Oral Candidiasis Associated with Aging and Salivary Hypofunction in Stomatitis Patients
Abstract
1. Introduction
2. Methods
2.1. Study Population and Study Design
2.2. Diagnosis of Oral Candidiasis
2.3. Clinical Characteristics
2.4. Measurement of Salivary Flow Rate and Diagnostic Criteria for Xerostomia
3. Statistical Analysis
4. Results
4.1. Demographics
4.2. Pain, Lesion Distribution, and Oral Hygiene
4.3. Salivary Flow Rate and Xerostomia
4.4. Systemic Diseases
4.5. Factors Associated with Oral Candidiasis
4.6. The Cutoff Value for Oral Candidiasis
4.7. Distribution of Oral Candidiasis and Xerostomia by Age
4.8. Generalized Linear Model for Oral Candidiasis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent Aphthous Stomatitis: A Review. J. Clin. Aesthet. Dermatol. 2017, 10, 26–36. [Google Scholar] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Deepa, A.; Nair, B.J.; Sivakumar, T.; Joseph, A.P. Uncommon opportunistic fungal infections of oral cavity: A review. J. Oral Maxillofac. Pathol. 2014, 18, 235–243. [Google Scholar] [CrossRef]
- Kaplan, S.; Xie, H.; Wang, J. Estimating the prevalence and incidence of multiple system atrophy in the USA: Insights from a national claims database. Park. Relat. Disord. 2023, 117, 105920. [Google Scholar] [CrossRef]
- Sheetal, A.; Hiremath, V.K.; Patil, A.G.; Sajjansetty, S.; Kumar, S.R. Malnutrition and its oral outcome—A review. J. Clin. Diagn. Res. 2013, 7, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Manoj, M.A.; Jain, A.; Madtha, S.A.; Cherian, T.M. Prevalence and risk factors of recurrent aphthous stomatitis among college students at Mangalore, India. PeerJ 2023, 11, e14998. [Google Scholar] [CrossRef]
- Qi, X.; Northridge, M.E.; Hu, M.; Wu, B. Oral health conditions and COVID-19: A systematic review and meta-analysis of the current evidence. Aging Health Res. 2022, 2, 100064. [Google Scholar] [CrossRef]
- Molek, M.; Florenly, F.; Lister, I.N.E.; Wahab, T.A.; Lister, C.; Fioni, F. Xerostomia and hyposalivation in association with oral candidiasis: A systematic review and meta-analysis. Evid.-Based Dent. 2022. [Google Scholar] [CrossRef]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, L.; Guida, A.; Petruzzi, M.; Serpico, R. Candida-associated denture stomatitis. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e139–e143. [Google Scholar] [CrossRef] [PubMed]
- Gleiznys, A.; Zdanavičienė, E.; Žilinskas, J. Candida albicans importance to denture wearers: A literature review. Stomatologija 2015, 17, 54–66. [Google Scholar]
- Snarr, B.D.; Drummond, R.A.; Lionakis, M.S. It’s all in your head: Antifungal immunity in the brain. Curr. Opin. Microbiol. 2020, 58, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.G.J.; Veringa, A.; Marriott, D.J.E.; Boonstra, J.M.; van der Elst, K.C.M.; Doukas, F.F.; McLachlan, A.J.; Alffenaar, J.C. Invasive Candidiasis in the Elderly: Considerations for Drug Therapy. Drugs Aging 2018, 35, 781–789. [Google Scholar] [CrossRef]
- Salvatori, O.; Puri, S.; Tati, S.; Edgerton, M. Innate Immunity and Saliva in Candida albicans-mediated Oral Diseases. J. Dent. Res. 2016, 95, 365–371. [Google Scholar] [CrossRef]
- Patel, M. Oral Cavity and Candida albicans: Colonisation to the Development of Infection. Pathogens 2022, 11, 335. [Google Scholar] [CrossRef]
- Kashyap, B.; Padala, S.R.; Kaur, G.; Kullaa, A. Candida albicans Induces Oral Microbial Dysbiosis and Promotes Oral Diseases. Microorganisms 2024, 12, 2138. [Google Scholar] [CrossRef]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Légeret, C.; Furlano, R. Oral ulcers in children—A clinical narrative overview. Ital. J. Pediatr. 2021, 47, 144. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Lucariello, A.; Colella, G.; De Luca, A.; Marinelli, P. Rapid identification of Candida species in oral rinse solutions by PCR. J. Clin. Pathol. 2007, 60, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef]
- Perić, M.; Miličić, B.; Kuzmanović Pfićer, J.; Živković, R.; Arsić Arsenijević, V. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida spp. Distribution. J. Fungi 2024, 10, 328. [Google Scholar] [CrossRef]
- Marcotte, H.; Lavoie, M.C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998, 62, 71–109. [Google Scholar] [CrossRef]
- Gonsalves, W.C.; Wrightson, A.S.; Henry, R.G. Common oral conditions in older persons. Am. Fam. Physician 2008, 78, 845–852. [Google Scholar]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Vissink, A.; Spijkervet, F.K.; Van Nieuw Amerongen, A. Aging and saliva: A review of the literature. Spec. Care Dent. 1996, 16, 95–103. [Google Scholar] [CrossRef]
- Abu Eid, R.; Sawair, F.; Landini, G.; Saku, T. Age and the architecture of oral mucosa. Age 2012, 34, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Dong, N.; Wu, L.; Zhang, X.; Li, H.; Wu, H.; Ward, N.; Yu, J.; Liu, H.; Wang, J.; et al. Promoting oral mucosal wound healing using a DCS-RuB(2)A(2) hydrogel based on a photoreactive antibacterial and sustained release of BMSCs. Bioact. Mater. 2023, 23, 53–68. [Google Scholar] [CrossRef]
- Lee, Y.H.; Won, J.H.; Auh, Q.S.; Noh, Y.K.; Lee, S.W. Prediction of xerostomia in elderly based on clinical characteristics and salivary flow rate with machine learning. Sci. Rep. 2024, 14, 3423. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, S.W.; Chung, S.C.; Kim, Y.K.; Kho, H.S. Analysis of residual saliva and minor salivary gland secretions in patients with dry mouth. Arch. Oral Biol. 2002, 47, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Gomar-Vercher, S.; Simón-Soro, A.; Montiel-Company, J.M.; Almerich-Silla, J.M.; Mira, A. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLoS ONE 2018, 13, e0198021. [Google Scholar] [CrossRef]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Bianchi, C.M.; Bianchi, H.A.; Tadano, T.; Paula, C.R.; Hoffmann-Santos, H.D.; Leite, D.P., Jr.; Hahn, R.C. Factors related to oral Candidiasis in elderly users and non-users of removable dental prostheses. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 17. [Google Scholar] [CrossRef]
- Kennedy, M.S.; Chang, E.B. The microbiome: Composition and locations. Prog. Mol. Biol. Transl. Sci. 2020, 176, 1–42. [Google Scholar] [CrossRef]
- Sureda, A.; Daglia, M.; Argüelles Castilla, S.; Sanadgol, N.; Fazel Nabavi, S.; Khan, H.; Belwal, T.; Jeandet, P.; Marchese, A.; Pistollato, F.; et al. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef]
- Laurent, M.; Gogly, B.; Tahmasebi, F.; Paillaud, E. Oropharyngeal candidiasis in elderly patients. Geriatr. Psychol. Neuropsychiatr. Vieil. 2011, 9, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Weerasuriya, N.; Snape, J. Oesophageal candidiasis in elderly patients: Risk factors, prevention and management. Drugs Aging 2008, 25, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Iandolo, A.; Amato, A.; Caggiano, M.; Raimondo, A.; Lembo, S.; Martina, S. Prevalence, Features and Degree of Association of Oral Lesions in COVID-19: A Systematic Review of Systematic Reviews. Int. J. Environ. Res. Public Health 2022, 19, 7486. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The power of saliva: Antimicrobial and beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef] [PubMed]
- Tamai, R.; Kiyoura, Y. Candida Infections: The Role of Saliva in Oral Health—A Narrative Review. Microorganisms 2025, 13, 717. [Google Scholar] [CrossRef] [PubMed]



| Stomatitis Without Oral Candidiasis (n = 178) | Stomatitis with Oral Candidiasis (n = 81) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) a | 57.73 ± 16.10 | 64.25 ± 14.66 | 0.002 ** |
| Sex b | |||
| Male | 46 (25.8%) | 12 (14.8%) | 0.048 * |
| Female | 132 (74.2%) | 69 (85.2%) | |
| Clinical symptoms | |||
| VAS (0–10) a | 3.47 ± 3.07 | 3.89 ± 2.82 | 0.279 |
| Symptom duration (months) a | 16.39 ± 30.45 | 12.65 ± 35.12 | 0.033 * |
| Chronicity b | 94 (52.8%) | 32 (39.5%) | 0.047 * |
| Lesion site | |||
| Tongue b | 99 (55.6%) | 43 (53.1%) | 0.788 |
| Buccal mucosa b | 17 (9.6%) | 11 (13.6%) | 0.333 |
| Lips b | 8 (4.5%) | 12 (14.8%) | 0.010 * |
| Gingiva b | 6 (3.4%) | 3 (3.7%) | 1.000 |
| Palate b | 7 (3.9%) | 1 (1.2%) | 0.245 |
| Entire mouth b | 68 (38.2%) | 28 (34.6%) | 0.575 |
| Number of lesion sites b | 1.12 ± 0.47 | 1.17 ± 0.52 | 0.418 |
| Oral hygiene b | |||
| Good | 39 (21.9%) | 13 (16.0%) | 0.547 |
| Acceptable | 126 (70.8%) | 62 (76.5%) | |
| Poor | 13 (7.3%) | 6 (7.4%) |
| Stomatitis Without Oral Candidiasis (n = 178) | Stomatitis with Oral Candidiasis (n = 81) | p-Value | |
|---|---|---|---|
| Salivary flow rate | |||
| UFR (mL/min) a | 0.47 ± 0.28 | 0.36 ± 0.32 | 0.006 ** |
| SFR (mL/min) a | 1.41 ± 0.69 | 1.21 ± 0.68 | 0.032 * |
| Xerostomia_UFR b | 33 (18.5%) | 40 (49.4%) | <0.001 *** |
| Xerostomia_SFR b | 19 (10.7%) | 22 (27.2%) | 0.002 ** |
| Systemic diseases | |||
| Hypertension b | 66 (37.1%) | 30 (37.0%) | 1.000 |
| Diabetes mellitus b | 21 (11.8%) | 11 (13.6%) | 0.687 |
| Osteoporosis b | 9 (5.1%) | 2 (2.5%) | 0.511 |
| Cardiovascular diseases b | 14 (7.9%) | 3 (3.7%) | 0.283 |
| Number of systemic diseases a | 0.62 ± 0.84 | 0.57 ± 0.71 | 0.619 |
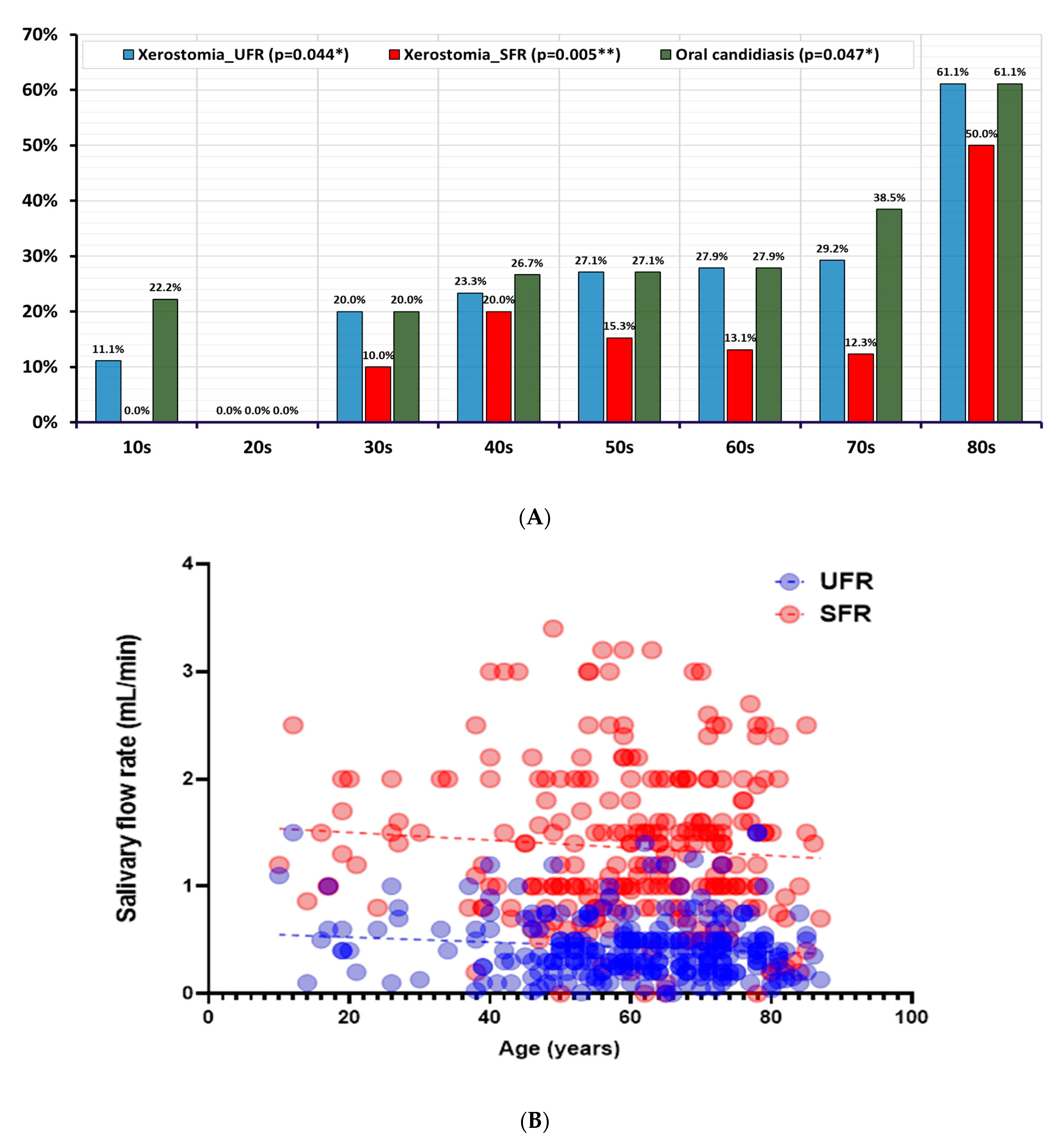
| 10s (n = 9) | 20s (n = 7) | 30s (n = 10) | 40s (n = 30) | 50s (n = 59) | 60s (n = 61) | 70s (n = 65) | 80s (n = 18) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Proportion of oral candidiasis | |||||||||
| Oral candidiasis | 2 (22.2%) | 0 (0.0%) | 2 (20.0%) | 8 (26.7%) | 16 (27.1%) | 17 (27.9%) | 25 (38.5%) | 11 (61.1%) | 0.047 * |
| Quantity of Candida albicans | |||||||||
| None | 7 (77.8%) | 6 (85.7%) | 8 (0.8%) | 22 (73.3%) | 43 (72.9%) | 44 (72.1%) | 40 (61.5%) | 7 (38.9%) | 0.238 |
| A few | 2 (22.2%) | 1 (14.3%) | 1 (0.1%) | 3 (0.1%) | 7 (11.9%) | 7 (11.5%) | 10 (15.4%) | 1 (5.6%) | |
| Moderate | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 2 (6.7%) | 5 (8.5%) | 3 (4.9%) | 5 (7.7%) | 4 (22.2%) | |
| Many | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (10.0%) | 4 (6.8%) | 7 (11.5%) | 10 (15.4%) | 6 (33.3%) | |
| Xerostomia | |||||||||
| Xerostomia_UFR | 1 (11.1%) | 0 (0.0%) | 2 (20.0%) | 7 (23.3%) | 18 (27.1%) | 17 (27.9%) | 19 (29.2%) | 11 (61.1%) | 0.044 * |
| Xerostomia_SFR | 0 (0.0%) | 0 (0.0%) | 1 (10.0%) | 6 (20.0%) | 9 (15.3%) | 8 (13.1%) | 8 (12.3%) | 9 (50.0%) | 0.005 ** |
| Parameter | B | SE | 95% CI Lower | 95% CI Upper | p-Value |
| Female [ref. = male] | 0.110 | 0.065 | −0.017 | 0.237 | 0.090 |
| Xerostomia_UFR [ref. = none] | 0.328 | 0.077 | 0.177 | 0.480 | <0.001 *** |
| Xerostomia_SFR [ref. = none] | 0.059 | 0.092 | −0.120 | 0.239 | 0.517 |
| Age | 0.001 | 0.009 | −0.017 | 0.019 | 0.907 |
| Symptom duration | 0.016 | 0.049 | −0.080 | 0.112 | 0.743 |
| Number of systemic diseases | 0.001 | 0.007 | −0.013 | 0.018 | 0.915 |
| Lips [ref. = none] | 0.280 | 0.099 | 0.085 | 0.475 | 0.005 ** |
| Constant | 0.178 | 0.016 | 0.150 | 0.212 | 0.168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Seo, S.; Kim, T.-S.; Lee, S.-w. Oral Candidiasis Associated with Aging and Salivary Hypofunction in Stomatitis Patients. J. Fungi 2025, 11, 574. https://doi.org/10.3390/jof11080574
Lee Y-H, Seo S, Kim T-S, Lee S-w. Oral Candidiasis Associated with Aging and Salivary Hypofunction in Stomatitis Patients. Journal of Fungi. 2025; 11(8):574. https://doi.org/10.3390/jof11080574
Chicago/Turabian StyleLee, Yeon-Hee, Solsol Seo, Tae-Seok Kim, and Sang-woo Lee. 2025. "Oral Candidiasis Associated with Aging and Salivary Hypofunction in Stomatitis Patients" Journal of Fungi 11, no. 8: 574. https://doi.org/10.3390/jof11080574
APA StyleLee, Y.-H., Seo, S., Kim, T.-S., & Lee, S.-w. (2025). Oral Candidiasis Associated with Aging and Salivary Hypofunction in Stomatitis Patients. Journal of Fungi, 11(8), 574. https://doi.org/10.3390/jof11080574






