Fungal Pathogens in Pet Dogs and Cats in Grenada: Identification and Antifungal Susceptibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Processing, and Isolation
2.2. Morphological and Biochemical Identification
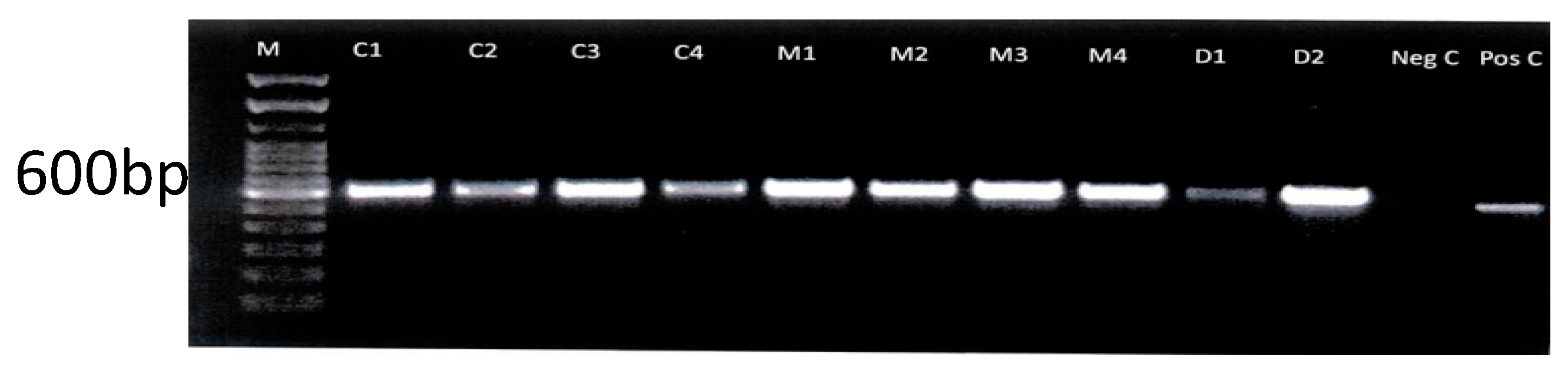
2.3. Molecular Confirmation of Isolates
2.4. Antifungal Susceptibility Testing
2.5. Data Analysis
3. Results
3.1. Morphological and Biochemical Identification
3.2. Molecular Confirmation of Isolates
3.3. Antifungal Susceptibility Results (AFST)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlotti, D.N. Canine and feline superficial fungal skin infections. Vet. Q. 1997, 19 (Suppl. S1), 45–46. [Google Scholar] [CrossRef] [PubMed]
- Chupia, V.; Ninsuwon, J.; Piyarungsri, K.; Sodarat, C.; Prachasilchai, W.; Suriyasathaporn, W.; Pikulkaew, S. Prevalence of Microsporum canis from Pet Cats in Small Animal Hospitals, Chiang Mai, Thailand. Vet. Sci. 2022, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Karacan Sever, N.; Üstün, T.; Omerovic, M.; Önol, M.; Zahiri, A.K.; Doğan, B. Prevalence of dermatophytes isolated from domestic animals in Ankara within a three-year period (2014–2017). Vet. J. Mehmet Akif Ersoy Univ. 2021, 6, 1–7. [Google Scholar] [CrossRef]
- Dworecka-Kaszak, B.; Biegańska, M.J.; Dąbrowska, I. Occurrence of various pathogenic and opportunistic fungi in skin diseases of domestic animals: A retrospective study. BMC Vet. Res. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; de Bosco, S.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56 (Suppl. S1), S165–S187. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Z.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Molecular Epidemiology, Genetic Diversity, and Antifungal Susceptibility of Major Pathogenic Dermatophytes Isolated From Human Dermatophytosis. Front. Microbiol. 2021, 12, 4643509. [Google Scholar] [CrossRef] [PubMed]
- Guillot, J.; Bond, R. Malassezia Yeasts in Veterinary Dermatology: An Updated Overview. Front. Cell. Infect. Microbiol. 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.P.; Carter, E.M.; Markey, B.; Carter, R.G. Clinical Veterinary Microbiology. Introduction to the pathogenic fungi. In Vet eBooks; Mosby International: London, UK, 1994; Chapter 38; pp. 367–394. [Google Scholar]
- White, T.C.; Findley, K.; Dawson, T.L., Jr.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the Skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.A.; Sorrell, T.C. Antifungal agents. Med. J. Aust. 2007, 187, 404–409. [Google Scholar] [CrossRef]
- Rayens, E.; Norris, K.A. Prevalence and Healthcare Burden of Fungal Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte Resistance to Antifungal Drugs: Mechanisms and Prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Chebil, W.; Haouas, N.; Eskes, E.; Vandecruys, P.; Belgacem, S.; Belhadj Ali, H.; Babba, H.; Van Dijck, P. In Vitro Assessment of Azole and Amphotericin B Susceptibilities of Malassezia spp. Isolated from Healthy and Lesioned Skin. J. Fungi 2022, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Releases First-Ever List of Health-Threatening Fungi. www.who.int. WHO. Available online: https://www.who.int/news/item/25-10-2022-who-releases-first-ever-list-of-health-threatening-fungi (accessed on 25 October 2022).
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Bures, A.; Pieper, J.B.; Bidot, W.A.; O’Dell, M.; Sander, W.E.; Maddox, C.W. Survey of dermatophytes in stray dogs and cats with and without skin lesions in Puerto Rico and confirmed with MALDI-TOF MS. PLoS ONE 2021, 16, e0257514. [Google Scholar] [CrossRef] [PubMed]
- Katiraee, F.; Kouchak Kosari, Y.; Soltani, M.; Shokri, H.; Hassan Minooieanhaghighi, M. Molecular identification and antifungal susceptibility patterns of dermatophytes isolated from companion animals with clinical symptoms of dermatophytosis. J. Vet. Res. 2021, 65, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 35, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Frías-De-León, M.G.; Martínez-Herrera, E.; Atoche-Diéguez, C.E.; Cespón, J.L.G.; Uribe, B.; Arenas, R.; Rodríguez-Cerdeira, C. Molecular identification of isolates of the Trichophyton mentagrophytes complex. Int. J. Med. Sci. 2020, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gräser, Y.; Kuijpers, A.F.A.; Presber, W.; Hoog, G.D. Molecular taxonomy of Trichophyton mentagrophytes and T. tonsurans. Med. Mycol. 1999, 37, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tamura, K.; Nei, M. MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Bioinformatics 1994, 10, 189–191. [Google Scholar] [CrossRef] [PubMed]
- NCCLS. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline. NCCLS Document M44-A; NCCLS. Clinical and Laboratory Standards Institute: Pennsylvania, PA, USA, 2008. [Google Scholar]
- Niae, S.; Yurayart, C.; Thengchaisri, N.; Sattasathuchana, P. Prevalence and in vitro antifungal susceptibility of commensal yeasts in the external ear canal of cats. BMC Vet. Res. 2021, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Copetti, M.V.; Santurio, J.M.; Cavalheiro, A.S.; Boeck, A.A.; Argenta, J.S.; Aguiar, L.C.; Alves, S.H. Dermatophytes isolated from dogs and cats suspected of dermatophytosis in Southern Brazil. Acta Sci. Vet. 2018, 34, 119–124. [Google Scholar] [CrossRef]
- Outerbridge, C.A. Mycologic Disorders of the Skin. Clin. Tech. Small Anim. Pract. 2006, 21, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Velegraki, A.; Cafarchia, C.; Gaitanis, G.; Iatta, R.; Boekhout, T. Malassezia Infections in Humans and Animals: Pathophysiology, Detection, and Treatment. PLoS Pathog. 2015, 11, e1004523. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Dokuzeylül, B.; Bakırel, U.; Or, M.E. Antifungal Resistance and Clinical Significance in Small Animals. German J. Vet. Res. 2022, 2, 28–36. [Google Scholar] [CrossRef]
- Lima, R.; Ribeiro, F.C.; Colombo, A.L.; de Almeida, J.N. The emerging threat antifungal-resistant Candida tropicalis in humans, animals, and environment. Front. Fungal Biol. 2022, 3, 957021. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.N.; Heick, T.M. Azole Use in Agriculture, Horticulture, and Wood Preservation–Is It Indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; An, N.; Yang, Y.; Yang, X.; Fan, Y.; Feng, J. Candida tropicalis distribution and drug resistance is correlated with ERG11 and UPC2 expression. Antimicrob. Resist. Infect. Control. 2021, 10, 54. [Google Scholar] [CrossRef] [PubMed]

| Animal | ||||
|---|---|---|---|---|
| Dog (n = 136) | Cat (n = 43) | Total (n = 179) | ||
| Age | <1 year old 1–5 years old >5 years old unknown | 15 (11%) 35 (25.7%) 47 (34.5%) 39 (28.6%) | 9 (20.9%) 19 (44.1%) 11 (25.5%) 4 (9.3%) | 24 (13.4%) 54 (30.1%) 58 (32.4%) 43 (24%) |
| Sex | Female Male Unknown | 77 (56.6%) 46 (33.8%) 13 (9.5%) | 18 ((41.8%) 25 (58.1) 0 (0%) | 95 (53%) 71 (39.65) 13 (7.2%) |
| Breed | Pot hound Mixed German shepherd Pitt bull Rottweiler Labrador Others Domestic short hair Siamese | 54 (39.7%) 55 (40.4%) 5 (3.6%) 6 (4.4%) 4 (2.9%) 7 (5.1%) 12 (8.8) | 42 (97.7%) 1 (2.3%) | |
| Variable | Categories | # Of Subjects | % Positive M. pachydermatis | % Positive C. tropicalis | % Positive Dermatophytes |
|---|---|---|---|---|---|
| Age | Young (<1 Year) | 24 | 16.6% | 4.1% | 0% |
| Adult (>1 Year) | 97 | 18.5% | 7.2% | 2% | |
| Species | Dog | 136 | 14.7% | 6.6% | 1.4% |
| Cat | 43 | 2.3% | 0% | 0% | |
| Ear samples | Dog | 234 | 11.5% | 3.4% | * |
| Cat | 32 | 6.2% | 0% | * | |
| Skin samples | Dog | 44 | 0% | 4.5% | * |
| Cat | 17 | 0% | 0% | * | |
| Hair samples | Dog | 57 | * | * | 3.6% |
| Cat | 21 | * | * | 0% | |
| p-value | 0.19 | 0.14 | 0.67 | ||
| Antifungal Drug | Malassezia (n = 56) | Candida (n = 21) | Dermatophyte (n = 2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #S | #I | #R | %R | #S | #I | #R | %R | #S | #I | #R | %R | |
| Voriconazole | 54 | 0 | 0 | 0% | 2 | 19 | 0 | 0% | 0 | 2 | 100% | |
| Itraconazole | 42 | * | 14 | 25% | 10 | * | 11 | 52% | 2 | 0 | 0% | |
| Fluconazole | 0 | 0 | 56 | 100% | 0 | 0 | 21 | 100% | * | * | 2 | 100% |
| Ketoconazole | 56 | * | 0 | 0% | 0 | * | 21 | 100% | * | * | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brathwaite, E.H.-A.; Kumar, K.; Dolphin-Bond, G.; Sylvester, W.; Amadi, V.; Alhassan, A. Fungal Pathogens in Pet Dogs and Cats in Grenada: Identification and Antifungal Susceptibility. J. Fungi 2025, 11, 590. https://doi.org/10.3390/jof11080590
Brathwaite EH-A, Kumar K, Dolphin-Bond G, Sylvester W, Amadi V, Alhassan A. Fungal Pathogens in Pet Dogs and Cats in Grenada: Identification and Antifungal Susceptibility. Journal of Fungi. 2025; 11(8):590. https://doi.org/10.3390/jof11080590
Chicago/Turabian StyleBrathwaite, Erica Hazel-Ann, Kamashi Kumar, Grace Dolphin-Bond, Wayne Sylvester, Victor Amadi, and Andy Alhassan. 2025. "Fungal Pathogens in Pet Dogs and Cats in Grenada: Identification and Antifungal Susceptibility" Journal of Fungi 11, no. 8: 590. https://doi.org/10.3390/jof11080590
APA StyleBrathwaite, E. H.-A., Kumar, K., Dolphin-Bond, G., Sylvester, W., Amadi, V., & Alhassan, A. (2025). Fungal Pathogens in Pet Dogs and Cats in Grenada: Identification and Antifungal Susceptibility. Journal of Fungi, 11(8), 590. https://doi.org/10.3390/jof11080590






