Breeding of High-Polysaccharide-Producing Volvariella volvacea Strains Based on Genome Shuffling Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Media
2.1.1. Original Microorganism
2.1.2. Culture Media and Culture Conditions
2.1.3. Solution Preparation
2.2. Determination of EPS Content
2.3. Determination of Dosage of the ARTP Mutagen
2.4. Screening of Mutant Strains
2.5. Protoplast Fusion
2.6. Inactivation of Parental Genes
2.7. Genome Shuffling
2.8. Transcriptomics and Metabolomics Analysis
2.8.1. Metabolomic Analysis
2.8.2. Transcriptomic Analysis
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Statistical Analysis
3. Results
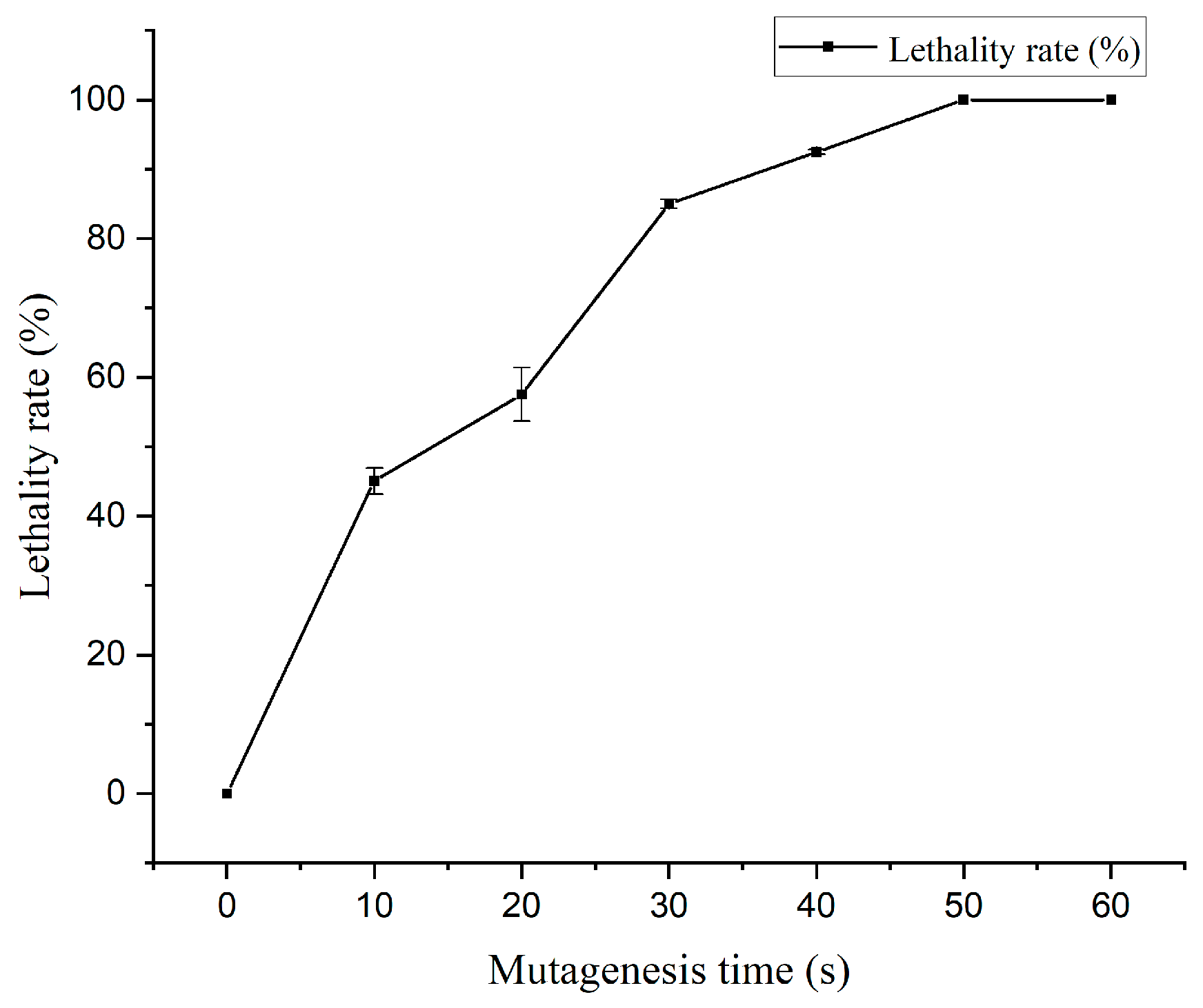
3.1. Effect of ARTP Mutagenesis Time on Lethality Rate
3.2. Screening of Mutagenic Strains
3.2.1. Primary Screening of Mutagenized Strains
3.2.2. Secondary Screening of Mutagenized Strains
3.3. Genome Shuffling
3.4. Comparative Metabolomics Analysis of V. volvacea
3.4.1. Metabolomics Multivariate Statistical Analysis
3.4.2. Inter-Group Differential Analysis
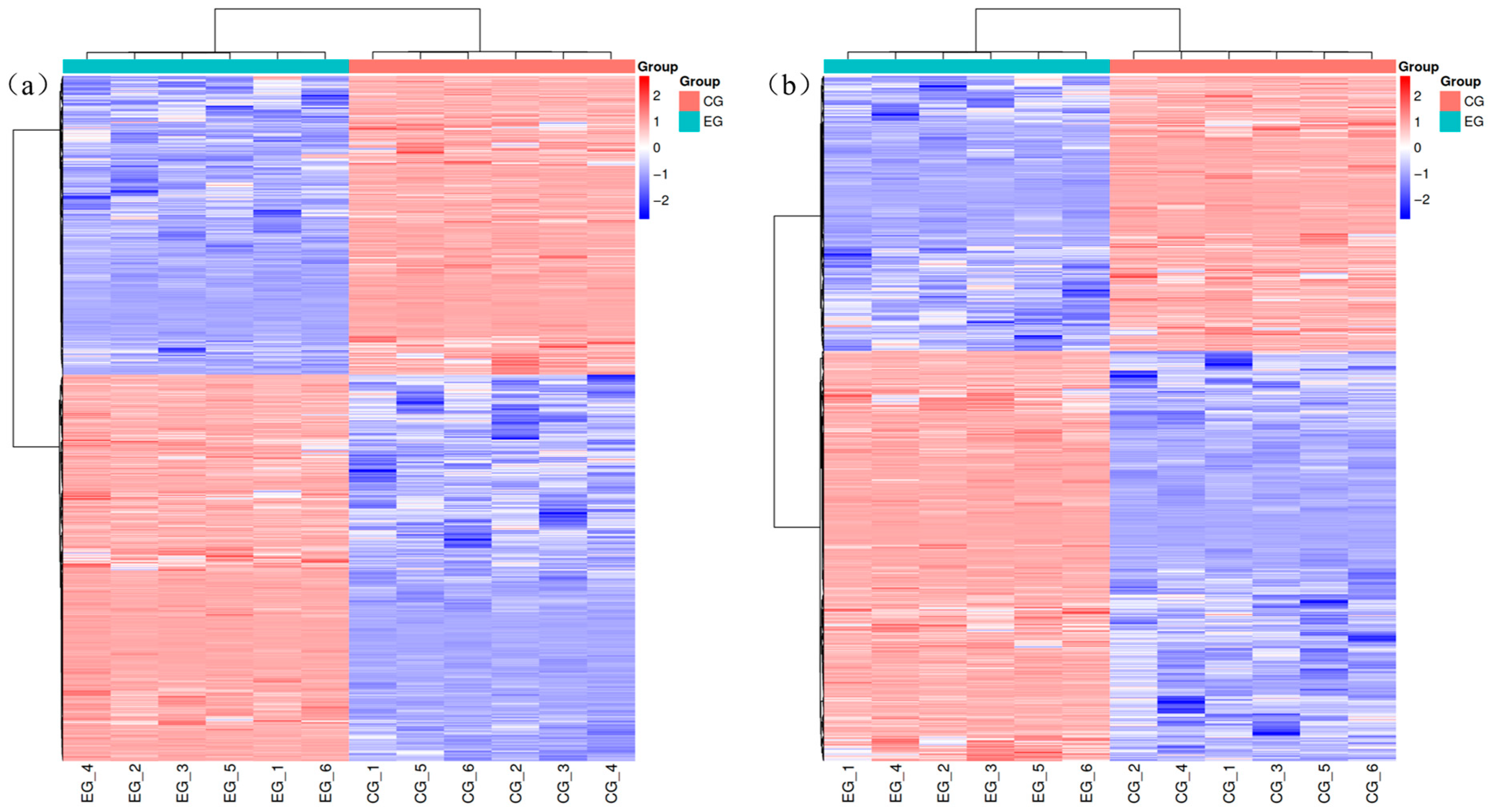
3.4.3. Cluster Analysis
3.4.4. Correlation Analysis of Differential Metabolites
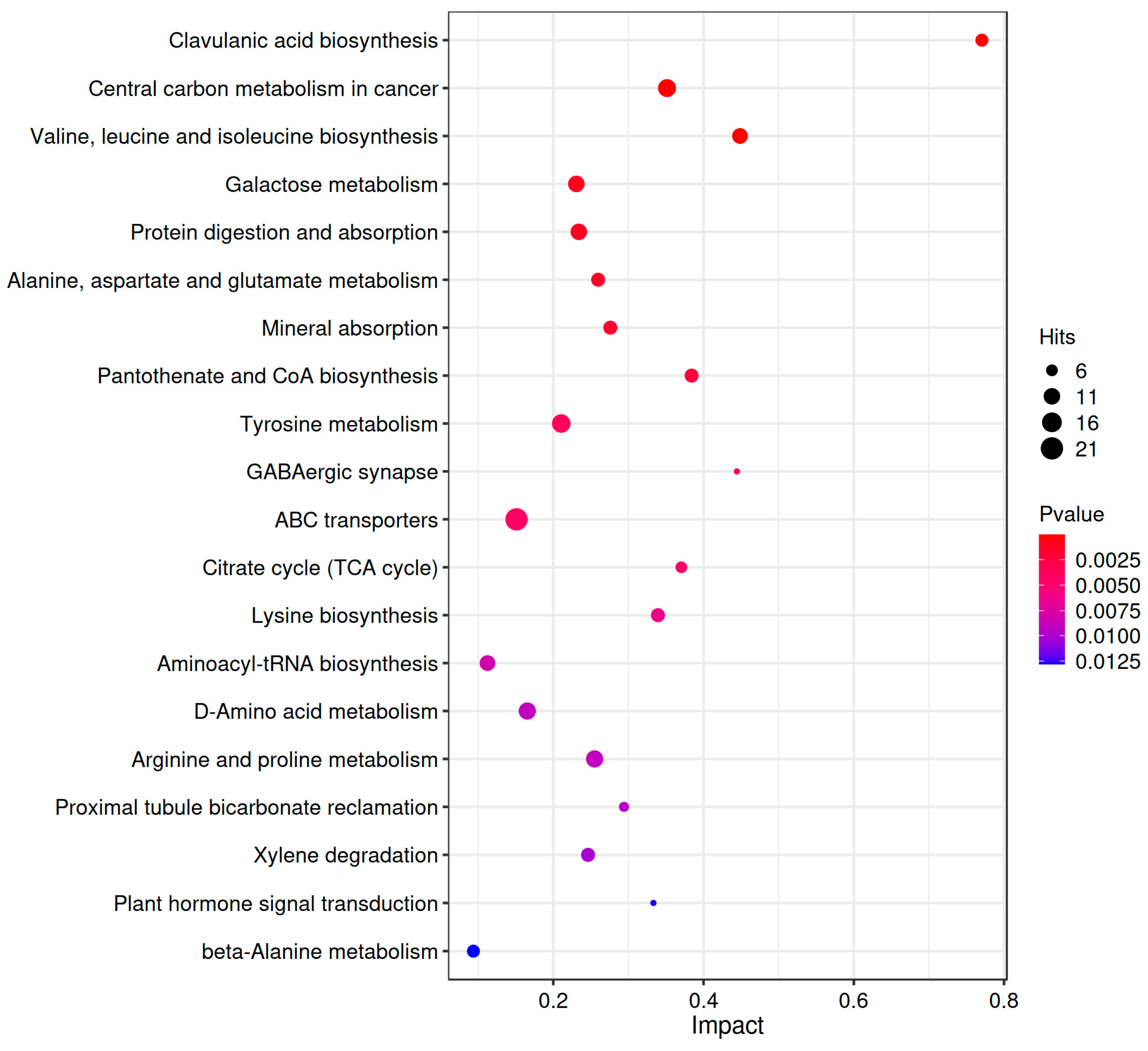
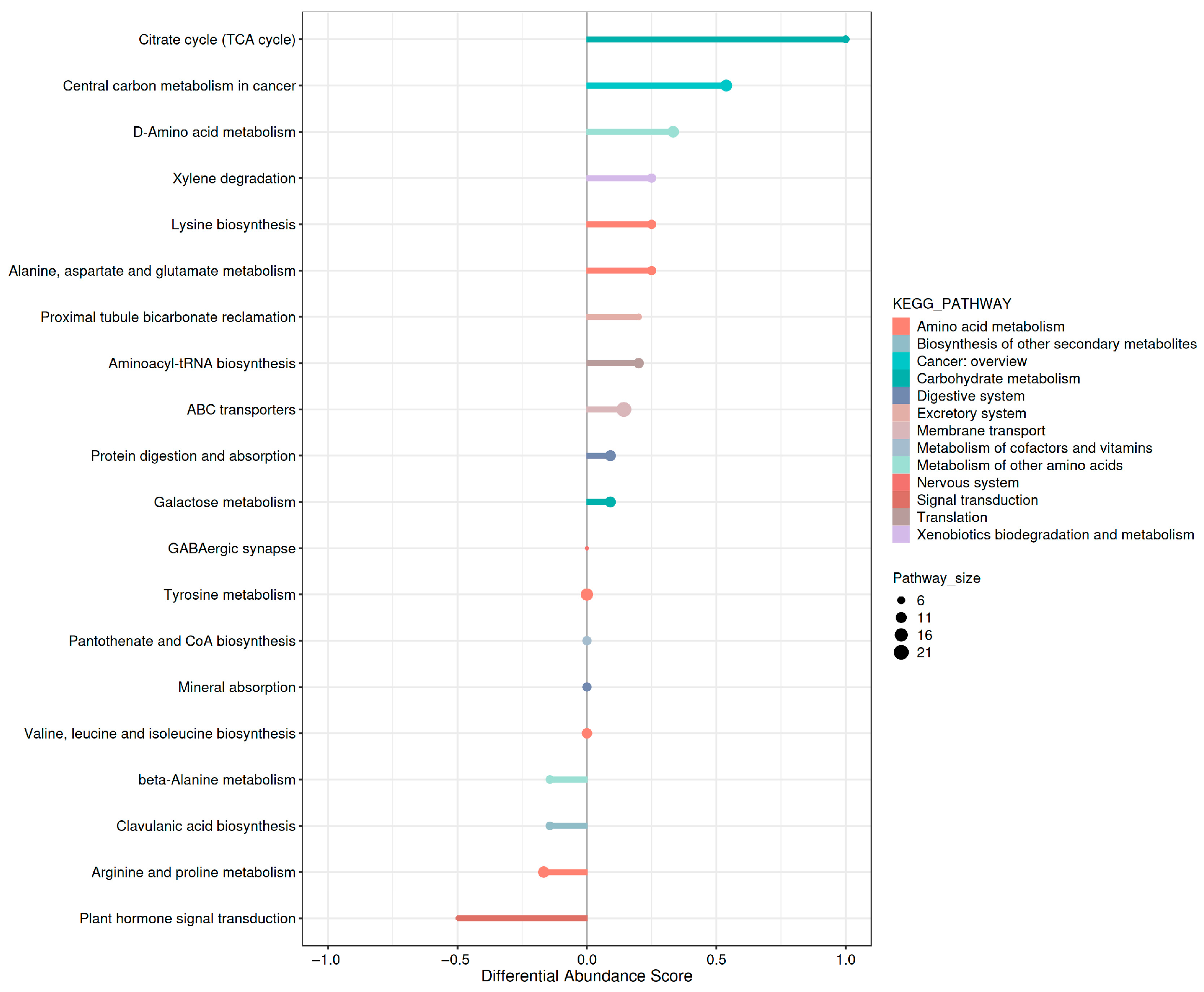
3.4.5. KEGG Pathway Enrichment Analysis of Differential Metabolites
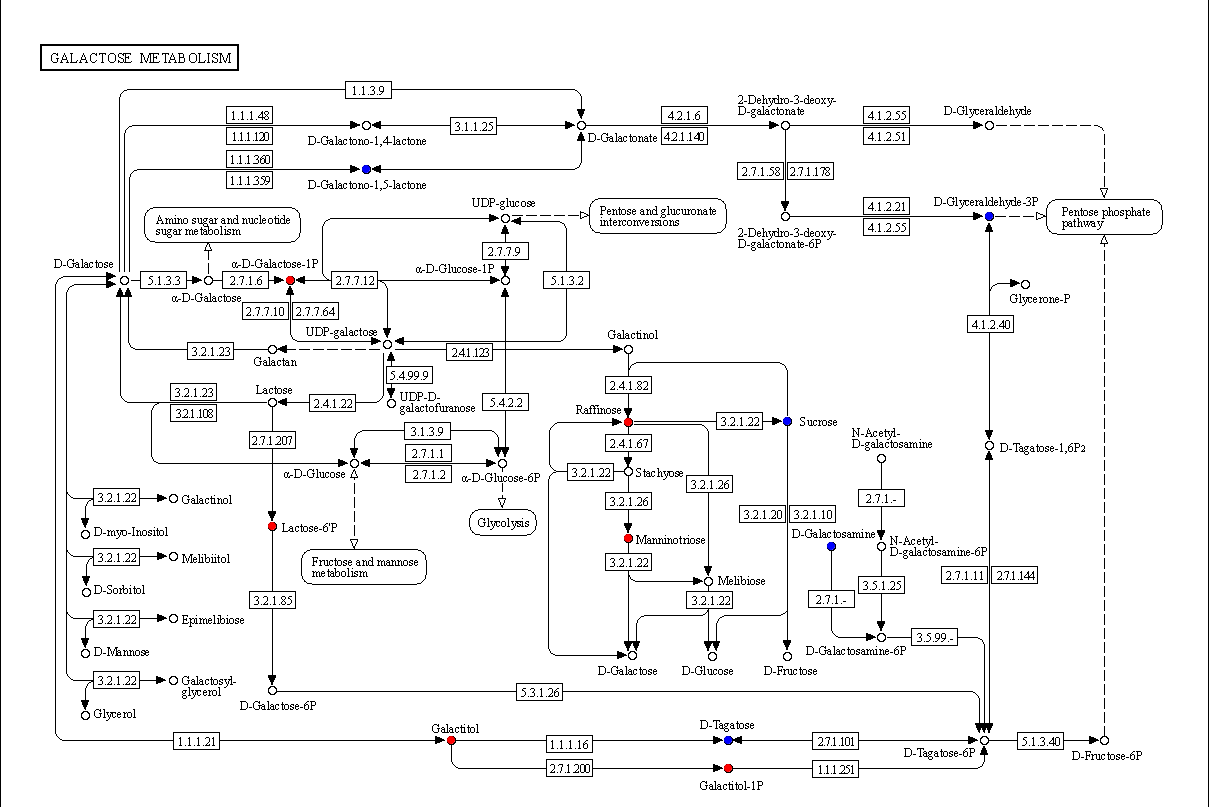
3.4.6. Global KEGG Pathway Variation Analysis
3.5. Transcriptomic Analysis
3.5.1. Sequencing Quality Analysis and Data Quality Control
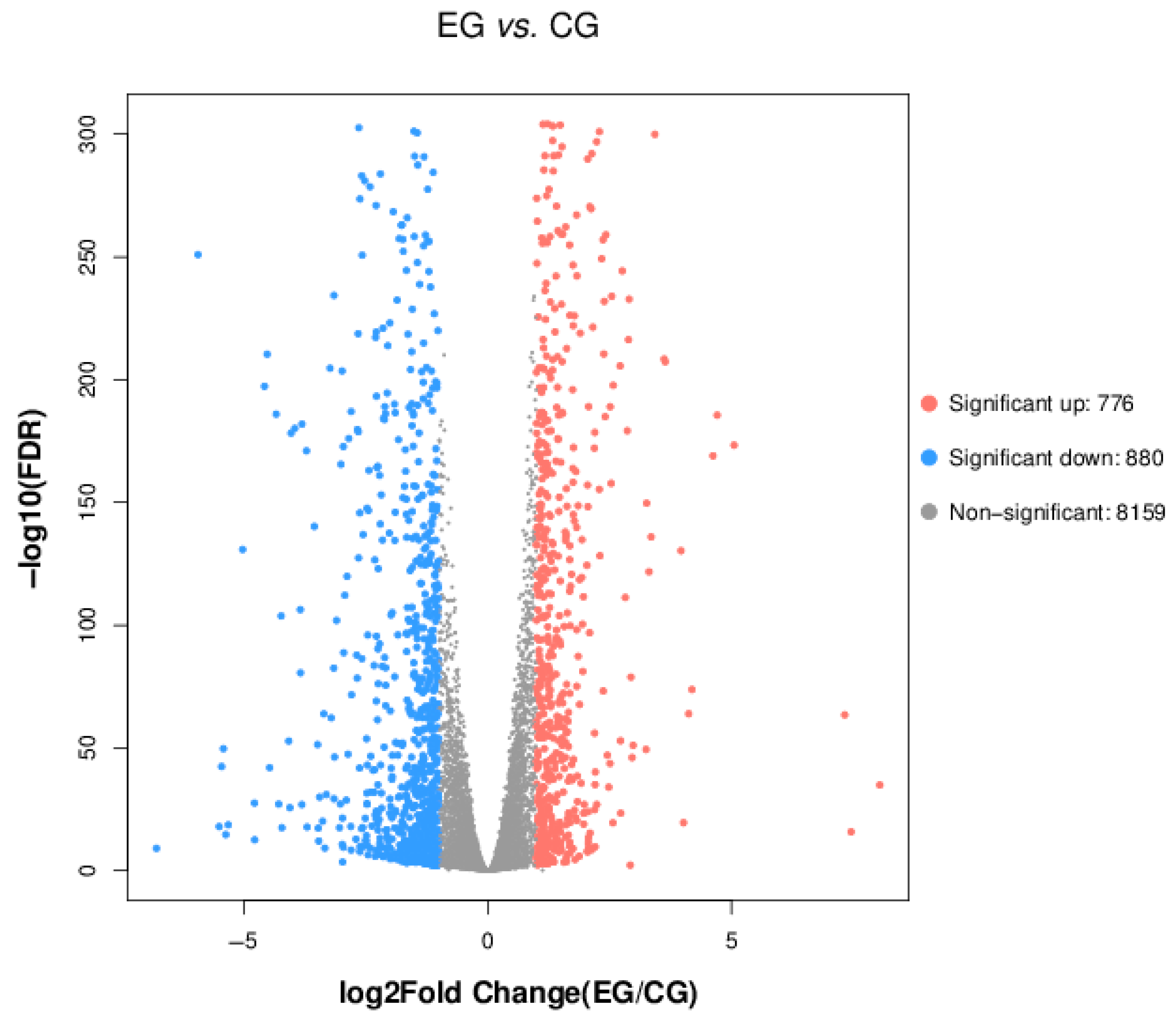
3.5.2. Analysis of Differentially Expressed Genes (DEGs)
3.5.3. Clustering Analysis
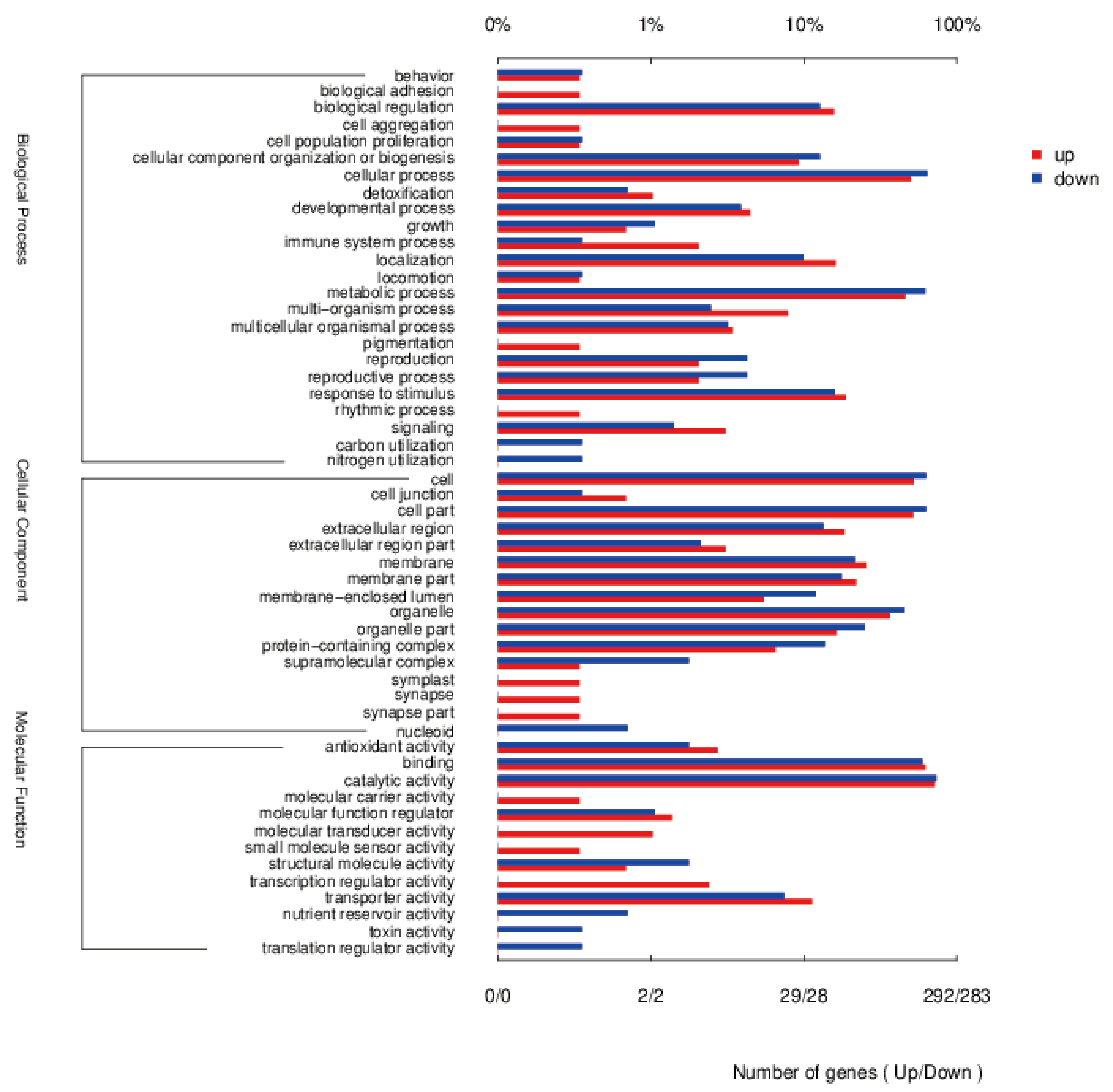
3.5.4. Enrichment Analysis of DEGs
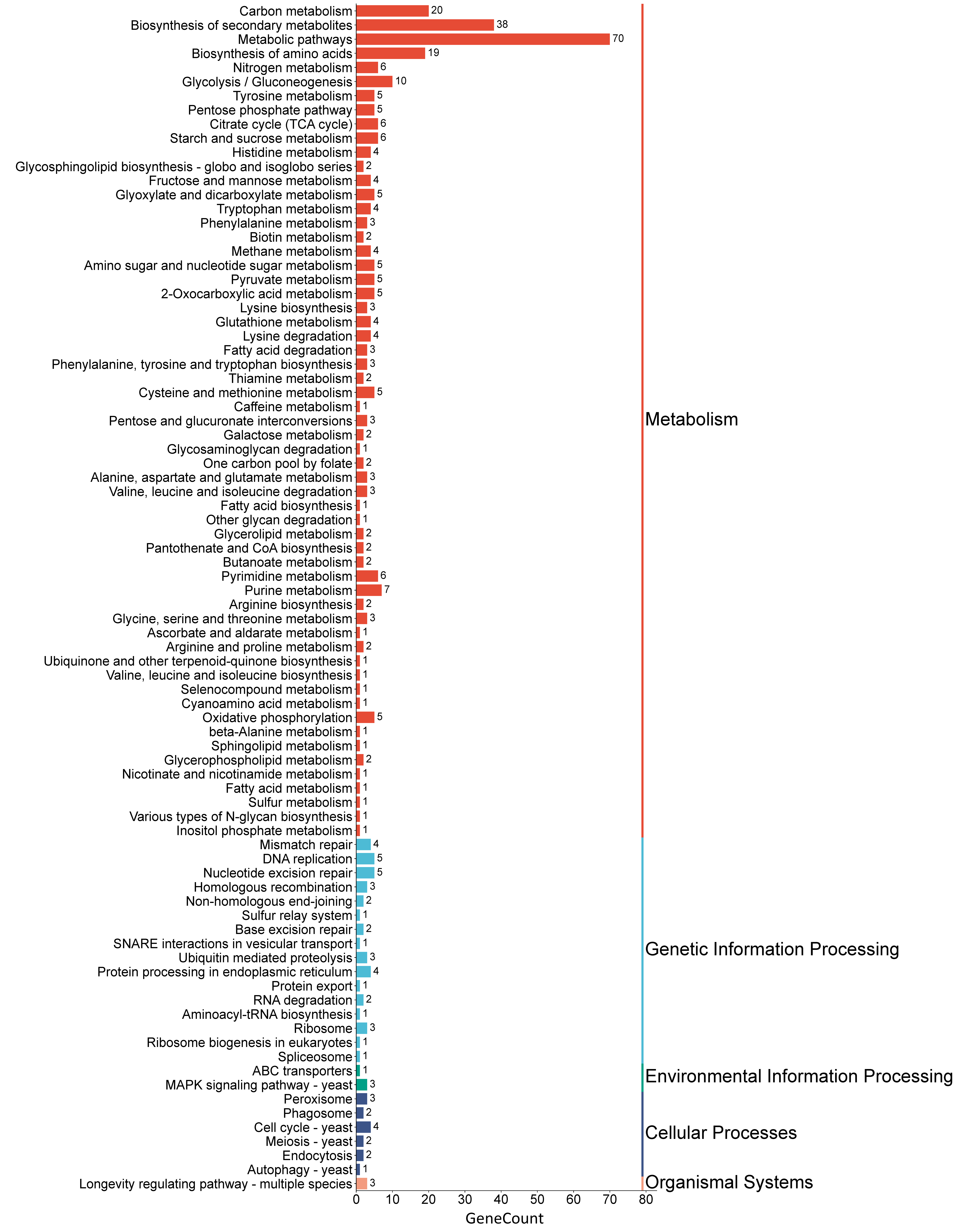
3.5.5. KEGG Pathway Enrichment Analysis
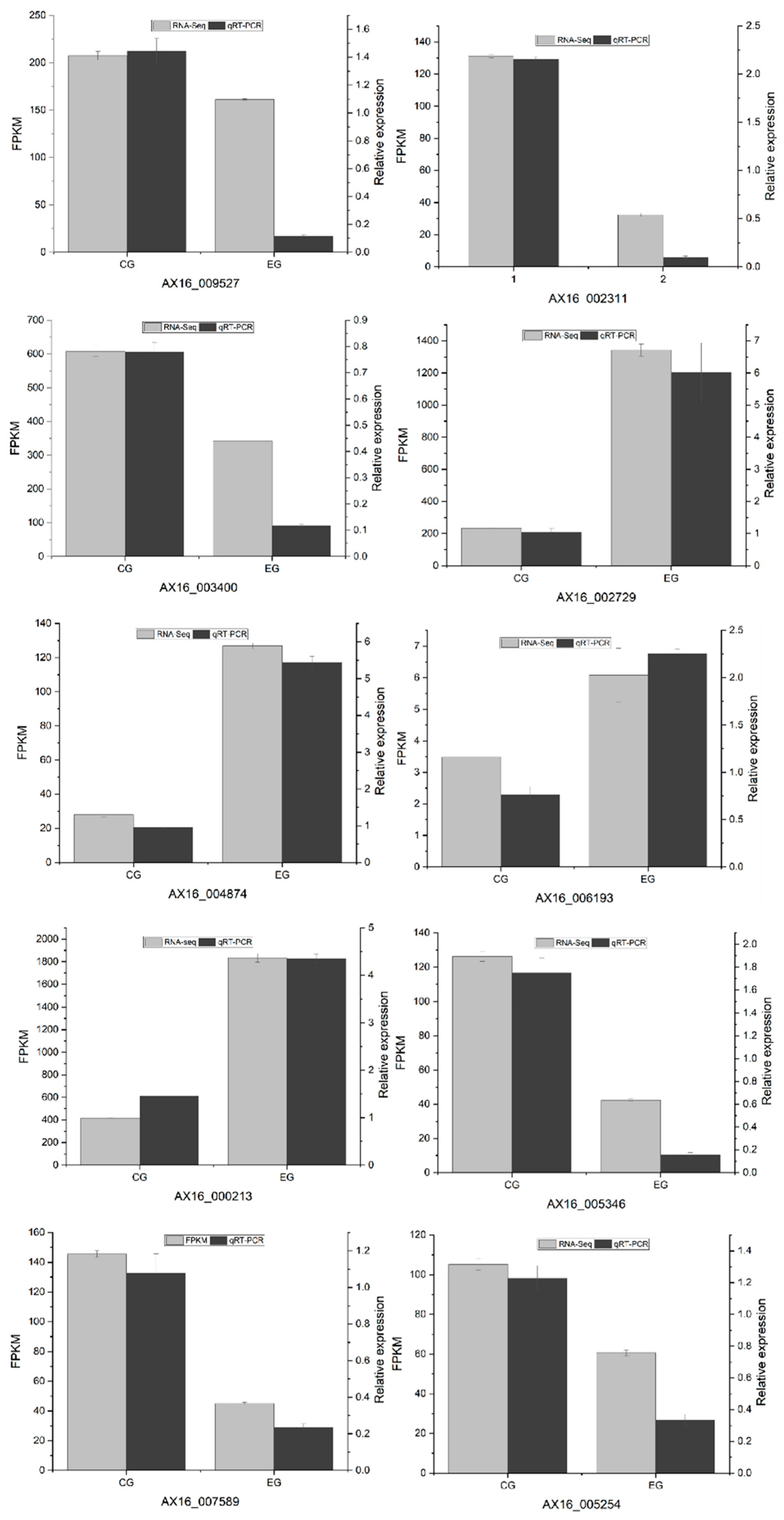
3.5.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ARTP | Atmospheric and room-temperature plasma |
| EPS | Exopolysaccharide |
| TCA | Tricarboxylic acid cycle |
| ROS | Reactive oxygen species |
| ARE | Antioxidant response element |
| PEG | Polyethylene glycol |
| PDA | Potato Dextrose Agar |
References
- Yan, J.J.; Tong, Z.J.; Liu, Y.Y.; Lin, Z.Y.; Long, Y.; Han, X.; Xu, W.N.; Huang, Q.H.; Tao, Y.X.; Xie, B.G. The NADPH oxidase in Volvariella volvacea and its differential expression in response to mycelial ageing and mechanical injury. Braz. J. Microbiol. 2020, 51, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, M.J.; Li, Z.P.; Zhao, Y.; Yang, H.L.; Zha, L.; Yu, C.X.; Wu, Y.J.; Song, X.X. The response of Volvariella volvacea to low-temperature stress based on metabonomics. Front. Microbiol. 2020, 11, 1787. [Google Scholar] [CrossRef]
- Joha, N.S.M.; Misran, A.; Mahmud, T.M.M.; Abdullah, S.; Mohamad, A. Physical quality, amino acid contents, and health risk assessment of straw mushroom (Volvariella volvacea) at different maturity stages. Int. Food Res. J. 2021, 28, 181–188. [Google Scholar] [CrossRef]
- Masitah; Candra, K.P.; Masruhim, M.; Kusumaningtyas, P. Mycochemicals and proximate composition of two different stages in fruiting body development of Volvariella volvaceae growing naturally on palm empty fruit bunches. J. Sustain. Sci. Manag. 2023, 18, 24–36. [Google Scholar] [CrossRef]
- Ali, S.; Yousaf, N.; Usman, M.; Javed, M.A.; Nawaz, M.; Ali, B.; Azam, M.; Ercisli, S.; Tirasci, S.; Ahmed, A.E. Volvariella volvacea (paddy straw mushroom): A mushroom with exceptional medicinal and nutritional properties. Heliyon 2024, 10, e39747. [Google Scholar] [CrossRef]
- Manikandan, K.; Manjit, S. Proximate composition of different mushroom varieties and effect of UV light exposure on vitamin D content in Agaricus bisporus and Volvariella volvace. Mushroom Res. 2016, 25, 1–8. [Google Scholar]
- Wang, L.; Zhang, X.; Niu, Y.; Ahmed, A.F.; Wang, J.M.; Kang, W.Y. Anticoagulant activity of two novel polysaccharides from flowers of Apocynum venetum L. Int. J. Biol. Macromol. 2019, 124, 1230–1237. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, W.; Li, J.; Lin, X.; Wang, Y. Preparation of animal polysaccharides nanofibers by electrospinning and their potential biomedical applications. J. Biomed. Mater. Res. Part A 2015, 103, 807–818. [Google Scholar] [CrossRef]
- Qamar, S.A.; Riasat, A.; Jahangeer, M.; Fatima, R.; Bilal, M.; Lqbal, H.M.N.; Mu, B.Z. Prospects of microbial polysaccharides-based hybrid constructs for biomimicking applications. J. Basic Microbiol. 2022, 62, 1319–1336. [Google Scholar] [CrossRef]
- Liang, L.H.; Su, Q.H.; Ma, Y.; Zhao, S.Z.; Zhang, H.J.; Gao, X.F. Research progress on the polysaccharide extraction and antibacterial activity. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yavuzturk, G.B.; Zeytuncu, B.; Korkut, S.; Ilyasoglu, G.; Turken, T.; Badawi, M.; Koyuncu, I.; Saeb, M.R. Polysaccharides in fabrication of membranes: A review. Carbohydr. Polym. 2022, 281, 119041. [Google Scholar] [CrossRef]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Pu, X.Y.; Ma, X.L.; Liu, L.; Ren, J.; Li, H.B.; Li, X.Y.; Yu, S.; Zhang, W.J.; Fan, W.B. Structural characterization and antioxidant activity in vitro of polysaccharides from Angelica and Astragalus. Carbohydr. Polym. 2016, 137, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, C.; Chen, Y.B.; Gu, M.; Cai, Z.K.; Chen, Q.; Wang, Z. Sulforaphane treatment of stress urinary incontinence via the Nrf2-ARE pathway in a rat model. Cell. Physiol. Biochem. 2017, 44, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, Q.F.; Zhang, T.; Chen, X.Q.; Wu, Z.Q.; Zhang, N.; Shao, Y.D.; Cheng, Y. Antibacterial activity and mechanism of green tea polysaccharide conjugates against Escherichia coli. Ind. Crops Prod. 2020, 152, 112464. [Google Scholar] [CrossRef]
- Xie, S.Z.; Liu, B.; Zhang, D.D.; Zha, X.Q.; Pan, L.H.; Luo, J.P. Intestinal immunomodulating activity and structural characterization of a new polysaccharide from stems of Dendrobium officinale. Food Funct. 2016, 7, 2789–2799. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Anti-inflammatory and anti-proliferative activities of natural and sulphonated polysaccharides from Pleurotus eryngii. J. Funct. Foods 2016, 23, 80–86. [Google Scholar] [CrossRef]
- Cheng, J.W.; Song, J.L.; Wei, H.L.; Wang, Y.B.; Huang, X.B.; Liu, Y.; Lu, N.; He, L.; Lv, G.Y.; Ding, H.M.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef]
- Vitak, T.; Yurkiv, B.; Wasser, S.; Nevo, E.; Sybirna, N. Effect of medicinal mushrooms on blood cells under conditions of diabetes mellitus. World J. Diabetes 2017, 8, 187–201. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Pan, R.; Zhu, Y.; Xiao, X.; Li, Y.; Li, C. Polysaccharides from Volvariella volvacea inhibit fat accumulation in C. elegans dependent on the aak-2/nhr-49-mediated pathway. J. Food Biochem. 2021, 45, e13912. [Google Scholar] [CrossRef]
- Huang, H.; Huang, G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Li, D.G.; Shen, J.; Ding, Q.; Wu, J.Y.; Chen, X.S. Recent progress of atmospheric and room-temperature plasma as a new and promising mutagenesis technology. Cell Biochem. Funct. 2024, 42, e3991. [Google Scholar] [CrossRef]
- Li, C.; Xia, Y.; Li, M.; Zhang, T. ARTP mutagenesis of phospholipase D-producing strain Streptomyces hiroshimensis SK43.001, and its enzymatic properties. Heliyon 2022, 8, e12587. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Yao, L.; Zheng, Y.; Yuan, J.; Wang, D. UV-ARTP-DES compound mutagenesis breeding improves natamycin production of Streptomyces natalensis HW-2 and reveals transcriptional changes by RNA-seq. Food Sci. Biotechnol. 2023, 32, 341–352. [Google Scholar] [CrossRef]
- Meng, Y.J.; Zhang, X.; Zhai, Y.F.; Li, Y.; Shao, Z.H.; Liu, S.S.; Zhang, C.; Xing, X.H.; Zheng, H. Identification of the mutual gliding locus as a factor for gut colonization in non-native bee hosts using the ARTP mutagenesis. Microbiome 2024, 12, 93. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Zhao, X.Y.; Lyu, Z.Y.; Gao, W.L.; Zhao, Q.W.; Chen, X.A.; Li, Y.Q. Daptomycin production enhancement by ARTP mutagenesis and fermentation optimization in Streptomyces roseosporus. J. Appl. Microbiol. 2023, 134, lxad230. [Google Scholar] [CrossRef]
- Ou, Y.; Li, Y.Q.; Feng, S.S.; Wang, Q.; Yang, H.L. Transcriptome analysis reveals an eicosapentaenoic acid accumulation mechanism in a Schizochytrium sp. Mutant. Microbiol. Spectr. 2023, 11, e0013023. [Google Scholar] [CrossRef]
- Liu, K.Y.; Fang, H.; Cui, F.J.; Nyabako, B.A.; Tao, T.L.; Zan, X.Y.; Chen, H.Y.; Sun, W.J. ARTP mutation and adaptive laboratory evolution improve probiotic performance of Bacillus coagulans. Appl. Microbiol. Biotechnol. 2020, 104, 6363–6373. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, J.; Wei, H.; Liu, Q.; Xu, W.; Bao, Y. Optimizing mycelial protein yield in Pleurotus djamor via ARTP mutagenesis and hybridization strategies. J. Biotechnol. 2024, 386, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Rao, J.W.; Meng, F.B.; Wang, Z.W.; Liu, D.Y.; Yu, H. Combination of mutagenesis and adaptive evolution to engineer salt-tolerant and aroma-producing yeast for soy sauce fermentation. J. Sci. Food Agric. 2021, 101, 4288–4297. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, C.; Liang, N.; Xiao, W.; Wang, Y.; Yao, M.; Yuan, Y. Adaptive evolution and metabolic engineering boost lycopene production in Saccharomyces cerevisiae via enhanced precursors supply and utilization. J. Agric. Food Chem. 2023, 71, 3821–3831. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Qi, X.; Ei-Shenody, R.A. Enhancing biomass and lipid productivity of a green microalga Parachlorella kessleri for biodiesel production using rapid mutation of atmospheric and room temperature plasma. Biotechnol. Biofuels Bioprod. 2022, 15, 122. [Google Scholar] [CrossRef]
- Magocha, T.A.; Zabed, H.; Yang, M.; Yun, J.; Zhang, H.; Qi, X. Improvement of industrially important microbial strains by genome shuffling: Current status and future prospects. Bioresour. Technol. 2018, 257, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Middendorf, M.; Bernt, M. Genome rearrangement analysis: Cut and join genome rearrangements and gene cluster preserving approaches. Methods Mol. Biol. 2024, 2802, 215–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xin, Q.H.; Ma, L.M.; Li, R.F.; Bian, K. Applications and research advance of genome shuffling for industrial microbial strains improvement. World J. Microbiol. Biotechnol. 2020, 36, 158. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Perry, K.; Vinci, V.A.; Powell, K.; Stemmer, W.P.; Del, C.S.B. Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature 2002, 415, 644–646. [Google Scholar] [CrossRef]
- Jetti, K.D.; Gns, R.R.; Garlapati, D.; Nammi, S.K. Improved ethanol productivity and ethanol tolerance through genome shuffling of Saccharomyces cerevisiae and Pichia stipitis. Int. Microbiol. 2019, 22, 247–254. [Google Scholar] [CrossRef]
- Chen, L.; Chong, X.Y.; Zhang, Y.Y.; Lv, Y.Y.; Hu, Y.S. Genome Shuffling of Bacillus velezensis for enhanced surfactin production and variation analysis. Curr. Microbiol. 2020, 77, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Zabed, H.M.; Zhang, Y.; Zhang, G.; Zhao, M.; Qi, X. Improving tolerance and 1,3-propanediol production of Clostridium butyricum using physical mutagenesis, adaptive evolution and genome shuffling. Bioresour. Technol. 2022, 363, 127967. [Google Scholar] [CrossRef] [PubMed]
- Buysse, J.A.N.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Breuil, C.; Saddler, J.N. Comparison of the 3,5-dinitrosalicylic acid and Nelson-Somogyi methods of assaying for reducing sugars and determining cellulase activity. Enzym. Microb. Technol. 1985, 7, 327–332. [Google Scholar] [CrossRef]
- Liu, W.M.; Yang, W.W.; Wu, J.; Chen, Y.; Wei, Z.C.; Wang, T.; Ampofo, K.A.; Ma, H.L.; Cui, F.J.; Yang, X.M.; et al. ARTP mutagenesis to improve mycelial polysaccharide production of Grifola frondosa using a mixture of wheat bran and rice bran as substrate. J. Food Qual. 2021, 2021, 6110743. [Google Scholar] [CrossRef]
- Li, Y.H.; Juo, J.J.; Ng, I.S. Current breakthroughs and advances in atmospheric room temperature plasma (ARTP) technology for biomanufacturing. Bioresour. Bioprocess. 2025, 12, 63. [Google Scholar] [CrossRef]
- Zhu, Z.R.; Ding, X.Z.; Rang, J.; Xia, L. Application and research progress of ARTP mutagenesis in actinomycetes breeding. Gene 2024, 929, 148837. [Google Scholar] [CrossRef]
- Zhao, L.T.; Ma, Z.B.; Yin, J.; Shi, G.Y.; Ding, Z.Y. Biological strategies for oligo/polysaccharide synthesis: Biocatalyst and microbial cell factory. Carbohydr. Polym. 2021, 258, 117695. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Hegedűs, T. A twist in the ABC: Regulation of ABC transporter trafficking and transport by FK506-binding proteins. FEBS Lett. 2020, 594, 3986–4000. [Google Scholar] [CrossRef]
- Sangthong, S.; Pintathong, P.; Pongsua, P.; Jirarat, A.; Chaiwut, P. Polysaccharides from Volvariella volvacea mushroom: Extraction, biological activities and cosmetic efficacy. J. Fungi 2022, 8, 572. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, M.; Zhang, J.; Ma, Z.; Cui, J.; Zhao, L.; Chen, L.; Shi, G.; Ding, Z. Refined regulation of polysaccharide biosynthesis in edible and medicinal fungi: From pathways to production. Carbohydr. Polym. 2025, 358, 123560. [Google Scholar] [CrossRef]
- Zhu, L.L.; Wu, D.; Zhang, H.N.; Li, Q.Z.; Zhang, Z.; Liu, Y.F.; Zhou, S.; Wang, W.H.; Li, Z.P.; Yang, Y. Effects of Atmospheric and Room Temperature Plasma (ARTP) Mutagenesis on Physicochemical Characteristics and Immune Activity In Vitro of Hericium erinaceus Polysaccharides. Molecules 2019, 24, 262. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhang, H.N.; Wu, D.; Zhang, Z.; Zhang, J.S.; Bao, D.P.; Yang, Y. Key metabolism pathways and regulatory mechanisms of high polysaccharide yielding in Hericium erinaceus. BMC Genom. 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Sun, J.S.; Ning, H.J.; Qin, Z.F.; Miao, Y.X.; Su, T.; Zhang, X.Q. De novo transcriptome sequencing and comprehensive analysis of the heat stress response genes in the basidiomycetes fungus Ganoderma lucidum. Gene 2018, 661, 139–151. [Google Scholar] [CrossRef] [PubMed]

| Passage Number | EPS Content (g/L) |
|---|---|
| 1 | 46.02 ± 0.47 |
| 2 | 46.11 ± 0.58 |
| 3 | 46.14 ± 0.53 |
| 4 | 46.78 ± 0.47 |
| 5 | 46.05 ± 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Su, Q.; Wang, Y.; Du, P.; Zhao, S.; Zhang, H.; Gao, X. Breeding of High-Polysaccharide-Producing Volvariella volvacea Strains Based on Genome Shuffling Technology. J. Fungi 2025, 11, 591. https://doi.org/10.3390/jof11080591
Liang L, Su Q, Wang Y, Du P, Zhao S, Zhang H, Gao X. Breeding of High-Polysaccharide-Producing Volvariella volvacea Strains Based on Genome Shuffling Technology. Journal of Fungi. 2025; 11(8):591. https://doi.org/10.3390/jof11080591
Chicago/Turabian StyleLiang, Lihui, Qihang Su, Yawei Wang, Peichen Du, Suzhen Zhao, Huanjie Zhang, and Xiaofeng Gao. 2025. "Breeding of High-Polysaccharide-Producing Volvariella volvacea Strains Based on Genome Shuffling Technology" Journal of Fungi 11, no. 8: 591. https://doi.org/10.3390/jof11080591
APA StyleLiang, L., Su, Q., Wang, Y., Du, P., Zhao, S., Zhang, H., & Gao, X. (2025). Breeding of High-Polysaccharide-Producing Volvariella volvacea Strains Based on Genome Shuffling Technology. Journal of Fungi, 11(8), 591. https://doi.org/10.3390/jof11080591






