Into the Wild: A Look at Candida albicans Outside the Clinical Setting
Abstract
1. Introduction
2. Candida albicans in Soil
3. Candida albicans in Plants
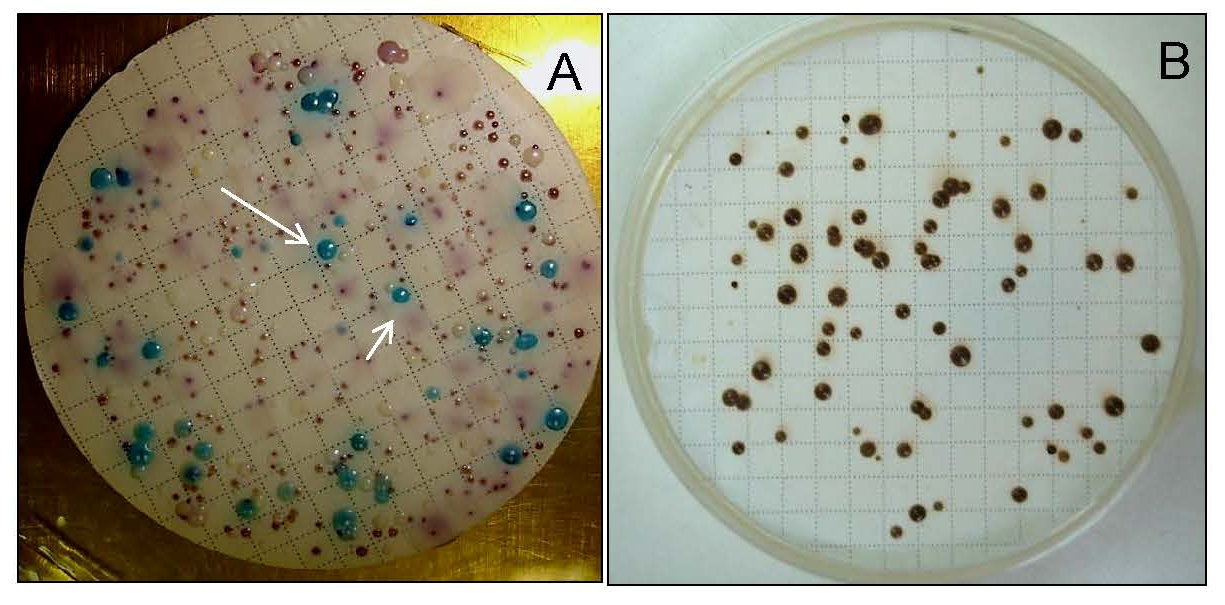
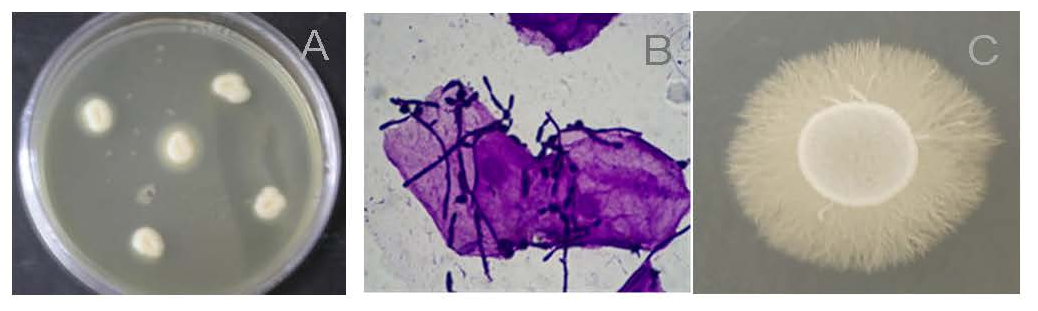
4. Candida albicans in Freshwater and Sea
5. Clinical Importance of the Isolation of Candida albicans in Natural Environments
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gow, N.; Yadav, B. Microbe Profile: Candida albicans: A shape-changing, opportunistic pathogenic fungus of humans: This article is part of the Microbe Profiles collection. Microbiol. 2017, 163(8), 1145–1147. [Google Scholar]
- Groenewald, M.; Hittinger, C.T.; Bensch, K.; Opulente, D.; Shen, X.-X.; Li, Y.; Liu, C.; LaBella, A.; Zhou, X.; Limtong, S.; et al. A genome-informed higher rank classification of the biotechnologically important fungal subphylum Saccharomycotina. Stud. Mycol. 2023, 105, 1–22. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73 (suppl1), i4–i13. [Google Scholar] [CrossRef]
- Opulente, D.A.; Langdon, Q.K.; Buh, K.V.; Haase, M.A.B.; Sylvester, K.; Moriarty, R.V.; Jarzyna, M.; Considine, S.L.; Schneider, R.M.; Hittinger, C.T. Pathogenic budding yeasts isolated outside of clinical settings. FEMS Yeast Res. 2019, 19, foz032. [Google Scholar] [CrossRef]
- Barnett, J.A. A history of research on yeasts 12: Medical yeasts part 1, Candida albicans. Yeast 2008, 25, 385–417. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.-A.; Boekhout, T.; Scorzetti, G.; Fell, J.W.; Kurtzman, C.P. 2011 Chapter 90 Candida Berkhout. In The Yeasts, 5th ed.; Kutrzman, C., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 1923; pp. 987–1278. [Google Scholar]
- Van Uden, N.; De Matos Faia, M.; Assis-Lopes, L. Isolation of Candida albicans from vegetable sources. J. Gen. Microbiol. 1956, 15, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Di Menna, M. Candida albicans from Grass Leaves. Nature 1958, 181, 1287–1288. [Google Scholar] [CrossRef]
- Robinson, H.A.; Pinharanda, A.; Bensasson, D. Summer temperature can predict the distribution of wild yeast populations. Ecol. Evol. 2016, 6, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Bensasson, D.; Dicks, J.; Ludwig, J.M. Diverse lineages of Candida albicans live on old oaks. Genetics 2019, 211, 277–288. [Google Scholar] [CrossRef]
- Kochkina, G.; Ivanushkina, N.; Ozerskaya, S. Ancient fungi in Antarctic permafrost environments. FEMS Microbiol. Ecol. 2012, 82, 501–509. [Google Scholar] [CrossRef]
- Lopes, M.R.; Lara, C.A.; Moura, M.E.F.; Uetanabaro, A.P.T.; Morais, P.B.; Vital, M.J.; Rosa, C.A. Characterization of the diversity and physiology of cellobiose-fermenting yeasts isolated from rotting wood in Brazilian ecosystems. Fungal Biol. 2018, 122, 668–676. [Google Scholar] [CrossRef]
- Maciel, N.O.; Johann, S.; Brandão, L.R.; Kucharíková, S.; Morais, C.G.; Oliveira, A.P.; Freitas, G.J.; Borelli, B.M.; Pellizzari, F.M.; A Santos, D.; et al. Occurrence, antifungal susceptibility, and virulence factors of opportunistic yeasts isolated from Brazilian beaches. Mem. Inst. Oswaldo Cruz. 2019, 114, e180566. [Google Scholar] [CrossRef]
- Stone, W.; Jones, B.L.; Wilsenach, J.; Botha, A. External ecological niche for Candida albicans within reducing, oxygen-limited zones of wetlands. Appl. Environ. Microbiol. 2012, 78, 2443–2445. [Google Scholar] [CrossRef]
- Ajello, L. Soil as natural reservoir for human pathogenic fungi. Science 1956, 123, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.L.; Beneke, E.S. Human pathogenic fungi recovered from Brasilian soil. Mycopathol. Mycol. Appl. 1964, 22, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mok, W.Y.; Luizão, R.C.; Do Socorro Barreto Da Silva, M.; Teixeira, M.F.; Muniz, E.G. Ecology of pathogenic yeasts in Amazonian soil. Appl. Environ. Microbiol. 1984, 47, 390–394. [Google Scholar] [CrossRef]
- Prigitano, A.; Trovato, L.; Esposto, M.C.; Brandão, J.; Cogliati, M.; Gatta, G.D.; Grancini, A.; Migliorisi, G.; Oliveri, S.; Romanò, L.; et al. Fungal diversity in lake and sea beaches of Italy: Relevance to human health. Sci. Total Environ. 2023, 859, 160417. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Dudášová, H.; Stratilová, E.; Balaščáková, M. Diversity of yeasts in the soil adjacent to fruit trees of the Rosaceae family. Yeast 2019, 36, 617–631. [Google Scholar] [CrossRef]
- Brandão, J.; Gangneux, J.P.; Arikan-Akdagli, S.; Esposto, M.C.; Brandão, J.; Cogliati, M.; Gatta, G.D.; Grancini, A.; Migliorisi, G.; Oliveri, S.; et al. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021, 781, 146598. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Shahzad, S.; Choudhary, M.I.; Khan, S.A.; Ahmad, A. Biodiversity of the endophytic fungi isolated from Calotropis procera (AIT.) R. BR. Pak. J. Bot. 2007, 39, 2233–2239. [Google Scholar]
- Guamán-Burneo, M.C.; Dussán, K.J.; Cadete, R.M.; Cheab, M.A.; Portero, P.; Carvajal-Barriga, E.J.; da Silva, S.S.; Rosa, C.A. Xylitol production by yeasts isolated from rotting wood in the Galápagos Islands, Ecuador, and description of Cyberlindnera galapagoensis f.a., sp. nov. Antonie Van Leeuwenhoek 2015, 108, 919–931. [Google Scholar] [CrossRef]
- Barros, K.O.; Alvarenga, F.B.M.; Magni, G.; Souza, G.F.L.; Abegg, M.A.; Palladino, F.; da Silva, S.S.; Rodrigues, R.C.L.B.; Sato, T.K.; Hittinger, C.T.; et al. The Brazilian Amazonian rainforest harbors a high diversity of yeasts associated with rotting wood, including many candidates for new yeast species. Yeast 2023, 40, 84–101. [Google Scholar] [CrossRef]
- Bakhiet, S.; Ahmed, W.; Mohammed, W. Significance of fungal species isolated from blue nile river and tuti island on drinking water quality. J. Appl. Life Sci. Int. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, K.; Biedunkiewicz, A.; Nowacka, K.; Glinka, P. Potentially pathogenic fungi of the Candida genus isolated from the Łyna River—A 20-year study. Ann. Parasitol. 2018, 64, 217–223. [Google Scholar]
- Monapathi, M.E.; Bezuidenhout, C.C.; Rhode, O.H.J. Efflux pumps genes of clinical origin are related to those from fluconazole-resistant Candida albicans isolates from environmental water. Water Sci. Technol. 2018, 77, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Cupozak-Pinheiro, W.J.; Araújo De Almeida-Apolonio, A.; Sasaki, M.H.; Maran, N.H. Candida species contamination in drinking groundwater from residence wells in three municipalities of midwestern Brazil and the potential human health risks. Microb. Pathog. 2022, 169, 105660. [Google Scholar] [CrossRef]
- Sautour, M.; Lemaître, J.; Ranjard, L.; Truntzer, C.; Basmaciyan, L.; Depret, G.; Hartmann, A.; Dalle, F. Detection and survival of Candida albicans in soils. Environ. DNA 2021, 3, 1093–1101. [Google Scholar] [CrossRef]
- Tanghe, A.; Carbrey, J.M.; Agre, P.; Thevelein, J.M.; Van Dijck, P. Aquaporin expression and freeze tolerance in Candida albicans. Appl. Environ. Microbiol. 2005, 71, 6434–6437. [Google Scholar] [CrossRef]
- Buck, J.D.; Bubucis, P.M. Membrane filter procedure for enumeration of Candida albicans in natural waters. Appl. Environ. Microbiol. 1978, 35, 237–242. [Google Scholar] [CrossRef]
- Pinto, K.C.; Hachich, E.M.; Sato, M.I.Z.; Di Bari, M.; Coelho, M.C.L.S.; Matté, M.H.; Lamparelli, C.C.; Razzolini, M.T.P. Microbiological quality assessment of sand and water from three selected beaches of South Coast, São Paulo State, Brazil. Water Sci. Technol. 2012, 66, 2475–2482. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Nosanchuk, J.D.; Malliaris, S.D.; Casadevall, A. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect. Immun. 2004, 72, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Fu, M.; Guimaraes, A.; Albuquerque, P. The ‘amoeboid predator-fungal animal virulence’ hypothesis. J. Fungi 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Lemos Tavares, P.; Carvalho Ribeiro, A.; Kercher Berte, F. The interaction between Sporothrix schenckii sensu stricto and Sporothrix brasiliensis with Acanthamoeba castellanii. Mycoses 2020, 63, 302–307. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.J.; Kutz, S.; Harvell, C.D. Climate change and infectious diseases: From evidence to a predictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Raffel, T.R.; Romansic, J.M.; Halstead, N.T.; McMahon, T.A.; Venesky, M.D.; Rohr, J.R. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 2013, 3, 146–151. [Google Scholar] [CrossRef]
- Maynard, J.; Van Hooidonk, R.; Eakin, C.M.; Puotinen, M.; Garren, M.; Williams, G.; Heron, S.F.; Lamb, J.; Weil, E.; Willis, B.; et al. Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nat. Clim. Change 2015, 5, 688–694. [Google Scholar] [CrossRef]
- Leach, M.D.; Farrer, R.A.; Tan, K.; Miao, Z.; Walker, L.A.; Cuomo, C.A.; Wheeler, R.T.; Brown, A.J.P.; Wong, K.H.; Cowen, L.E. Hsf1 and Hsp90 orchestrate temperature-dependent global transcriptional remodelling and chromatin architecture in Candida albicans. Nat. Commun. 2016, 7, 11704. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Medeiros, A.O.; Kohler, L.M.; Hamdan, J.S.; Missagia, B.S.; Barbosa, F.A.; Rosa, C.A. Diversity and antifungal susceptibility of yeasts from tropical freshwater environments in Southeastern Brazil. Water Res. 2008, 42, 3921–3929. [Google Scholar] [CrossRef]
- Brandão, L.R.; Medeiros, A.O.; Duarte, M.C.; Barbosa, A.C.; Rosa, C.A. Diversity and antifungal susceptibility of yeasts isolated by multiple-tube fermentation from three freshwater lakes in Brazil. J. Wather Health 2010, 8, 279–289. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Castelo Branco, D.S.C.M.; Duarte, G.P.S.; Paiva, M.A.; Teixeira, C.E.; Zeferino, J.P.; Monteiro, A.J.; Cordeiro, R.A.; Sidrim, J.J.; Rocha, M.F. Yeast microbiota of raptors: A possible tool for environmental monitoring: Use of Candida spp. for environmental monitoring. Environ. Microb. Rep. 2012, 4, 189–193. [Google Scholar] [CrossRef]
- Castelo-Branco, D.S.C.M.; Brilhante, R.S.N.; Paiva, M.A.N.; Teixeira, C.E.C.; Caetano, E.P.; Ribeiro, J.F.; Cordeiro, R.A.; Sidrim, J.J.C.; Monteiro, A.J.; Rocha, M.F.G. Azole-resistant Candida albicans from a wild Brazilian porcupine (Coendou prehensilis): A sign of an environmental imbalance? Med. Mycol. 2013, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Paiva, M.A.N.; Sampaio, C.M.S.; Castelo-Branco, D.S.C.M.; Alencar, L.P.; Bandeira, T.J.P.G.; Cordeiro, R.A.; Neto, W.d.A.P.; Moreira, J.L.B.; Sidrim, J.J.C.; et al. Surveillance of azole resistance among Candida spp. As a strategy for the indirect monitoring of freshwater environments. Water Air Soil. Pollut. 2015, 226, 52. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Alencar, L.P.; Paiva, M.A.N.; Melo, L.M.; Bandeira, S.P.; Ponte, Y.B.; Sales, J.A.; Guedes, G.M.M.; Castelo-Branco, D.S.C.M.; Bandeira, T.J.; et al. Cross-resistance to fluconazole induced by exposure to the agricultural azole tetraconazole: An environmental resistance school? Mycoses 2016, 59, 281–290. [Google Scholar] [CrossRef] [PubMed]

| Number of Isolates | Substract/Habitat | Site | City/Country | Reference |
|---|---|---|---|---|
| 1 | Soil | Jefferson | Iowa, USA | Opulente et al. [4] |
| 1 | Soil | Northeastern | Wisconsin, USA | Opulente et al. [4] |
| 1 | Soil | Northeastern | Wisconsin, USA | Opulente et al. [4] |
| 1 | Plant Matter Soil | Mesa Canyon San Antonio | Texas, USA | Opulente et al. [4] |
| 1 | Plant Matter Soil/Duff | Mesa Cyn San Antonio, TX | Texas, USA | Opulente et al. [4] |
| 1 | Soil | Shelby County | Tenesesse, USA | Ajello et al. [15] |
| 8 | Soils containing cow and hog dung | Farms near Belo Horizonte | Minas Gerais, Brazil | Rogers and Beneke [16] |
| 8 | Chicken house soils | Farms near Belo Horizonte | Minas Gerais, Brazil | Rogers and Beneke [16] |
| 2 | Cave soil | Itatiaia Park area | Minas Gerais, Brasil | Rogers and Beneke [16] |
| 1 | Chicken yard soil | Piracicaba and Vicinity | Piracicaba, São Paulo | Rogers and Beneke [16] |
| 1 | Sand near steps | Santos beach area | Santos, Brasil | Rogers and Beneke [16] |
| 1 | Park flower bed soil | São Paulo | São Paulo, Brazil | Rogers and Beneke [16] |
| 1 | Amazonian soils | Brazilian Amazon Basin | Brazilian Amazon Basin, Brazil | Mok et al. [17] |
| 3 | Sand | Beach | Paraná and Rio de Janeiro, Brazil | Maciel et al. [13] |
| 28.3 CFU/g | Sands | Sicilian Costal beach | Italy | Prigitano et al. [18] |
| 2 | Soil near peach tree | Southwest of Slovakia | Slovakia | Vadkertiová et al. [19] |
| 1.7 CFU/g | Sands | Irlanda | Irlanda | Brandao et al. [20] |
| 5.0 CFU/g | Sands | Black sea | Romania | Brandao et al. [20] |
| 27.1 CFU/g | Sands | Mediterranean | Serbia | Brandao et al. [20] |
| 3.3 CFU/g | Sands | Mediterranean | Turkey | Brandao et al. [20] |
| 1 | Bark tree | Wyalusing State Park | Wisconsin, USA | Opulente et al. [4] |
| 1 | Plant Matter—Fruit; Ericaceae Berry | West Sand Island | Oregon, USA | Opulente et al. [4] |
| 1 | Plant Matter—Fruit; Ericaceae Berry | Hungarian Falls | Michigan, USA | Opulente et al. [4] |
| 1 | Plant Matter—Fruit; Ericaceae Berry | Hungarian Falls | Michigan, USA | Opulente et al. [4] |
| 1 | Flower of African tulip tree (Spathodea campanulate, Bignoniaceae) | Rarontohga | Cook Islands | Lachance et al. [6] |
| 1 | Fruit of Stenocereus hystrix (Cactaceae) | - | Jamaica | Lachance et al. [6] |
| 1 | Leaves of Myrtus communis | Near the top of a hill (300 m. high) near Vermoil | Estremadura, Portugual | Van Uden et al. [7] |
| 2 | Flowers of furze (Ulex sp.) | Near the top of a hill (300 m. high) near Vermoil | Estremadura, Portugual | Van Uden et al. [7] |
| 3 | Oak | New Forest, North Europe | UK | Robinson et al. [9] |
| 1 | Steam of Calotropis procera (Ait.) R. Br. | Karachi University campus | Pakistan | Khan et al. [21] |
| 2 | Rotting wood samples | Galápagos Archipelago | Ecuador | Guama’n-Burneo et al. [22] |
| 1 | Rotting wood | Brazilian Amazonian rainforests | Brazil | Barros et al. [23] |
| 3 | Rotting wood | Atlantic Rainforest | Brazil | Lopes et al. [12] |
| 1 | Rotting wood | Cerrado | Brazil | Lopes et al. [12] |
| 1 | Rotting wood | Amazonian rainforests | Brazil | Lopes et al. [12] |
| 2 | Seawater | Recreational beaches | Paraná, Brazil | Maciel et al. [13] |
| 5 CFU/ml | Water | Sicilian Costal beach | Italy | Prigitano et al. [18] |
| 3.8 CFU/ml | Water | Northwest Europe | Irland | Brandao et al. [20] |
| 3.8 CFU/mL | Water | Mediterranean | Serbia | Brandao et al. [20] |
| 25 and 10% * | Water | Blue Nile River | Sudan | Bakhiet et al. [24] |
| ** | Water | Łyna River | Olsztyn, Poland | Kulesza et al. [25] |
| 37 | Water | North West Province Rivers | South Africa | Monopathi et al. [26] |
| 1 | Water | Groundwater for human consumption from wells | Mato Grosso do Sul, Brazil | Cupozak-Pinheiro et al. [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valério, A.D.; de Menezes, G.C.A.; Rosa, C.A.; Johann, S. Into the Wild: A Look at Candida albicans Outside the Clinical Setting. J. Fungi 2025, 11, 622. https://doi.org/10.3390/jof11090622
Valério AD, de Menezes GCA, Rosa CA, Johann S. Into the Wild: A Look at Candida albicans Outside the Clinical Setting. Journal of Fungi. 2025; 11(9):622. https://doi.org/10.3390/jof11090622
Chicago/Turabian StyleValério, Aline Dias, Graciéle Cunha Alves de Menezes, Carlos Augusto Rosa, and Susana Johann. 2025. "Into the Wild: A Look at Candida albicans Outside the Clinical Setting" Journal of Fungi 11, no. 9: 622. https://doi.org/10.3390/jof11090622
APA StyleValério, A. D., de Menezes, G. C. A., Rosa, C. A., & Johann, S. (2025). Into the Wild: A Look at Candida albicans Outside the Clinical Setting. Journal of Fungi, 11(9), 622. https://doi.org/10.3390/jof11090622







