Genomic Analysis of Laccaria Genomes at High Altitude
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi Material, Genome Sequencing, and Assembly
2.2. Genome Assembly and Annotation
2.3. Gene Family Expansion and Contraction
2.4. Positively Selected and Species-Specific Genes
3. Results and Discussion
3.1. Genome Assembly and Annotation
3.2. Functional Annotations
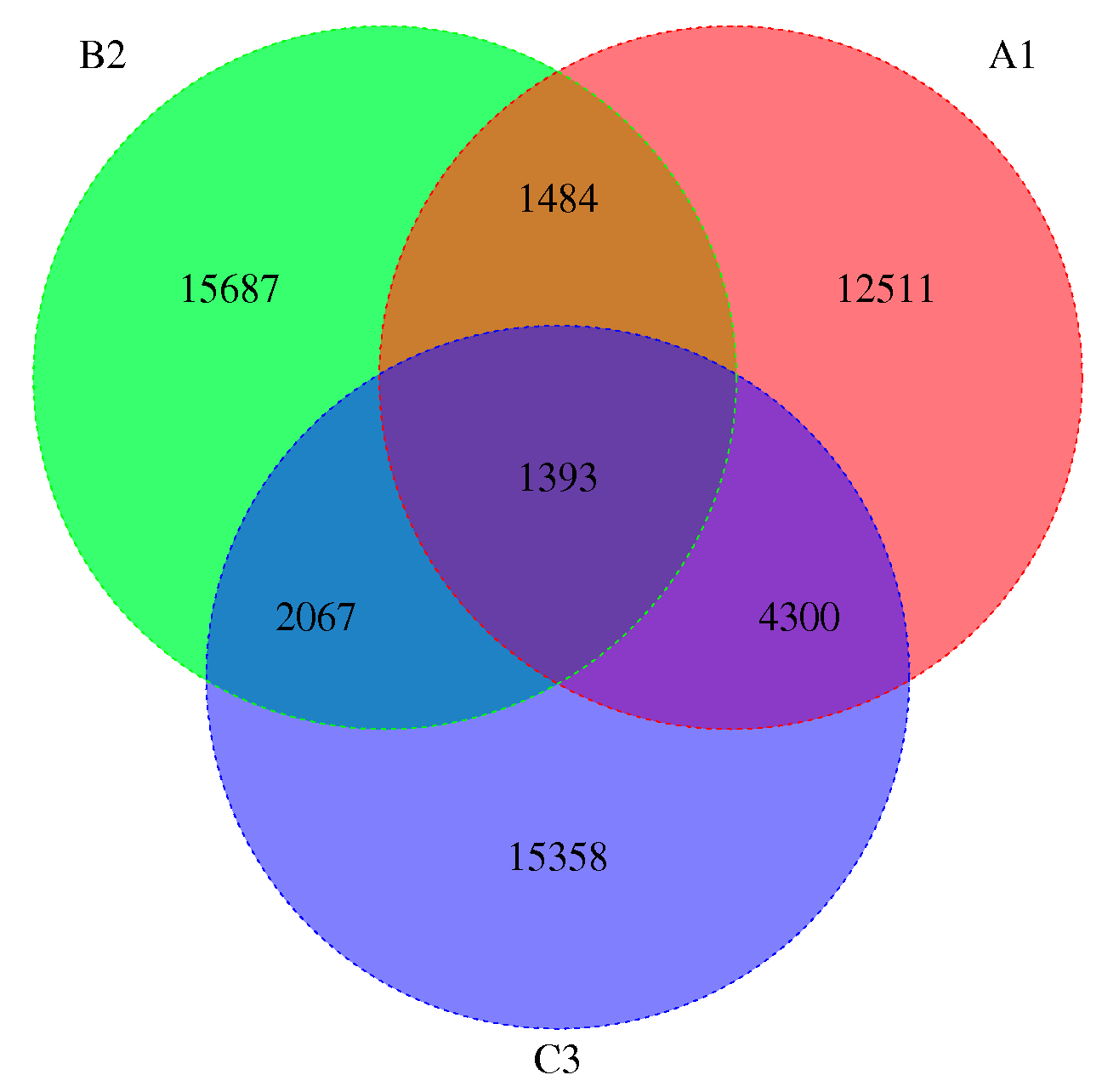
3.3. Gene Family Expansion and Contraction
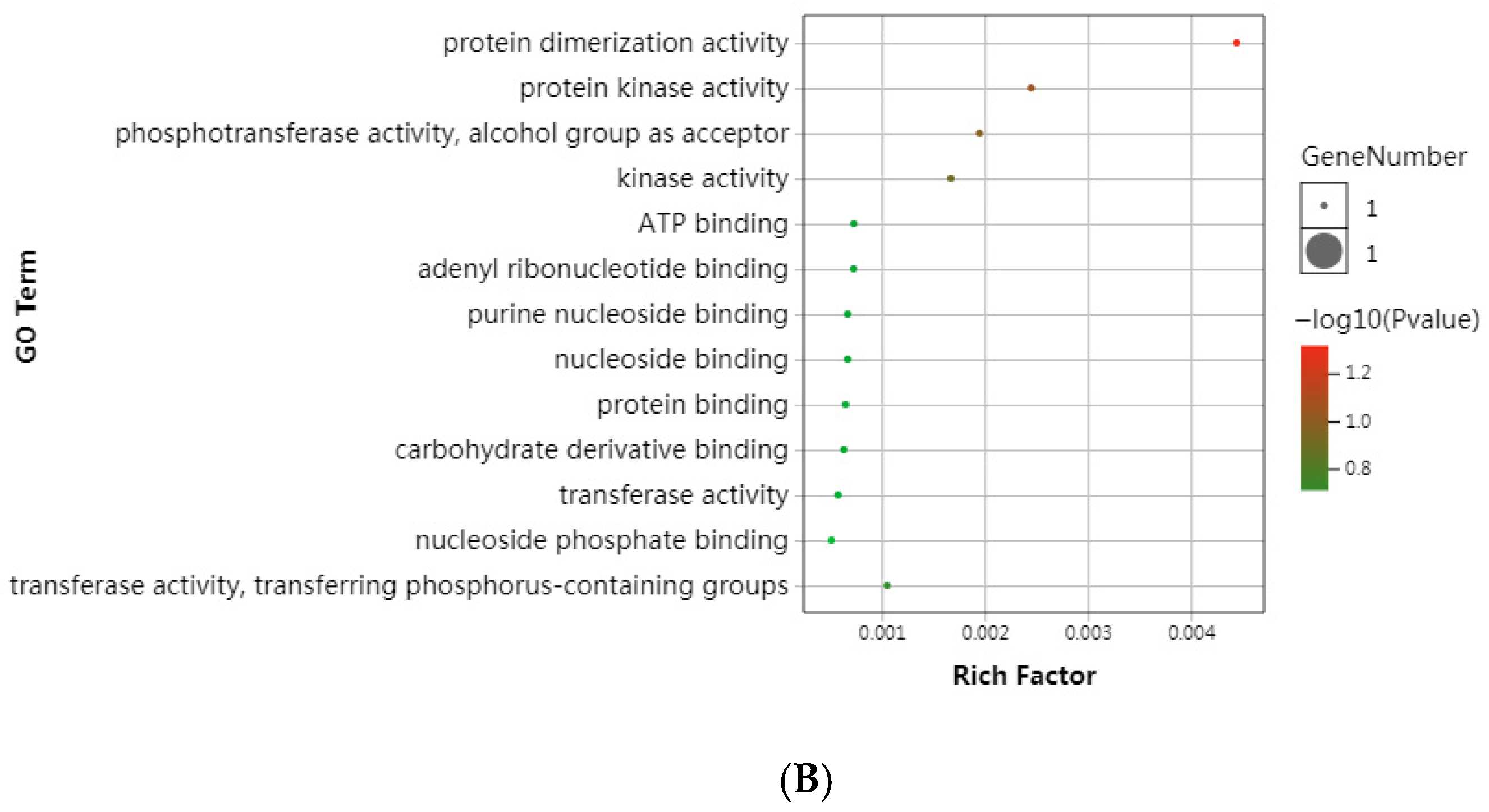
3.4. Positive Selection in Single-Copy Genes
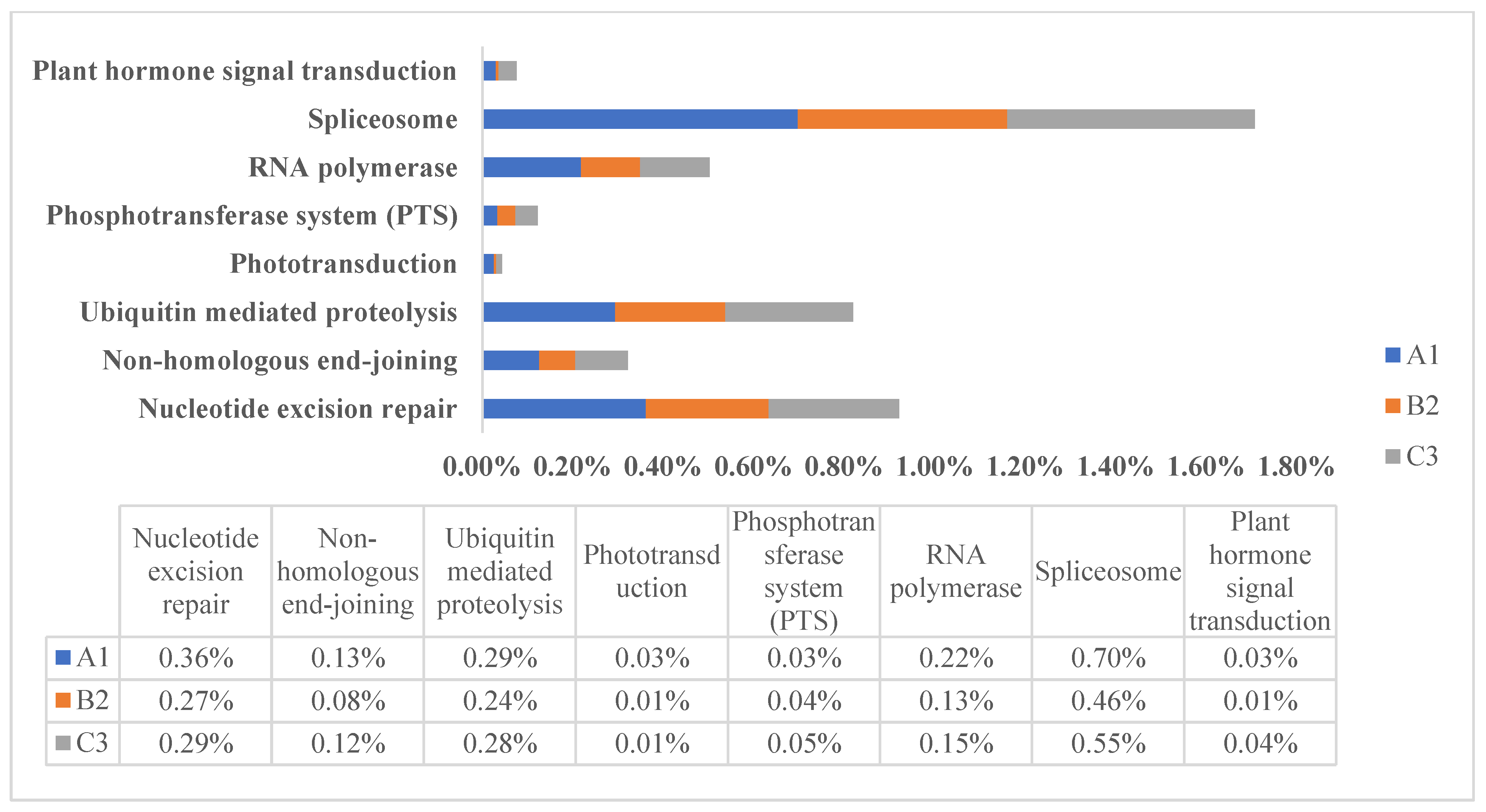
3.5. Species-Specific Gene of High-Altitude Adaptation
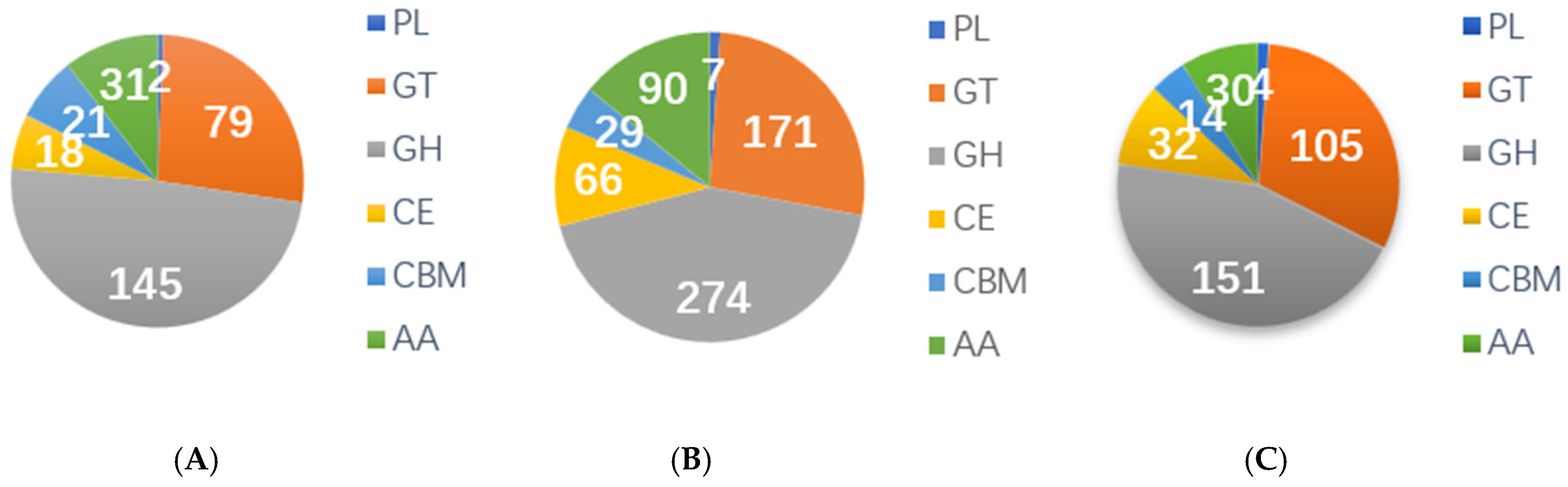
3.6. CAZymes Gene of High-Altitude Adaptation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QTP | Qinghai–Tibet Plateau |
| PCR | Polymerase chain reaction |
| LTR | Long terminal repeat |
| DNA | Deoxyribonucleic acid |
| PASA | Primer-aligned sequence analysis |
| RNA | Ribonucleic acid |
| CAZymes | Carbohydrate-active enzymes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KOG | Clusters of orthologous groups |
| NR | Non-Redundant Protein Database |
| SEA | Singular enrichment analysis |
| GO | Gene Ontology |
| MAPK | Mitogen-Activated Protein Kinase |
| NLR | NOD-like receptor |
| GHs | Glycoside hydrolases |
References
- Xing, Y.; Ree, R.H. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl. Acad. Sci. USA 2017, 114, E3444–E3451. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Huang, Y.; Qi, J.; Qu, M.; Jiang, C.; Lin, P.; Li, R.; Song, L.; Yonezawa, T.; Hasegawa, M.; et al. The genome and transcriptome of Trichormus sp.NMC-1: Insights into adaptation to extreme environments on the Qinghai-Tibet Plateau. Sci. Rep. 2016, 6, 92404. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Wang, Q.; Han, X.; Guan, Y.; Sun, H.; Zhong, Y.; Huang, J.; Zhang, T. Transcriptome sequencing of Crucihimalaya himalaica (Brassicaceae) reveals how Arabidopsis close relative adapt to the Qinghai-Tibet Plateau. Sci. Rep. 2016, 6, 21729. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1443–1476. [Google Scholar] [CrossRef]
- Grande, F.D.; Sharma, R.; Meiser, A.; Rolshausen, G.; Büdel, B.; Mishra, B.; Thines, M.; Otte, J.; Pfenninger, M.; Schmitt, I. Adaptive differentiation coincides with local bioclimatic conditions along an elevational cline in populations of a lichen-forming fungus. BMC Evol. Biol. 2017, 17, 93. [Google Scholar] [CrossRef]
- Satriawan, H.; Teoh, T.C.; Rizman-Idid, M.; Krishnan, A.; Abu Bakar, N.; Alias, S.A. Polar Fungi Pseudogymnoascus: Secondary Metabolites and Ecological Significance. Chiang Mai J. Sci. 2024, 51, e2024043. [Google Scholar] [CrossRef]
- Milo, S.; Namawejje, R.; Krispin, R.; Covo, S. Dynamic responses of Fusarium mangiferae to ultra-violet radiation. Fungal Biol. 2024, 128, 1714–1723. [Google Scholar] [CrossRef]
- Cheng, F.; Li, M.; Ren, Y.; Hou, L.; Gao, T.; He, P.; Deng, X.; Lu, J. Soil Fungal Community Characteristics at Timberlines of Sejila Mountain in Southeast Tibet, China. J. Fungi 2023, 9, 596. [Google Scholar] [CrossRef]
- Han, B.; Yu, Q.; Han, Q.; Wang, S.; Su, W.; Qu, J.; Li, H. Precipitation weakens the gravesoil fungal richness and species interactions in the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2023, 189, 104958. [Google Scholar] [CrossRef]
- Gao, X.; Wang, S.; Wang, Y.-F.; Li, S.; Wu, S.-X.; Yan, R.-G.; Zhang, Y.-W.; Wan, R.-D.; He, Z.; Song, R.-D.; et al. Long read genome assemblies complemented by single cell RNA-sequencing reveal genetic and cellular mechanisms underlying the adaptive evolution of yak. Nat. Commun. 2022, 13, 4887. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, P.; Zhao, S.; Chen, Z.; Li, X.; Zhang, W.; Liu, C.; Yang, Y.; Li, X.; Liu, X. Population transcriptomics uncover the relative roles of positive selection and differential expression in Batrachium bungei adaptation to the Qinghai–Tibetan plateau. Plant Cell Rep. 2023, 42, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Yu, B. Mycoflora Biodiversity and Genetic Variation of Macrofungiin Alpine Meadow of Tibet. Ph.D. Thesis, Northeast Normal University, Changchun, China, 2021. [Google Scholar]
- Khan, A.M.; Bhadauria, S. Molecular characterization of keratin degrading fungi isolated from semi-arid soil by PCR using ITS4 and ITS5 primers. J. King Saud Univ. Sci. 2019, 31, 1418–1423. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.; Taylor, J.W.; Innis, M.A.; Gelfand, D.H.; Sninsky, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Birney, E.; Durbin, R. Using GeneWise in the Drosophila annotation experiment. Genome Res. 2000, 10, 547–548. [Google Scholar] [CrossRef]
- Saha, S.; Bridges, S.; Magbanua, Z.V.; Peterson, D.G. Empirical comparison of ab initio repeat finding programs. Nucleic Acids Res. 2008, 36, 2284–2294. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Staerfeldt, H.-H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Gardner, P.P.; Daub, J.; Tate, J.G.; Nawrocki, E.P.; Kolbe, D.L.; Lindgreen, S.; Wilkinson, A.C.; Finn, R.D.; Griffiths-Jones, S.; Eddy, S.R.; et al. Rfam: Updates to the RNA families database. Nucleic Acids Res. 2009, 37 (Suppl. 1), D136–D140. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Burge, S.W.; Bateman, A.; Daub, J.; Eberhardt, R.Y.; Eddy, S.R.; Floden, E.W.; Gardner, P.P.; Jones, T.A.; Tate, J.; et al. Rfam 12.0: Updates to the RNA families database. Nucleic Acids Res. 2015, 43, D130–D137. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32 (Suppl. 1), D277–D280. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34 (Suppl. 1), D354–D357. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jaroszewski, L.; Godzik, A. Tolerating some redundancy significantly speeds up clustering of large protein databases. Bioinformatics 2002, 18, 77–82. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37 (Suppl. 1), D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.B. dbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012, 40, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Han, M.V.; Thomas, G.W.; Lugo-Martinez, J.; Hahn, M.W. Estimating gene gain and loss rates in the presence of error in genome assembly and annotation using CAFÉ 3. Mol. Biol. Evol. 2013, 30, 1987–1997. [Google Scholar] [CrossRef]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, W64–W70. [Google Scholar] [CrossRef]
- Li, L.; Stoeckert, C.J.; Roos, D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. Int. J. Nat. Synth. Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Damianov, A.; Lin, C.H.; Huang, J.; Zhou, L.; Jami-Alahmadi, Y.; Peyda, P.; Wohlschlegel, J.; Black, D.L. The splicing regulators RBM5 and RBM10 are subunits of the U2 snRNP engaged with intron branch sites on chromatin. Mol. Cell 2024, 84, 24. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, A.; Yadav, N.; Yadav, D.K. Current perspectives of ubiquitination and SUMOylation in abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 993194. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Bao, Q.; Wang, Z.; He, S.; Qu, X.; Tang, Y.; Song, B.; Huang, J.; Yi, G. Uncovering Evolutionary Adaptations in Common Warthogs through Genomic Analyses. Genes 2024, 15, 166. [Google Scholar] [CrossRef]
- Xia, X.-M.; Du, H.-L.; Hu, X.-D.; Wu, J.-J.; Yang, F.-S.; Li, C.-L.; Huang, S.-X.; Wang, Q.; Liang, C.; Wang, X.-Q. Genomic insights into adaptive evolution of the species-rich cosmopolitan plant genus Rhododendron. Cell Rep. 2024, 43, 114745. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, M.; Li, D.; You, M.; Yan, J.; Bai, S. Metabolomic Analysis of Elymus sibiricus Exposed to UV-B Radiation Stress. Molecules 2024, 29, 5133. [Google Scholar] [CrossRef]
- Zhang, T.; Qiao, Q.; Novikova, P.Y.; Wang, Q.; Yue, J.; Guan, Y.; Ming, S.; Liu, T.; De, J.; Liu, Y.; et al. Genome of Crucihimalaya himalaica, a close relative of Arabidopsis, shows ecological adaptation to high altitude. Proc. Natl. Acad. Sci. USA 2019, 116, 7137–7146. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zuo, H.; Zheng, W.; Zhang, S.; Huang, Y.; Pingcuo, G.; Ying, H.; Zhao, F.; Li, Y.; et al. Genomic basis of high-altitude adaptation in Tibetan prunus fruit trees. Curr. Biol. 2021, 31, 3848–3860.e8. [Google Scholar] [CrossRef]
- Liu, M.; Sun, W.; Ma, Z.; Guo, C.; Chen, J.; Wu, Q.; Wang, X.; Chen, H. Integrated network analyses identify MYB4R1 neofunctionalization in the UV-B adaptation of Tartary buckwheat. Plant Commun. 2022, 3, 100414. [Google Scholar] [CrossRef]
- Li, J.T.; Gao, Y.D.; Xie, L.; Deng, C.; Shi, P.; Guan, M.L.; Huang, S.; Ren, J.L.; Wu, D.D.; Ding, L.; et al. Comparative genomic investigation of high-elevation adaptation in ectothermic snakes. Proc. Natl. Acad. Sci. USA 2018, 115, 8406–8411. [Google Scholar] [CrossRef]
- Ge, R.L.; Cai, Q.; Shen, Y.Y.; San, A.; Ma, L.; Zhang, Y.; Yi, X.; Chen, Y.; Yang, L.; Huang, Y.; et al. Draft genome sequence of the Tibetan antelope. Nat. Commun. 2013, 4, 1858. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Long, H.; Wang, Z.; Zhao, S.; Tang, Y.; Huang, Z.; Wang, Y.; Xu, Q.; Mao, L.; Deng, G.; et al. The draft genome of Tibetan hulless barley reveals adaptive patterns to the high stressful Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2015, 112, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gou, W.; Wang, X.; Zhang, Y.; Ma, J.; Zhang, H.; Zhang, Y.; Zhang, H. Genome resequencing identifies unique adaptations of Tibetan chickens to hypoxia and high-dose ultraviolet radiation in high-altitude environments. Genome Biol. Evol. 2016, 8, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Habib, Y.J.; Yao, C.; Wan, H. Toll-like receptor expression in Pacific white shrimp (Litopenaeus vannamei) reveals differential responses after fungal (Fusarium solani) infection. Aquacult. Int. 2024, 32, 5719–5736. [Google Scholar]
- Cheang, W.K.; Wong, G.R.; Rahim, A.N.; Kethiravan, D.; Harikrishna, J.A.; Tan, B.C.; Ramakrishnan, N.; Mazumdar, P. Synergistic Effects of UV-B and UV-C in Suppressing Sclerotinia sclerotiorum Infection in Tomato Plants. J. Crop. Health 2024, 76, 1383–1402. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, W.; He, J.; Li, D.Z.; Li, H.T. Positive selection and relaxed purifying selection contribute to rapid evolution of male-biased genes in a dioecious flowering plant. eLife 2024, 12, RP89941. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The yak genome and adaptation to life at high altitude. Nat Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, 490–495. [Google Scholar] [CrossRef]
- Pervaiz, T.; Liu, T.; Fang, X.; Ren, Y.; Li, X.; Liu, Z.; Fiaz, M.; Fang, J.; Shangguan, L. Identification of GH17 gene family in Vitis vinifera and expression analysis of GH17 under various adversities. Physiol. Mol. Biol. Plants 2021, 27, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Chauhan, H.; Singh, K. Glycoside hydrolases reveals their differential role in response to drought and salt stress in potato (Solanum tuberosum). Funct. Plant Biol. 2024, 51, FP24114. [Google Scholar] [CrossRef]
- Wen, T.-T.; Qian, Z.-Y.; Sun, L.; Cui, F.-J.; Zan, X.-Y.; Meng, L.-J.; Sun, W.-J. Fungal β-1, 3-glucanosyltransferases: A comprehensive review on classification, catalytic mechanism and functional role. Int. J. Biol. Macromol. 2025, 289, 138651. [Google Scholar] [CrossRef] [PubMed]
- Baltar, F.; Zhao, Z.; Herndl, G.J. Potential and expression of carbohydrate utilization by marine fungi in the global ocean. Microbiome 2021, 9, 106. [Google Scholar] [CrossRef]


| Sp. | A1 (High Altitude) (L. bicolor) | B2 (Low Altitude) (L. tortilis) | C3 (High Altitude) (L. tortilis) |
|---|---|---|---|
| Genome size (bp) | 109,399,439 | 104,534,143 | 120,365,163 |
| No. of contigs | 64,686 | 34,057 | 73,143 |
| GC content (%) | 47.03 | 47.79 | 47.66 |
| No. of scaffolds | 63,946 | 33,829 | 72,483 |
| GC content (%) | 47.03 | 47.79 | 47.66 |
| N50 contig length (bp) | 2245 | 6000 | 2096 |
| N50 scaffold length (bp) | 2264 | 6031 | 2109 |
| N90 contig length (bp) | 755 | 1158 | 767 |
| N90 scaffold length (bp) | 762 | 1164 | 773 |
| No. of protein-coding genes | 23,719 | 25,283 | 27,546 |
| Sp. | No. of Repeat (in Genome%) | No. of Repbase (in Genome%) | No. of TRF (in Genome%) | No. LTR (in Genome%) | No. LTR (in Repeat%) |
|---|---|---|---|---|---|
| A1 (L. bicolor, high altitude) | |||||
| 115,354 (14.6) | 37,284 (8.6) | 78,070 (6.0) | 31,810 (7.96) | 27.58% | |
| B2 (L. tortilis, low altitude) | |||||
| 80,603 (10.2) | 23,862 (5.2) | 56,741 (5.0) | 19,464 (4.67) | 24.15 | |
| C3 (L. tortilis, high altitude) | |||||
| 115,278 (13.5) | 37,938 (8.0) | 77,340 (5.4) | 32,046 (7.41) | 27.8 | |
| Sp. | A1 L. bicolor High-Altitude | C3 L. tortilis High-Altitude |
|---|---|---|
| No. of expansion gene family | 364 | 94 |
| No. of contraction gene family | 122 | 134 |
| GO Term | Gene Ratio | Bg Ratio | p-Value | Rich Factor | |
|---|---|---|---|---|---|
| Expand | Translational termination | 5/2503 | 13/31,381 | 0.002 | 4.82 |
| Gene Families | Proton-transporting two-sector ATPase complex, catalytic domain | 6/2503 | 16/31,381 | 0.001 | 4.70 |
| Vesicle coat | 5/2503 | 20/31,381 | 0.018 | 3.13 | |
| Cytoplasmic vesicle membrane | 5/2503 | 20/31,381 | 0.018 | 3.13 | |
| Transcription from RNA polymerase III promoter | 5/2503 | 21/31,381 | 0.022 | 2.99 | |
| Actin binding | 7/2503 | 30/31,381 | 0.008 | 2.93 | |
| RNA polymerase II transcription factor activity, sequence-specific DNA binding | 5/2503 | 22/31,381 | 0.027 | 2.85 | |
| Membrane coat | 13/2503 | 66/31,381 | 0.002 | 2.47 | |
| Asexual reproduction | 6/2503 | 32/31,381 | 0.039 | 2.35 | |
| Oxidoreductase activity, acting on the CH-CH group of donors, NAD or NADP as acceptor | 8/2503 | 49/31,381 | 0.039 | 2.05 | |
| Contract | Helicase activity | 5/70 | 425/31,381 | 0.003 | 5.27 |
| Gene Families | Transporter activity | 5/70 | 648/31,381 | 0.018 | 3.46 |
| KEGG Term | Gene Ratio | Bg Ratio | p-Value | Rich Factor | |
|---|---|---|---|---|---|
| Expand gene families | Amino acid metabolism | 4/114 | 33/3198 | 0.03 | 3.40 |
| Signal transduction | 2/114 | 8/3198 | 0.01 | 7.00 | |
| Contract gene families | Plant–pathogen interaction | 1/3 | 27/2038 | 0.03 | 50.30 |
| Toll-like receptor signaling pathway | 1/3 | 21/2038 | 0.01 | 32.30 | |
| One carbon pool by folate | 1/3 | 17/2038 | 0.02 | 40.00 |
| GO Term | Gene Ratio | Bg Ratio | p- Value | Rich Factor | |
|---|---|---|---|---|---|
| Expand | Antiporter activity | 5/1474 | 35/45,484 | 0.005 | 4.41 |
| gene families | Water-soluble vitamin biosynthetic process | 7/1474 | 67/45,484 | 0.006 | 3.22 |
| Vitamin biosynthetic process | 7/1474 | 67/45,484 | 0.006 | 3.22 | |
| Secondary active transmembrane transporter activity | 5/1474 | 59/45,484 | 0.042 | 2.62 | |
| Response to stress | 7/1474 | 66/45,484 | 9.90 × 10−10 | 3.27 | |
| Coenzyme biosynthetic process | 6/1474 | 76/45,484 | 0.037 | 2.44 | |
| Base excision repair | 11/1471 | 176/45,484 | 0.03 | 2.00 | |
| Contract | DNA integration | 10/416 | 431/45,484 | 0.008 | 2.54 |
| gene families | DNA binding | 26/416 | 1814/45,484 | 0.022 | 1.57 |
| Cellular aromatic compound metabolic process | 55/416 | 4661/45,484 | 0.047 | 1.29 |
| KEGG Term | Gene Ratio | Bg Ratio | p- Value | Rich Factor | |
|---|---|---|---|---|---|
| Expand gene families | Amino acid metabolism | 4/114 | 33/3198 | 0.03 | 3.40 |
| Signal transduction | 2/114 | 8/3198 | 0.01 | 7.00 | |
| Contract gene families | Galactose metabolism | 2/31 | 32/3198 | 0.037663 | 6.45 |
| Proteasome | 2/31 | 32/3198 | 0.037663 | 6.45 | |
| Inositol phosphate metabolism | 2/31 | 36/3198 | 0.046711 | 5.73 | |
| Citrate cycle (TCA cycle) | 3/31 | 64/3198 | 0.023059 | 4.83 | |
| Carbon metabolism | 2/31 | 265/3198 | 0.011524 | 2.72 |
| GO-Id | GO Term | Gene Ratio | Bg Ratio | p -Value | Rich Factor |
|---|---|---|---|---|---|
| GO: 0000123 | Histone acetyltransferase complex | 10/18,185 | 26/127,883 | 2.00 × 10−3 | 2.70 |
| GO: 0071805 | Potassium ion transmembrane transport | 5/18,185 | 13/127,883 | 2.80 × 10−2 | 2.70 |
| GO: 0080134 | Regulation of response to stress | 6/18,185 | 16/127,883 | 1.80 × 10−2 | 2.64 |
| GO: 0019888 | Protein phosphatase regulator activity | 17/18,185 | 66/127,883 | 9.60 × 10−3 | 1.81 |
| GO: 0051607 | Defense response to virus | 10/18,185 | 39/127,883 | 4.30 × 10−2 | 1.80 |
| GO: 0019899 | Enzyme binding | 30/18,185 | 134/127,883 | 7.20 × 10−3 | 1.57 |
| GO: 0000003 | Reproduction | 73/18,185 | 356/127,883 | 7.80 × 10−4 | 1.44 |
| GO: 0016310 | Phosphorylation | 199/18,185 | 1175/127,883 | 5.30 × 10−3 | 1.19 |
| GO: 0098796 | Membrane protein complex | 146/18,185 | 894/127,883 | 4.20 × 10−2 | 1.15 |
| GO: 0016301 | Kinase activity | 356/18,185 | 2276/127,883 | 3.00 × 10−2 | 1.10 |
| GO-Id | GO Term | Gene Ratio | Bg Ratio | p-Value | Rich Factor |
|---|---|---|---|---|---|
| GO: 0030076 | Light-harvesting complex | 6/28,632 | 9/127,883 | 5.60 × 10−3 | 2.97 |
| GO: 0006744 | Ubiquinone biosynthetic process | 7/28,632 | 12/127,883 | 7.60 × 10−3 | 2.61 |
| GO: 0071555 | Cell wall organization | 11/28,632 | 22/127,883 | 4.10 × 10−3 | 2.23 |
| GO: 0098533 | ATPase dependent transmembrane transport complex | 19/28,632 | 51/127,883 | 1.20 × 10−2 | 1.66 |
| GO: 0001510 | RNA methylation | 31/28,632 | 92/127,883 | 8.60 × 10−3 | 1.5 |
| GO: 0016407 | Acetyltransferase activity | 81/28,632 | 243/127,883 | 6.20 × 10−5 | 1.49 |
| GO: 0015171 | Amino acid transmembrane transporter activity | 37/28,632 | 111/127,883 | 5.40 × 10−3 | 1.49 |
| GO: 0016775 | Phosphotransferase activity, nitrogenous group as acceptor | 120/28,632 | 366/127,883 | 3.30 × 10−6 | 1.46 |
| GO: 0019954 | Asexual reproduction | 38/28,632 | 125/127,883 | 2.30 × 10−2 | 1.36 |
| GO: 0038023 | Signaling receptor activity | 161/28,632 | 566/127,883 | 4.60 × 10−4 | 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Mu, Y.; Hu, J.; Chen, M.; Xing, J. Genomic Analysis of Laccaria Genomes at High Altitude. J. Fungi 2025, 11, 592. https://doi.org/10.3390/jof11080592
Bao Y, Mu Y, Hu J, Chen M, Xing J. Genomic Analysis of Laccaria Genomes at High Altitude. Journal of Fungi. 2025; 11(8):592. https://doi.org/10.3390/jof11080592
Chicago/Turabian StyleBao, Yu, Ye Mu, Jinghuan Hu, Mengchao Chen, and Jing Xing. 2025. "Genomic Analysis of Laccaria Genomes at High Altitude" Journal of Fungi 11, no. 8: 592. https://doi.org/10.3390/jof11080592
APA StyleBao, Y., Mu, Y., Hu, J., Chen, M., & Xing, J. (2025). Genomic Analysis of Laccaria Genomes at High Altitude. Journal of Fungi, 11(8), 592. https://doi.org/10.3390/jof11080592






