Pulmonary Aspergillosis in Immunocompromised Critically Ill Patients: Prevalence, Risk Factors, Clinical Features and Diagnosis—A Narrative Review
Abstract
1. Introduction
2. Microbiology, Transmission and Pathogenesis
3. Immunocompromised Patients
3.1. Hematologic Patients
3.2. HIV Patients
3.3. Organ Recipients
3.4. Liver Transplant Recipients
3.5. Renal Transplant Recipients
3.6. Lung Transplant Recipients
3.7. Heart Transplant Recipients
4. Coinfections
4.1. COVID-Associated Pulmonary Aspergillosis (CAPA)
4.2. Influenza-Associated Pulmonary Aspergillosis (IAPA)
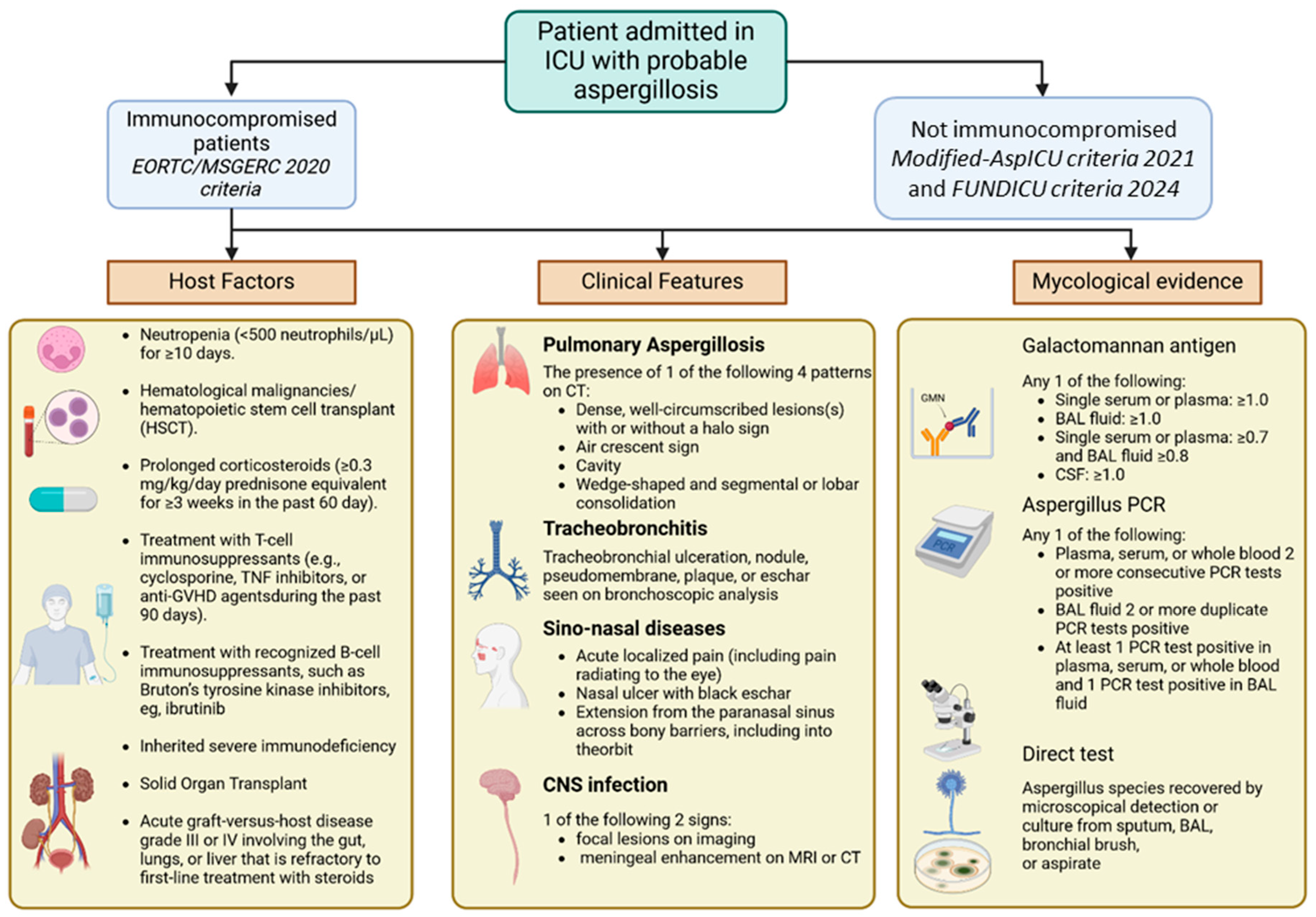
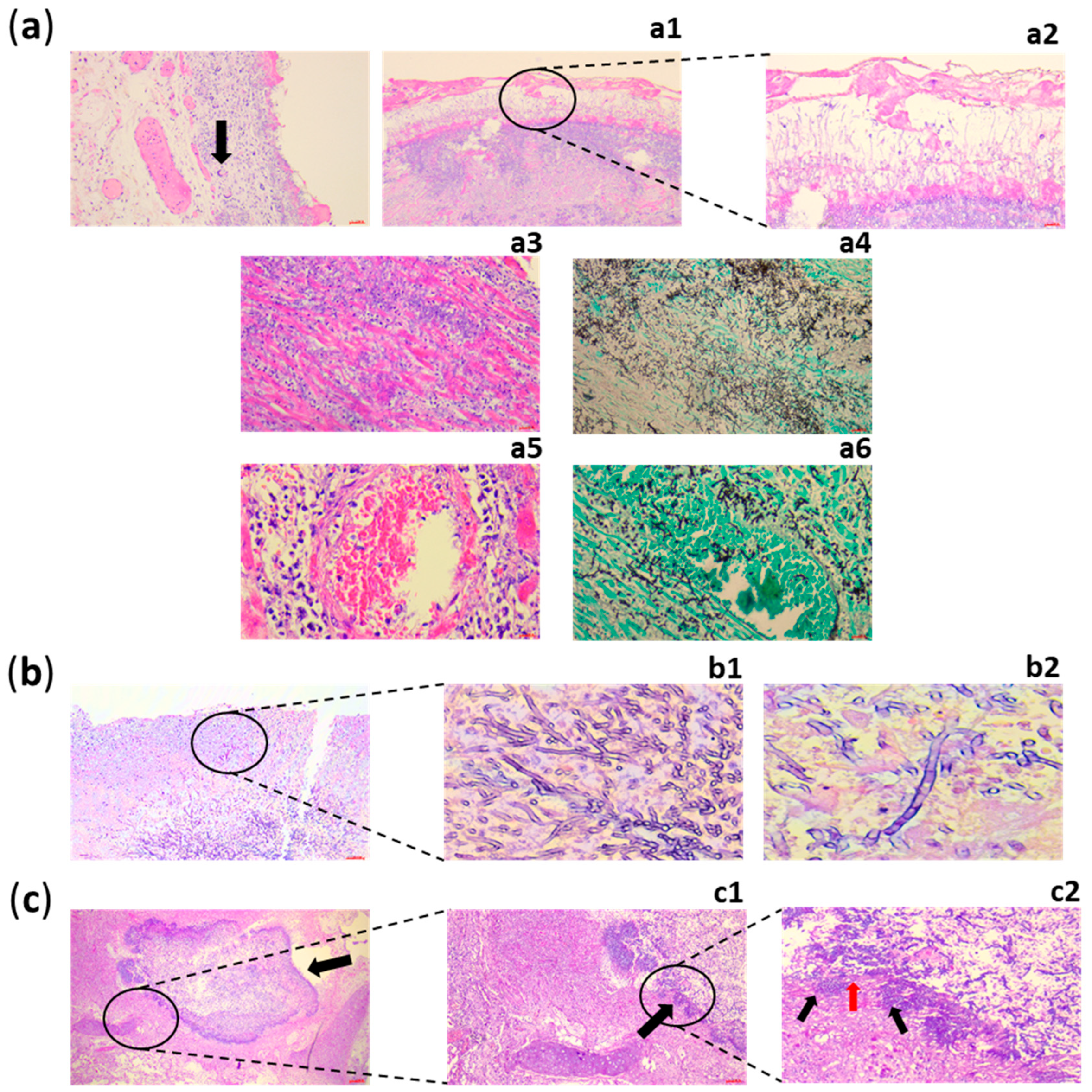
5. Diagnosis
6. Antifungal Prophylaxis and Pre-Emptive Treatment Versus Antifungal Therapy
7. Antifungal Resistance
7.1. Amphotericin B
7.2. Azoles
7.3. Echinocandins
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamoth, F. Aspergillus Fumigatus-Related Species in Clinical Practice. Front. Microbiol. 2016, 7, 683. [Google Scholar] [CrossRef]
- Shi, C.; Shan, Q.; Xia, J.; Wang, L.; Wang, L.; Qiu, L.; Xie, Y.; Lin, N.; Wang, L. Incidence, Risk Factors and Mortality of Invasive Pulmonary Aspergillosis in Patients with Influenza: A Systematic Review and Meta-analysis. Mycoses 2022, 65, 152–163. [Google Scholar] [CrossRef]
- Calderón-Parra, J.; Mills-Sanchez, P.; Moreno-Torres, V.; Tejado-Bravo, S.; Romero-Sánchez, I.; Balandin-Moreno, B.; Calvo-Salvador, M.; Portero-Azorín, F.; García-Masedo, S.; Muñez-Rubio, E.; et al. COVID-19-associated Pulmonary Aspergillosis (CAPA): Risk Factors and Development of a Predictive Score for Critically Ill COVID-19 Patients. Mycoses 2022, 65, 541–550. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Chamilos, G. Aspergillus Fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Geremia, N.; Giovagnorio, F.; Colpani, A.; De Vito, A.; Caruana, G.; Meloni, M.C.; Madeddu, G.; Panese, S.; Parisi, S.G. What Do We Know about Cryptic Aspergillosis? Microorganisms 2024, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Karakatsanis, S.; Roumpakis, C.; Korantanis, K.; Sambatakou, H.; Sipsas, N.V.; Tsirigotis, P.; Pagoni, M.; Meletiadis, J. A Prospective Multicenter Cohort Surveillance Study of Invasive Aspergillosis in Patients with Hematologic Malignancies in Greece: Impact of the Revised EORTC/MSGERC 2020 Criteria. J. Fungi 2021, 7, 27. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Malesu, V.K. Aspergillosis: A Growing Global Crisis. Available online: https://www.news-medical.net/health/Aspergillosis-A-Growing-Global-Crisis.aspx (accessed on 19 June 2025).
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and Managing COVID-19-Associated Pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus Criteria for Research and Clinical Guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Stemler, J.; de Jonge, N.; Skoetz, N.; Sinkó, J.; Brüggemann, R.J.; Busca, A.; Ben-Ami, R.; Ráčil, Z.; Piechotta, V.; Lewis, R.; et al. Antifungal Prophylaxis in Adult Patients with Acute Myeloid Leukaemia Treated with Novel Targeted Therapies: A Systematic Review and Expert Consensus Recommendation from the European Hematology Association. Lancet Haematol. 2022, 9, e361–e373. [Google Scholar] [CrossRef] [PubMed]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive Aspergillosis in Patients Admitted to the Intensive Care Unit with Severe Influenza: A Retrospective Cohort Study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Gibbons, J.G.; Rokas, A. The Function and Evolution of the Aspergillus Genome. Trends Microbiol. 2013, 21, 14–22. [Google Scholar] [CrossRef]
- Kousha, M.; Tadi, R.; Soubani, A.O. Pulmonary Aspergillosis: A Clinical Review. Eur. Respir. Rev. 2011, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P. Aspergillus Fumigatus and Aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef]
- Sugui, J.A.; Kwon-Chung, K.J.; Juvvadi, P.R.; Latgé, J.-P.; Steinbach, W.J. Aspergillus Fumigatus and Related Species. Cold Spring Harb. Perspect. Med. 2015, 5, a019786. [Google Scholar] [CrossRef]
- Zhou, S.; Ismail, M.A.I.; Buil, J.B.; Gabr, A.; Verweij, P.E.; Mahgoub, E.-S.; de Hoog, S.; Kang, Y.; Ahmed, S.A. Fungi Involved in Rhinosinusitis in Arid Regions: Insights from Molecular Identification and Antifungal Susceptibility. Microbiol. Spectr. 2023, 11, e01831-23. [Google Scholar] [CrossRef]
- Studies in Mycology No. 59. Available online: https://www.studiesinmycology.org/index.php/issue/61-studies-in-mycology-no-59 (accessed on 15 June 2025).
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus Fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef]
- Tischler, B.Y.; Hohl, T.M. Menacing Mold: Recent Advances in Aspergillus Pathogenesis and Host Defense. J. Mol. Biol. 2019, 431, 4229–4246. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A.R. Interactions of Fungal Pathogens with Phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Margalit, A.; Kavanagh, K. The Innate Immune Response to Aspergillus Fumigatus at the Alveolar Surface. FEMS Microbiol. Rev. 2015, 39, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; Wang, X.; Becker, K.L.; Aimanianda, V.; Valsecchi, I.; Gresnigt, M.S.; Netea, M.G.; Latge, J.-P.; Gow, N.A.R.; van de Veerdonk, F.L. Immunomodulatory Function of Chitosan Is Dependent on Complement Receptor 3. Cell Surf. 2025, 14, 100146. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef]
- Espinosa, V.; Jhingran, A.; Dutta, O.; Kasahara, S.; Donnelly, R.; Du, P.; Rosenfeld, J.; Leiner, I.; Chen, C.-C.; Ron, Y.; et al. Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung. PLoS Pathog. 2014, 10, e1003940. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Peri, G.; Delneste, Y.; Frémaux, I.; Doni, A.; Moalli, F.; Garlanda, C.; Romani, L.; Gascan, H.; Bellocchio, S.; et al. The Humoral Pattern Recognition Receptor PTX3 Is Stored in Neutrophil Granules and Localizes in Extracellular Traps. J. Exp. Med. 2007, 204, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Nett, J.E. Neutrophil Extracellular Traps in Fungal Infection. Semin. Cell Dev. Biol. 2019, 89, 47–57. [Google Scholar] [CrossRef]
- Bozza, S.; Gaziano, R.; Lipford, G.B.; Montagnoli, C.; Bacci, A.; Di Francesco, P.; Kurup, V.P.; Wagner, H.; Romani, L. Vaccination of Mice against Invasive Aspergillosis with Recombinant Aspergillus Proteins and CpG Oligodeoxynucleotides as Adjuvants. Microbes Infect. 2002, 4, 1281–1290. [Google Scholar] [CrossRef]
- Hohl, T.M. Immune Responses to Invasive Aspergillosis: New Understanding and Therapeutic Opportunities. Curr. Opin. Infect. Dis. 2017, 30, 364–371. [Google Scholar] [CrossRef]
- Heung, L.J.; Wiesner, D.L.; Wang, K.; Rivera, A.; Hohl, T.M. Immunity to Fungi in the Lung. Semin. Immunol. 2023, 66, 101728. [Google Scholar] [CrossRef]
- Dewi, I.M.W.; van de Veerdonk, F.L.; Gresnigt, M.S. The Multifaceted Role of T-Helper Responses in Host Defense against Aspergillus Fumigatus. J. Fungi 2017, 3, 55. [Google Scholar] [CrossRef]
- Romani, L. Immunity to Fungal Infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Afessa, B. The Intensive Care Support of Patients with Malignancy: Do Everything That Can Be Done. Intensive Care Med. 2006, 32, 3–5. [Google Scholar] [CrossRef]
- Shariati, A.; Moradabadi, A.; Chegini, Z.; Khoshbayan, A.; Didehdar, M. An Overview of the Management of the Most Important Invasive Fungal Infections in Patients with Blood Malignancies. Infect. Drug Resist. 2020, 13, 2329–2354. [Google Scholar] [CrossRef] [PubMed]
- Bays, D.J.; Thompson, G.R. Fungal Infections of the Stem Cell Transplant Recipient and Hematologic Malignancy Patients. Infect. Dis. Clin. N. Am. 2019, 33, 545–566. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Mokart, D.; Pène, F.; Lambert, J.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Laisne, L.-M.; et al. Outcomes of Critically Ill Patients with Hematologic Malignancies: Prospective Multicenter Data from France and Belgium℄A Groupe de Recherche Respiratoire En Réanimation Onco-Hématologique Study. J. Clin. Oncol. 2013, 31, 2810–2818. [Google Scholar] [CrossRef]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A Clinical Algorithm to Diagnose Invasive Pulmonary Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Colombo, A.L.; de Almeida Júnior, J.N.; Slavin, M.A.; Chen, S.C.-A.; Sorrell, T.C. Candida and Invasive Mould Diseases in Non-Neutropenic Critically Ill Patients and Patients with Haematological Cancer. Lancet Infect. Dis. 2017, 17, e344–e356. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Lipton, J.H.; Vesole, D.H.; Chandrasekar, P.; Langston, A.; Tarantolo, S.R.; Greinix, H.; Morais de Azevedo, W.; Reddy, V.; Boparai, N.; et al. Posaconazole or Fluconazole for Prophylaxis in Severe Graft-versus-Host Disease. N. Engl. J. Med. 2007, 356, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.-T.; et al. Posaconazole vs. Fluconazole or Itraconazole Prophylaxis in Patients with Neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef]
- Crawford, J.; Dale, D.C.; Lyman, G.H. Chemotherapy-Induced Neutropenia: Risks, Consequences, and New Directions for Its Management. Cancer 2004, 100, 228–237. [Google Scholar] [CrossRef]
- Newburger, P.E.; Dale, D.C. Evaluation and Management of Patients with Isolated Neutropenia. Semin. Hematol. 2013, 50, 198–206. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Georgiadou, S.P.; Wierda, W.G.; Wright, S.; Albert, N.D.; Ferrajoli, A.; Keating, M.; Lewis, R.E. Impaired Bactericidal but Not Fungicidal Activity of Polymorphonuclear Neutrophils in Patients with Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2013, 54, 1730–1733. [Google Scholar] [CrossRef]
- Beyer, M.; Kochanek, M.; Darabi, K.; Popov, A.; Jensen, M.; Endl, E.; Knolle, P.A.; Thomas, R.K.; von Bergwelt-Baildon, M.; Debey, S.; et al. Reduced Frequencies and Suppressive Function of CD4+CD25hi Regulatory T Cells in Patients with Chronic Lymphocytic Leukemia after Therapy with Fludarabine. Blood 2005, 106, 2018–2025. [Google Scholar] [CrossRef]
- Neofytos, D.; Lu, K.; Hatfield-Seung, A.; Blackford, A.; Marr, K.A.; Treadway, S.; Ostrander, D.; Nussenblatt, V.; Karp, J. Epidemiology, Outcomes, and Risk Factors of Invasive Fungal Infections in Adult Patients with Acute Myelogenous Leukemia after Induction Chemotherapy. Diagn. Microbiol. Infect. Dis. 2013, 75, 144–149. [Google Scholar] [CrossRef]
- Douglas, A.P.; Smibert, O.C.; Bajel, A.; Halliday, C.L.; Lavee, O.; McMullan, B.; Yong, M.K.; van Hal, S.J.; Chen, S.C.-A. Australasian Antifungal Guidelines Steering Committee Consensus Guidelines for the Diagnosis and Management of Invasive Aspergillosis, 2021. Intern. Med. J. 2021, 51 (Suppl. 7), 143–176. [Google Scholar] [CrossRef]
- Lamoth, F.; Calandra, T. Pulmonary Aspergillosis: Diagnosis and Treatment. Eur. Respir. Rev. 2022, 31, 220114. [Google Scholar] [CrossRef] [PubMed]
- Bukkems, L.M.P.; van Dommelen, L.; Regis, M.; van den Heuvel, E.; Nieuwenhuizen, L. The Use of Galactomannan Antigen Assays for the Diagnosis of Invasive Pulmonary Aspergillosis in the Hematological Patient: A Systematic Review and Meta-Analysis. J. Fungi 2023, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Rüping, M.J.G.T.; Vehreschild, J.J.; Cornely, O.A. Patients at High Risk of Invasive Fungal Infections: When and How to Treat. Drugs 2008, 68, 1941–1962. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Tobón, A.; Restrepo, A.; Queiroz-Telles, F.; Nucci, M. Epidemiology of Endemic Systemic Fungal Infections in Latin America. Med. Mycol. 2011, 49, 785–798. [Google Scholar] [CrossRef]
- Buchacz, K.; Baker, R.K.; Palella, F.J.; Chmiel, J.S.; Lichtenstein, K.A.; Novak, R.M.; Wood, K.C.; Brooks, J.T. HOPS Investigators AIDS-Defining Opportunistic Illnesses in US Patients, 1994–2007: A Cohort Study. AIDS 2010, 24, 1549–1559. [Google Scholar] [CrossRef]
- Antinori, S.; Nebuloni, M.; Magni, C.; Fasan, M.; Adorni, F.; Viola, A.; Corbellino, M.; Galli, M.; Vago, G.; Parravicini, C.; et al. Trends in the Postmortem Diagnosis of Opportunistic Invasive Fungal Infections in Patients with AIDS: A Retrospective Study of 1,630 Autopsies Performed between 1984 and 2002. Am. J. Clin. Pathol. 2009, 132, 221–227. [Google Scholar] [CrossRef]
- Kosmidis, C.; Denning, D.W. The Clinical Spectrum of Pulmonary Aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef]
- Goncer Rodriguez, I. Aspergillosis in AIDS. Aspergillus and Aspergillosis. Available online: https://www.Aspergillus.org.uk/new_treatment/aspergillosis-in-aids/ (accessed on 17 January 2025).
- Keating, J.J.; Rogers, T.; Petrou, M.; Cartledge, J.D.; Woodrow, D.; Nelson, M.; Hawkins, D.A.; Gazzard, B.G. Management of Pulmonary Aspergillosis in AIDS: An Emerging Clinical Problem. J. Clin. Pathol. 1994, 47, 805–809. [Google Scholar] [CrossRef]
- Holding, K.J.; Dworkin, M.S.; Wan, P.C.; Hanson, D.L.; Klevens, R.M.; Jones, J.L.; Sullivan, P.S. Aspergillosis among People Infected with Human Immunodeficiency Virus: Incidence and Survival. Adult and Adolescent Spectrum of HIV Disease Project. Clin. Infect. Dis. 2000, 31, 1253–1257. [Google Scholar] [CrossRef]
- Tong, K.B.; Lau, C.J.; Murtagh, K.; Layton, A.J.; Seifeldin, R. The Economic Impact of Aspergillosis: Analysis of Hospital Expenditures across Patient Subgroups. Int. J. Infect. Dis. 2009, 13, 24–36. [Google Scholar] [CrossRef]
- Baddley, J.W. Clinical Risk Factors for Invasive Aspergillosis. Med. Mycol. 2011, 49 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.T.; Sais, G.J.; Frank, I.; Gefter, W.B.; Aronchick, J.M.; Miller, W.T. Pulmonary Aspergillosis in Patients with AIDS. Clinical and Radiographic Correlations. Chest 1994, 105, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.M.; Lipman, M.C.; Johnson, M.A.; Prentice, H.G. Respiratory Infections in Immunocompromised Patients. Curr. Opin. Pulm. Med. 1999, 5, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Barlam, T.F.; Flanigan, T.; Rich, J.D. Pulmonary Aspergillosis and Invasive Disease in AIDS: Review of 342 Cases. Chest 1998, 114, 251–262. [Google Scholar] [CrossRef]
- Woitas, R.P.; Rockstroh, J.K.; Theisen, A.; Leutner, C.; Sauerbruch, T.; Spengler, U. Changing Role of Invasive Aspergillosis in AIDS—A Case Control Study. J. Infect. 1998, 37, 116–122. [Google Scholar] [CrossRef]
- Wallace, J.M.; Lim, R.; Browdy, B.L.; Hopewell, P.C.; Glassroth, J.; Rosen, M.J.; Reichman, L.B.; Kvale, P.A. Risk Factors and Outcomes Associated with Identification of Aspergillus in Respiratory Specimens from Persons with HIV Disease. Pulmonary Complications of HIV Infection Study Group. Chest 1998, 114, 131–137. [Google Scholar] [CrossRef]
- Doumbo, S.N.; Cissoko, Y.; Dama, S.; Niangaly, A.; Garango, A.; Konaté, A.; Koné, A.; Traoré, B.; Thera, M.; Djimde, A.; et al. The Estimated Burden of Fungal Diseases in Mali. J. Mycol. Med. 2023, 33, 101333. [Google Scholar] [CrossRef]
- Truda, V.S.S.; Falci, D.R.; Porfírio, F.M.V.; de Santos, D.W.d.C.L.; Junior, F.I.O.; Pasqualotto, A.C.; Puga, F.G.; Bollela, V.R.; Junior, J.N.A.; Ferreira, P.R.A.; et al. A Contemporary Investigation of Burden and Natural History of Aspergillosis in People Living with HIV/AIDS. Mycoses 2023, 66, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Yerbanga, I.W.; Nakanabo Diallo, S.; Rouamba, T.; Denis, O.; Rodriguez-Villalobos, H.; Montesinos, I.; Bamba, S. A Systematic Review of Epidemiology, Risk Factors, Diagnosis, Antifungal Resistance, and Management of Invasive Aspergillosis in Africa. J. Mycol. Med. 2023, 33, 101328. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Morgan, E.F. Quantifying Deaths from Aspergillosis in HIV Positive People. J. Fungi 2022, 8, 1131. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Agvald-Ohman, C.; Akova, M.; Alastruey-Izquierdo, A.; Arikan-Akdagli, S.; Azoulay, E.; Blot, S.; Cornely, O.A.; Cuenca-Estrella, M.; et al. Invasive Fungal Diseases in Adult Patients in Intensive Care Unit (FUNDICU): 2024 Consensus Definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC, and ISHAM. Intensive Care Med. 2024, 50, 502–515. [Google Scholar] [CrossRef]
- Loreto, E.S.; Tondolo, J.S.M.; Loreto, E.S.; Tondolo, J.S.M. Introductory Chapter: Epidemiology of Invasive Fungal Infection—An Overview. In Fungal Infection; IntechOpen: London, UK, 2019; ISBN 978-1-83880-469-5. [Google Scholar]
- Singh, N.; Husain, S. AST Infectious Diseases Community of Practice Aspergillosis in Solid Organ Transplantation. Am. J. Transpl. 2013, 13 (Suppl. 4), 228–241. [Google Scholar] [CrossRef]
- Lum, L.; Lee, A.; Vu, M.; Strasser, S.; Davis, R. Epidemiology and Risk Factors for Invasive Fungal Disease in Liver Transplant Recipients in a Tertiary Transplant Center. Transpl. Infect. Dis. 2020, 22, e13361. [Google Scholar] [CrossRef]
- Gioia, F.; Filigheddu, E.; Corbella, L.; Fernández-Ruiz, M.; López-Medrano, F.; Pérez-Ayala, A.; Aguado, J.M.; Fariñas, M.C.; Arnaiz, F.; Calvo, J.; et al. Invasive Aspergillosis in Solid Organ Transplantation: Diagnostic Challenges and Differences in Outcome in a Spanish National Cohort (Diaspersot Study). Mycoses 2021, 64, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Shinoda, M.; Uno, S.; Obara, H.; Kitago, M.; Abe, Y.; Hishida, T.; Yagi, H.; Hasegawa, Y.; Kitagawa, Y. Invasive Pulmonary Aspergillosis after Liver Transplantation: Lessons from Successfully Treated Cases and Review of the Literature. Surg. Today 2021, 51, 1361–1370. [Google Scholar] [CrossRef]
- Singh, N.; Avery, R.K.; Munoz, P.; Pruett, T.L.; Alexander, B.; Jacobs, R.; Tollemar, J.G.; Dominguez, E.A.; Yu, C.M.; Paterson, D.L.; et al. Trends in Risk Profiles for and Mortality Associated with Invasive Aspergillosis among Liver Transplant Recipients. Clin. Infect. Dis. 2003, 36, 46–52. [Google Scholar] [CrossRef]
- Farges, C.; Cointault, O.; Murris, M.; Lavayssiere, L.; Lakhdar-Ghazal, S.; Del Bello, A.; Hebral, A.-L.; Esposito, L.; Nogier, M.-B.; Sallusto, F.; et al. Outcomes of Solid Organ Transplant Recipients with Invasive Aspergillosis and Other Mold Infections. Transpl. Infect. Dis. 2020, 22, e13200. [Google Scholar] [CrossRef]
- Fortún, J.; Martín-Dávila, P.; Moreno, S.; De Vicente, E.; Nuño, J.; Candelas, A.; Bárcena, R.; García, M. Risk Factors for Invasive Aspergillosis in Liver Transplant Recipients. Liver Transpl. 2002, 8, 1065–1070. [Google Scholar] [CrossRef]
- Kusne, S.; Dummer, J.S.; Singh, N.; Makowka, L.; Esquivel, C.; Starzl, T.E.; Ho, M. Fungal Infections After Liver Transplantation. Transpl. Proc. 1988, 20, 650–651. [Google Scholar]
- Neofytos, D.; Chatzis, O.; Nasioudis, D.; Boely Janke, E.; Doco Lecompte, T.; Garzoni, C.; Berger, C.; Cussini, A.; Boggian, K.; Khanna, N.; et al. Epidemiology, Risk Factors and Outcomes of Invasive Aspergillosis in Solid Organ Transplant Recipients in the Swiss Transplant Cohort Study. Transpl. Infect. Dis. 2018, 20, e12898. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-Associated Immune Dysfunction: Distinctive Features and Clinical Relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- de la Garza, R.G.; Sarobe, P.; Merino, J.; Lasarte, J.J.; D’Avola, D.; Belsue, V.; Delgado, J.A.; Silva, L.; Iñarrairaegui, M.; Sangro, B.; et al. Trial of Complete Weaning from Immunosuppression for Liver Transplant Recipients: Factors Predictive of Tolerance. Liver Transpl. 2013, 19, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.; Varghese, J.; Venkataraman, J.; Rela, M. Matching Donor to Recipient in Liver Transplantation: Relevance in Clinical Practice. World J. Hepatol. 2013, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Arnow, P.M.; Bonham, A.; Dominguez, E.; Paterson, D.L.; Pankey, G.A.; Wagener, M.M.; Yu, V.L. Invasive Aspergillosis in Liver Transplant Recipients in the 1990s. Transplantation 1997, 64, 716–720. [Google Scholar] [CrossRef]
- Gayowski, T.; Marino, I.R.; Singh, N.; Doyle, H.; Wagener, M.; Fung, J.J.; Starzl, T.E. Orthotopic Liver Transplantation in High-Risk Patients: Risk Factors Associated with Mortality and Infectious Morbidity. Transplantation 1998, 65, 499–504. [Google Scholar] [CrossRef]
- Saliba, F.; Delvart, V.; Ichaï, P.; Kassis, N.; Botterel, F.; Mihaila, L.; Azoulay, D.; Adam, R.; Castaing, D.; Bretagne, S.; et al. Fungal Infections after Liver Transplantation: Outcomes and Risk Factors Revisited in the MELD Era. Clin. Transpl. 2013, 27, E454–E461. [Google Scholar] [CrossRef]
- Melenotte, C.; Aimanianda, V.; Slavin, M.; Aguado, J.M.; Armstrong-James, D.; Chen, Y.-C.; Husain, S.; Van Delden, C.; Saliba, F.; Lefort, A.; et al. Invasive Aspergillosis in Liver Transplant Recipients. Transpl. Infect. Dis. 2023, 25, e14049. [Google Scholar] [CrossRef] [PubMed]
- Gavalda, J.; Len, O.; San Juan, R.; Aguado, J.M.; Fortun, J.; Lumbreras, C.; Moreno, A.; Munoz, P.; Blanes, M.; Ramos, A.; et al. Risk Factors for Invasive Aspergillosis in Solid-Organ Transplant Recipients: A Case-Control Study. Clin. Infect. Dis. 2005, 41, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bonham, C.A.; Dominguez, E.A.; Fukui, M.B.; Paterson, D.L.; Pankey, G.A.; Wagener, M.M.; Fung, J.J.; Singh, N. Central Nervous System Lesions in Liver Transplant Recipients: Prospective Assessment of Indications for Biopsy and Implications for Management. Transplantation 1998, 66, 1596–1604. [Google Scholar] [CrossRef]
- Altiparmak, M.R.; Apaydin, S.; Trablus, S.; Serdengecti, K.; Ataman, R.; Ozturk, R.; Erek, E. Systemic Fungal Infections after Renal Transplantation. Scand. J. Infect. Dis. 2002, 34, 284–288. [Google Scholar] [CrossRef]
- Brown, R.S.; Lake, J.R.; Katzman, B.A.; Ascher, N.L.; Somberg, K.A.; Emond, J.C.; Roberts, J.P. Incidence and Significance of Aspergillus Cultures Following Liver and Kidney Transplantation. Transplantation 1996, 61, 666–669. [Google Scholar] [CrossRef]
- Neofytos, D.; Garcia-Vidal, C.; Lamoth, F.; Lichtenstern, C.; Perrella, A.; Vehreschild, J.J. Invasive Aspergillosis in Solid Organ Transplant Patients: Diagnosis, Prophylaxis, Treatment, and Assessment of Response. BMC Infect. Dis. 2021, 21, 296. [Google Scholar] [CrossRef] [PubMed]
- Lortholary, O.; Gangneux, J.-P.; Sitbon, K.; Lebeau, B.; de Monbrison, F.; Le Strat, Y.; Coignard, B.; Dromer, F.; Bretagne, S. French Mycosis Study Group Epidemiological Trends in Invasive Aspergillosis in France: The SAIF Network (2005–2007). Clin. Microbiol. Infect. 2011, 17, 1882–1889. [Google Scholar] [CrossRef]
- Fishman, J.A.; Issa, N.C. Infection in Organ Transplantation: Risk Factors and Evolving Patterns of Infection. Infect. Dis. Clin. N. Am. 2010, 24, 273–283. [Google Scholar] [CrossRef]
- Eckardt, K.-U.; Kasiske, B.L.; Zeier, M.G. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transpl. 2009, 9 (Suppl. 3), S1–S155. [Google Scholar] [CrossRef]
- Ojo, A.O.; Hanson, J.A.; Wolfe, R.A.; Leichtman, A.B.; Agodoa, L.Y.; Port, F.K. Long-Term Survival in Renal Transplant Recipients with Graft Function. Kidney Int. 2000, 57, 307–313. [Google Scholar] [CrossRef]
- López-Medrano, F.; Fernández-Ruiz, M.; Silva, J.T.; Carver, P.L.; van Delden, C.; Merino, E.; Pérez-Saez, M.J.; Montero, M.; Coussement, J.; de Abreu Mazzolin, M.; et al. Multinational Case-Control Study of Risk Factors for the Development of Late Invasive Pulmonary Aspergillosis Following Kidney Transplantation. Clin. Microbiol. Infect. 2018, 24, 192–198. [Google Scholar] [CrossRef]
- Panackal, A.A.; Dahlman, A.; Keil, K.T.; Peterson, C.L.; Mascola, L.; Mirza, S.; Phelan, M.; Lasker, B.A.; Brandt, M.E.; Carpenter, J.; et al. Outbreak of Invasive Aspergillosis among Renal Transplant Recipients. Transplantation 2003, 75, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Paterson, D.L.; Studer, S.; Pilewski, J.; Crespo, M.; Zaldonis, D.; Shutt, K.; Pakstis, D.L.; Zeevi, A.; Johnson, B.; et al. Voriconazole Prophylaxis in Lung Transplant Recipients. Am. J. Transpl. 2006, 6, 3008–3016. [Google Scholar] [CrossRef]
- Solé, A.; Morant, P.; Salavert, M.; Pemán, J.; Morales, P. Valencia Lung Transplant Group Aspergillus Infections in Lung Transplant Recipients: Risk Factors and Outcome. Clin. Microbiol. Infect. 2005, 11, 359–365. [Google Scholar] [CrossRef]
- Aguilar, C.A.; Hamandi, B.; Fegbeutel, C.; Silveira, F.P.; Verschuuren, E.A.; Ussetti, P.; Chin-Hong, P.V.; Sole, A.; Holmes-Liew, C.; Billaud, E.M.; et al. Clinical Risk Factors for Invasive Aspergillosis in Lung Transplant Recipients: Results of an International Cohort Study. J. Heart Lung Transpl. 2018, 37, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Krenke, R.; Grabczak, E.M. Tracheobronchial Manifestations of Aspergillus Infections. Sci. World J. 2011, 11, 2310–2329. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Shullo, M.A.; Silveira, F.P.; Kwak, E.J.; Abdel Massih, R.C.; Toyoda, Y.; Bermudez, C.A.; Bhama, J.K.; Kormos, R.L.; et al. Invasive Aspergillosis among Heart Transplant Recipients Is Rare but Causes Rapid Death Due to Septic Shock and Multiple Organ Dysfunction Syndrome. Scand. J. Infect. Dis. 2012, 44, 982–986. [Google Scholar] [CrossRef]
- Muñoz, P.; Cerón, I.; Valerio, M.; Palomo, J.; Villa, A.; Eworo, A.; Fernández-Yáñez, J.; Guinea, J.; Bouza, E. Invasive Aspergillosis among Heart Transplant Recipients: A 24-Year Perspective. J. Heart Lung Transpl. 2014, 33, 278–288. [Google Scholar] [CrossRef]
- Montoya, J.G.; Chaparro, S.V.; Celis, D.; Cortés, J.A.; Leung, A.N.; Robbins, R.C.; Stevens, D.A. Invasive Aspergillosis in the Setting of Cardiac Transplantation. Clin. Infect. Dis. 2003, 37 (Suppl. 3), S281–S292. [Google Scholar] [CrossRef]
- Muñoz, P.; Singh, N.; Bouza, E. Treatment of Solid Organ Transplant Patients with Invasive Fungal Infections: Should a Combination of Antifungal Drugs Be Used? Curr. Opin. Infect. Dis. 2006, 19, 365–370. [Google Scholar] [CrossRef]
- Lu, L.Y.; Lee, H.M.; Burke, A.; Li Bassi, G.; Torres, A.; Fraser, J.F.; Fanning, J.P. Prevalence, Risk Factors, Clinical Features, and Outcome of Influenza-Associated Pulmonary Aspergillosis in Critically Ill Patients: A Systematic Review and Meta-Analysis. Chest 2024, 165, 540–558. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Map. Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html. (accessed on 21 January 2025).
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 Infection: The Role of Cytokines in COVID-19 Disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Gil-Etayo, F.J.; Suàrez-Fernández, P.; Cabrera-Marante, O.; Arroyo, D.; Garcinuño, S.; Naranjo, L.; Pleguezuelo, D.E.; Allende, L.M.; Mancebo, E.; Lalueza, A.; et al. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front. Cell Infect. Microbiol. 2021, 11, 624483. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients with COVID-19: A Prospective Study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose Coronavirus Disease 2019-Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Montrucchio, G.; Lupia, T.; Lombardo, D.; Stroffolini, G.; Corcione, S.; De Rosa, F.G.; Brazzi, L. Risk Factors for Invasive Aspergillosis in ICU Patients with COVID-19: Current Insights and New Key Elements. Ann. Intensive Care 2021, 11, 136. [Google Scholar] [CrossRef]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The Vascular Endothelium: The Cornerstone of Organ Dysfunction in Severe SARS-CoV-2 Infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.; Jeong, H.W.; Ahn, J.Y.; Choi, S.J.; Lee, H.; Choi, B.; Nam, S.K.; Sa, M.; Kwon, J.-S.; et al. Immunophenotyping of COVID-19 and Influenza Highlights the Role of Type I Interferons in Development of Severe COVID-19. Sci. Immunol. 2020, 5, eabd1554. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Mishra, T.; Kamaraj, S.; Punetha, S.; Sengupta, O.; Joshi, Y.; Vuppu, S.; Vaghela, D.; Vora, L. Post-COVID-19 Fungal Infection in the Aged Population. Vaccines 2023, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and Fungal Coinfection among Hospitalized Patients with COVID-19: A Retrospective Cohort Study in a UK Secondary-Care Setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of Co-Infections and Superinfections in Hospitalized Patients with COVID-19: A Retrospective Cohort Study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Paul, M.; Sasidharan, J.; Taneja, J.; Chatterjee, K.; Abbas, S.Z.; Chowdhury, V.; Das, A. Invasive Mucormycosis and Aspergillosis Coinfection Associated with Post-COVID-19 Pneumonia in a Tertiary Care Hospital. Med. Mycol. J. 2022, 63, 59–64. [Google Scholar] [CrossRef]
- Seyedjavadi, S.S.; Bagheri, P.; Nasiri, M.J.; Razzaghi-Abyaneh, M.; Goudarzi, M. Fungal Infection in Co-Infected Patients with COVID-19: An Overview of Case Reports/Case Series and Systematic Review. Front. Microbiol. 2022, 13, 888452. [Google Scholar] [CrossRef]
- Swain, S.; Ray, A.; Sarda, R.; Vyas, S.; Singh, G.; Jorwal, P.; Kodan, P.; Khanna, P.; Xess, I.; Sinha, S.; et al. COVID-19-Associated Subacute Invasive Pulmonary Aspergillosis. Mycoses 2022, 65, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-Associated Fungal Infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef]
- Reizine, F.; Pinceaux, K.; Lederlin, M.; Autier, B.; Guegan, H.; Gacouin, A.; Luque-Paz, D.; Boglione-Kerrien, C.; Bacle, A.; Le Daré, B.; et al. Influenza- and COVID-19-Associated Pulmonary Aspergillosis: Are the Pictures Different? J. Fungi 2021, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of Influenza-Associated Pulmonary Aspergillosis in ICU Patients and Proposal for a Case Definition: An Expert Opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Waldeck, F.; Boroli, F.; Suh, N.; Wendel Garcia, P.D.; Flury, D.; Notter, J.; Iten, A.; Kaiser, L.; Schrenzel, J.; Boggian, K.; et al. Influenza-Associated Aspergillosis in Critically-Ill Patients-a Retrospective Bicentric Cohort Study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1915–1923. [Google Scholar] [CrossRef]
- Waldeck, F.; Boroli, F.; Zingg, S.; Walti, L.N.; Wendel-Garcia, P.D.; Conen, A.; Pagani, J.-L.; Boggian, K.; Schnorf, M.; Siegemund, M.; et al. Higher Risk for Influenza-Associated Pulmonary Aspergillosis (IAPA) in Asthmatic Patients: A Swiss Multicenter Cohort Study on IAPA in Critically Ill Influenza Patients. Influenza Other Respir. Viruses 2023, 17, e13059. [Google Scholar] [CrossRef] [PubMed]
- Seldeslachts, L.; Staels, F.; Gkountzinopoulou, M.; Jacobs, C.; Tielemans, B.; Vanhoffelen, E.; Reséndiz-Sharpe, A.; De Herdt, L.; Haughton, J.; Prezzemolo, T.; et al. Damping Excessive Viral-Induced IFN-γ Rescues the Impaired Anti-Aspergillus Host Immune Response in Influenza-Associated Pulmonary Aspergillosis. eBioMedicine 2024, 108, 105347. [Google Scholar] [CrossRef]
- Ahmad, A.; Singh, R.B.; Nickolich, K.L.; Pilewski, M.J.; Ngeow, C.; Frempong-Manso, K.; Robinson, K.M. Restoration of Type 17 Immune Signaling Is Not Sufficient for Protection during Influenza-Associated Pulmonary Aspergillosis. Front. Immunol. 2025, 16. [Google Scholar] [CrossRef]
- Pantaleón García, J.; Wurster, S.; Albert, N.D.; Bharadwaj, U.; Bhoda, K.; Kulkarni, V.K.; Ntita, M.; Rodríguez Carstens, P.; Burch-Eapen, M.; Covarrubias López, D.; et al. Immunotherapy with Nebulized Pattern Recognition Receptor Agonists Restores Severe Immune Paralysis and Improves Outcomes in Mice with Influenza-Associated Pulmonary Aspergillosis. mBio 2025, 16, e04061-24. [Google Scholar] [CrossRef] [PubMed]
- Vanderbeke, L.; Janssen, N.A.F.; Bergmans, D.C.J.J.; Bourgeois, M.; Buil, J.B.; Debaveye, Y.; Depuydt, P.; Feys, S.; Hermans, G.; Hoiting, O.; et al. Posaconazole for Prevention of Invasive Pulmonary Aspergillosis in Critically Ill Influenza Patients (POSA-FLU): A Randomised, Open-Label, Proof-of-Concept Trial. Intensive Care Med. 2021, 47, 674–686. [Google Scholar] [CrossRef]
- Azoulay, É.; Afessa, B. Diagnostic Criteria for Invasive Pulmonary Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2012, 186, 8–10. [Google Scholar] [CrossRef]
- Ascioglu, S.; Rex, J.H.; de Pauw, B.; Bennett, J.E.; Bille, J.; Crokaert, F.; Denning, D.W.; Donnelly, J.P.; Edwards, J.E.; Erjavec, Z.; et al. Defining Opportunistic Invasive Fungal Infections in Immunocompromised Patients with Cancer and Hematopoietic Stem Cell Transplants: An International Consensus. Clin. Infect. Dis. 2002, 34, 7–14. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; Nam, H.H.; Hoenigl, M. Invasive Aspergillosis in Critically Ill Patients: Review of Definitions and Diagnostic Approaches. Mycoses 2021, 64, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-T.; Chen, Y.; Li, S.; Wan, X.-X.; Weng, L.; Peng, J.-M.; Du, B. A Comparison of Diagnostic Criteria for Invasive Pulmonary Aspergillosis in Critically Ill Patients. Infect. Dis. Ther. 2023, 12, 1641–1653. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Resch, G.; Nachbaur, D.; Mayr, A.; Gastl, G.; Auberger, J.; Bialek, R.; Freund, M.C. The Value of Computed Tomography-Guided Percutaneous Lung Biopsy for Diagnosis of Invasive Fungal Infection in Immunocompromised Patients. Clin. Infect. Dis. 2007, 45, e101–e104. [Google Scholar] [CrossRef]
- Lass-Flörl, C. How to Make a Fast Diagnosis in Invasive Aspergillosis. Med. Mycol. 2019, 57, S155–S160. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Bailly, É.; Chandenier, J. Diagnosis of Invasive Pulmonary Aspergillosis: Updates and Recommendations. Med. Mal. Infect. 2014, 44, 89–101. [Google Scholar] [CrossRef]
- Garg, A.; Bhalla, A.S.; Naranje, P.; Vyas, S.; Garg, M. Decoding the Guidelines of Invasive Pulmonary Aspergillosis in Critical Care Setting: Imaging Perspective. Indian J. Radiol. Imaging 2023, 33, 382–391. [Google Scholar] [CrossRef]
- Casutt, A.; Lamoth, F.; Lortholary, O.; Prior, J.O.; Tonglet, A.; Manuel, O.; Bergeron, A.; Beigelman-Aubry, C. Atypical Imaging Patterns during Lung Invasive Mould Diseases: Lessons for Clinicians. Eur. Respir. Rev. 2023, 32, 230086. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Raina, V.; Kumar, L.; Sharma, A.; Bakhshi, S.; Iqbal, S. Serum Galactomannan Assay for Diagnosis of Probable Invasive Aspergillosis in Acute Leukemia and Hematopoietic Stem Cell Transplantation. Indian. J. Med. Paediatr. Oncol. 2013, 34, 74–79. [Google Scholar] [CrossRef]
- Shin, D.W.; Cho, J.; Choi, K.S.; Lee, J.; Choi, Y.; Choi, S.J.; Kim, S.-A.; Moon, S.M.; Kim, E.S.; Kim, H.B.; et al. False-Positive Results of Galactomannan Assays in Patients Administered Glucose-Containing Solutions. Sci. Rep. 2024, 14, 2552. [Google Scholar] [CrossRef]
- Lamoth, F.; Akan, H.; Andes, D.; Cruciani, M.; Marchetti, O.; Ostrosky-Zeichner, L.; Racil, Z.; Clancy, C.J. Assessment of the Role of 1,3-β-d-Glucan Testing for the Diagnosis of Invasive Fungal Infections in Adults. Clin. Infect. Dis. 2021, 72, S102–S108. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Posso, R.B.; Barnes, R.A. Analytical and Clinical Evaluation of the PathoNostics AsperGenius Assay for Detection of Invasive Aspergillosis and Resistance to Azole Antifungal Drugs during Testing of Serum Samples. J. Clin. Microbiol. 2015, 53, 2115–2121. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Lagrou, K.; Rodrigues, C.S.; Campos, C.F.; Bernal-Martínez, L.; Rodrigues, F.; Silvestre, R.; Alcazar-Fuoli, L.; Maertens, J.A.; Cunha, C.; et al. Evaluation of Bronchoalveolar Lavage Fluid Cytokines as Biomarkers for Invasive Pulmonary Aspergillosis in At-Risk Patients. Front. Microbiol. 2017, 8, 2362. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef]
- Orasch, T.; Prattes, J.; Faserl, K.; Eigl, S.; Düttmann, W.; Lindner, H.; Haas, H.; Hoenigl, M. Bronchoalveolar Lavage Triacetylfusarinine C (TAFC) Determination for Diagnosis of Invasive Pulmonary Aspergillosis in Patients with Hematological Malignancies. J. Infect. 2017, 75, 370–373. [Google Scholar] [CrossRef]
- Koo, S.; Thomas, H.R.; Daniels, S.D.; Lynch, R.C.; Fortier, S.M.; Shea, M.M.; Rearden, P.; Comolli, J.C.; Baden, L.R.; Marty, F.M. A Breath Fungal Secondary Metabolite Signature to Diagnose Invasive Aspergillosis. Clin. Infect. Dis. 2014, 59, 1733–1740. [Google Scholar] [CrossRef]
- Machata, S.; Müller, M.M.; Lehmann, R.; Sieber, P.; Panagiotou, G.; Carvalho, A.; Cunha, C.; Lagrou, K.; Maertens, J.; Slevogt, H.; et al. Proteome Analysis of Bronchoalveolar Lavage Fluids Reveals Host and Fungal Proteins Highly Expressed during Invasive Pulmonary Aspergillosis in Mice and Humans. Virulence 2020, 11, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Lestrade, P.P.A.; Meis, J.F.; Melchers, W.J.G.; Verweij, P.E. Triazole Resistance in Aspergillus Fumigatus: Recent Insights and Challenges for Patient Management. Clin. Microbiol. Infect. 2019, 25, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Boucher, H.W.; Herbrecht, R.; Denning, D.W.; Lortholary, O.; Ribaud, P.; Rubin, R.H.; Wingard, J.R.; DePauw, B.; Schlamm, H.T.; et al. Strategy of Following Voriconazole versus Amphotericin B Therapy with Other Licensed Antifungal Therapy for Primary Treatment of Invasive Aspergillosis: Impact of Other Therapies on Outcome. Clin. Infect. Dis. 2005, 41, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus Voriconazole for Primary Treatment of Invasive Mould Disease Caused by Aspergillus and Other Filamentous Fungi (SECURE): A Phase 3, Randomised-Controlled, Non-Inferiority Trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Maertens, J.A.; Rahav, G.; Lee, D.-G.; Ponce-de-León, A.; Ramírez Sánchez, I.C.; Klimko, N.; Sonet, A.; Haider, S.; Diego Vélez, J.; Raad, I.; et al. Posaconazole versus Voriconazole for Primary Treatment of Invasive Aspergillosis: A Phase 3, Randomised, Controlled, Non-Inferiority Trial. Lancet 2021, 397, 499–509. [Google Scholar] [CrossRef]
- Sanford Guide AMT: Aspergillosis, Invasive. Available online: https://web.sanfordguide.com/en/sanford-guide-online/disease-clinical-condition/aspergillosis-invasive (accessed on 17 July 2025).
- Walsh, T.J.; Raad, I.; Patterson, T.F.; Chandrasekar, P.; Donowitz, G.R.; Graybill, R.; Greene, R.E.; Hachem, R.; Hadley, S.; Herbrecht, R.; et al. Treatment of Invasive Aspergillosis with Posaconazole in Patients Who Are Refractory to or Intolerant of Conventional Therapy: An Externally Controlled Trial. Clin. Infect. Dis. 2007, 44, 2–12. [Google Scholar] [CrossRef]
- Viscoli, C.; Herbrecht, R.; Akan, H.; Baila, L.; Sonet, A.; Gallamini, A.; Giagounidis, A.; Marchetti, O.; Martino, R.; Meert, L.; et al. An EORTC Phase II Study of Caspofungin as First-Line Therapy of Invasive Aspergillosis in Haematological Patients. J. Antimicrob. Chemother. 2009, 64, 1274–1281. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Ratanatharathorn, V.; Young, J.-A.; Raymond, J.; Laverdière, M.; Denning, D.W.; Patterson, T.F.; Facklam, D.; Kovanda, L.; Arnold, L.; et al. Micafungin Alone or in Combination with Other Systemic Antifungal Therapies in Hematopoietic Stem Cell Transplant Recipients with Invasive Aspergillosis. Transpl. Infect. Dis. 2009, 11, 89–93. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Haas, A.; Cornely, O.A. Invasive Aspergillosis: Epidemiology, Diagnosis and Management in Immunocompromised Patients. Drugs 2007, 67, 1567–1601. [Google Scholar] [CrossRef]
- De Francesco, M.A. Drug-Resistant Aspergillus Spp.: A Literature Review of Its Resistance Mechanisms and Its Prevalence in Europe. Pathogens 2023, 12, 1305. [Google Scholar] [CrossRef]
- Fakhim, H.; Badali, H.; Dannaoui, E.; Nasirian, M.; Jahangiri, F.; Raei, M.; Vaseghi, N.; Ahmadikia, K.; Vaezi, A. Trends in the Prevalence of Amphotericin B-Resistance (AmBR) among Clinical Isolates of Aspergillus Species. J. Med. Mycol. 2022, 32, 101310. [Google Scholar] [CrossRef]
- Kaya, A.; Kaya, S.Y.; Balkan, İ.İ.; Alkan, S.; Kurt, A.F.; Elverdi, T.; Ürkmez, S.; Öngören, Ş.; Aygün, G. Amphotericin B Resistant Aspergillus spp.: Report of Two Cases. Infect. Dis. Clin. Microbiol. 2024, 6, 343–348. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Azole-Resistant Aspergillosis: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S436–S444. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Epidemiology and Prevalence of Azole-Resistant Aspergillus Fumigatus: What Is Our Understanding of the Situation? Curr. Fungal Infect. Rep. 2023, 17, 177–187. [Google Scholar] [CrossRef]
- Verweij, P.E.; Lucas, J.A.; Arendrup, M.C.; Bowyer, P.; Brinkmann, A.J.F.; Denning, D.W.; Dyer, P.S.; Fisher, M.C.; Geenen, P.L.; Gisi, U.; et al. The One Health Problem of Azole Resistance in Aspergillus fumigatus: Current Insights and Future Research Agenda. Fungal Biol. Rev. 2020, 34, 202–214. [Google Scholar] [CrossRef]
- Dickwella Widanage, M.C.; Gautam, I.; Sarkar, D.; Mentink-Vigier, F.; Vermaas, J.V.; Ding, S.-Y.; Lipton, A.S.; Fontaine, T.; Latgé, J.-P.; Wang, P.; et al. Adaptative Survival of Aspergillus Fumigatus to Echinocandins Arises from Cell Wall Remodeling beyond Β−1,3-Glucan Synthesis Inhibition. Nat. Commun. 2024, 15, 6382. [Google Scholar] [CrossRef]
- CDC Antimicrobial-Resistant Aspergillus. Available online: https://www.cdc.gov/aspergillosis/php/guidance/index.html (accessed on 17 July 2025).
- Maertens, J.A.; Thompson, G.R.; Spec, A.; Donovan, F.M.; Hammond, S.P.; Bruns, A.H.W.; Rahav, G.; Shoham, S.; Johnson, R.; Rijnders, B.; et al. Olorofim for the Treatment of Invasive Fungal Diseases in Patients with Few or No Therapeutic Options: A Single-Arm, Open-Label, Phase 2b Study. Lancet Infect. Dis. 2025. [Google Scholar] [CrossRef]

| Sample/Test | Sensitivity | Specificity |
|---|---|---|
| Serum/plasma (proven/probable vs. no IPA) | 76% | 92% |
| Serum/plasma (proven/probable/possible vs. no IPA) | 45% | 91% |
| BAL (proven/probable vs. no IPA) | 80% | 95% |
| BAL (proven/probable/possible vs. no IPA) | 49% | 95% |
| EORTC/MSGERC (2020) | Modified AspICU (2021) | FUNDICU (2024) | |
|---|---|---|---|
| Target population | Severely immunocompromised (e.g., neutropenic, HSCT, hematologic malignancies) | Non-neutropenic ICU patients | All critically ill ICU patients, included non-classically immunocompromised |
| Diagnostic categories | Possible, Probable, Proven | Proven, Probable, Colonization | Possible, Probable, Proven |
| Required clinical criteria | Not central: diagnosis mostly relies on host factors, imaging and microbiological criteria | Yes: fever, respiratory symptoms, worsening oxygenation | Yes: detailed ICU-adapted criteria (e.g., sepsis, new infiltrates, secretions) |
| Radiological criteria | Yes: typical signs such as halo, air crescent, cavitation | Yes: infiltrates or new lesions consistent with infection | Yes: including HRCT or other imaging compatible with IPA |
| Microbiological criteria | Positive culture from sterile site or BAL GM index ≥ 0.5 in serum GM index ≥ 1.0 in BAL Positive PCR histopathology showing hyphae with tissue damage | BAL/tracheal culture GM in BAL (ODI ≥1.0) PCR optional | Culture GM (BAL ODI ≥ 1.0, serum ODI ≥ 0.5) BDG (>80 pg/mL) PCR on blood or BAL histopathology if available |
| Included host factors | Strict: Neutropenia (ANC < 500 cells/μL for >10 days), allogeneic HSCT, prolonged corticosteroids, T-cell immunosuppressants, inherited immunodeficiencies, solid organ transplant, recent chemotherapy, GVHD, or treatment with anti-cytokine biologics | ICU-specific: prolonged mechanical ventilation, ARDS, chronic lung disease | Broad: includes viral pneumonia, immunotherapy, ARDS, prolonged ICU stay, chronic lung disease |
| Advanced diagnostic techniques (PCR, BDG, GM) | Yes (GM, BDG, PCR, histopathology) | Partially, BAL GM included, PCR optional | Yes, required for Probable classification |
| Recognition of viral co-infections | No | Partially: influenza included, COVID-19 not systematically | Yes: COVID-19 and influenza recognized |
| Validation and intended use | Standard in trials and guidelines for immunocompromised patients | Used in ICU research, CAPA/IAPA definitions | Built by international consensus, intended for ICU clinical research and standardization |
| GM in Serum Samples | GM in BAL Samples | 1,3-β-D-Glucan | Molecular Methods (PCR) | Lateral Flow Device (LFD) Assay |
|---|---|---|---|---|
| Best performance in neutropenic patients (ODI > 0.5 in two samples) | Useful in non-neutropenic patients (no positive serum GM) | Nonspecific marker | Detection of several Aspergillus spp. in immunocompromised individuals | Aspergillus-specific monoclonal antibody for the detection of an extracellular glycoprotein secreted by Aspergillus spp., during active growth |
| Screening test in patient at risk | Cut-off not established: 0.5 USA versus 1.0 Europe in relation to patients’ risk | High NPV, useful as screening in high-risk patients | Early diagnosis with a high NPV, high sensitivity | Serum and BAL are the samples |
| Serum GM present 5–8 days before clinical manifestations | Performance test depends on technique used for BAL procedure (sterile saline volume instilled during bronchoscopy, volume and type of collected BAL fluid), mold prophylaxis or therapy, risk of fungal colonization | It allows to quantify and to recognize Aspergillus species | Cross-reactivity with Penicillium | |
| Serum GM should not be used on patients at risk but on mold active prophylaxis | Major sensitivity than serum GM assay with a high PPV | High cost and technical difficulties | Rapid and cost-effective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocci, M.G.; Cascarano, L.; Capecchi, G.; Lesci, A.; Sabatini, V.; Rubino, D.; Stazi, G.V.; Garotto, G.; Carrara, S.; Vulcano, A.; et al. Pulmonary Aspergillosis in Immunocompromised Critically Ill Patients: Prevalence, Risk Factors, Clinical Features and Diagnosis—A Narrative Review. J. Fungi 2025, 11, 617. https://doi.org/10.3390/jof11090617
Bocci MG, Cascarano L, Capecchi G, Lesci A, Sabatini V, Rubino D, Stazi GV, Garotto G, Carrara S, Vulcano A, et al. Pulmonary Aspergillosis in Immunocompromised Critically Ill Patients: Prevalence, Risk Factors, Clinical Features and Diagnosis—A Narrative Review. Journal of Fungi. 2025; 11(9):617. https://doi.org/10.3390/jof11090617
Chicago/Turabian StyleBocci, Maria Grazia, Laura Cascarano, Giulia Capecchi, Antonio Lesci, Valerio Sabatini, Dorotea Rubino, Giulia Valeria Stazi, Gabriele Garotto, Stefania Carrara, Antonella Vulcano, and et al. 2025. "Pulmonary Aspergillosis in Immunocompromised Critically Ill Patients: Prevalence, Risk Factors, Clinical Features and Diagnosis—A Narrative Review" Journal of Fungi 11, no. 9: 617. https://doi.org/10.3390/jof11090617
APA StyleBocci, M. G., Cascarano, L., Capecchi, G., Lesci, A., Sabatini, V., Rubino, D., Stazi, G. V., Garotto, G., Carrara, S., Vulcano, A., Gori, C., Del Nonno, F., Colombo, D., Falasca, L., Caraffa, E., Cicalini, S., & Fontana, C. (2025). Pulmonary Aspergillosis in Immunocompromised Critically Ill Patients: Prevalence, Risk Factors, Clinical Features and Diagnosis—A Narrative Review. Journal of Fungi, 11(9), 617. https://doi.org/10.3390/jof11090617






