.3390/jof11090656
Chicago/Turabian StyleWelagedara, Udari, Jessica Price, Raquel Posso, Matt Backx, and P. Lewis White. 2025. "The Prognostic Value of (1→3)-β-D-Glucan in COVID-19 Patients with and Without Secondary Fungal Disease" Journal of Fungi 11, no. 9: 656. https://doi.org/10.3390/jof11090656
APA StyleWelagedara, U., Price, J., Posso, R., Backx, M., & White, P. L. (2025). The Prognostic Value of (1→3)-β-D-Glucan in COVID-19 Patients with and Without Secondary Fungal Disease. Journal of Fungi, 11(9), 656. https://doi.org/10.3390/jof11090656







