Molecular and Morphological Evidence Reveals Four New Neocosmospora Species from Dragon Trees in Yunnan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Strains Isolation
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Phylogenetic Analyses
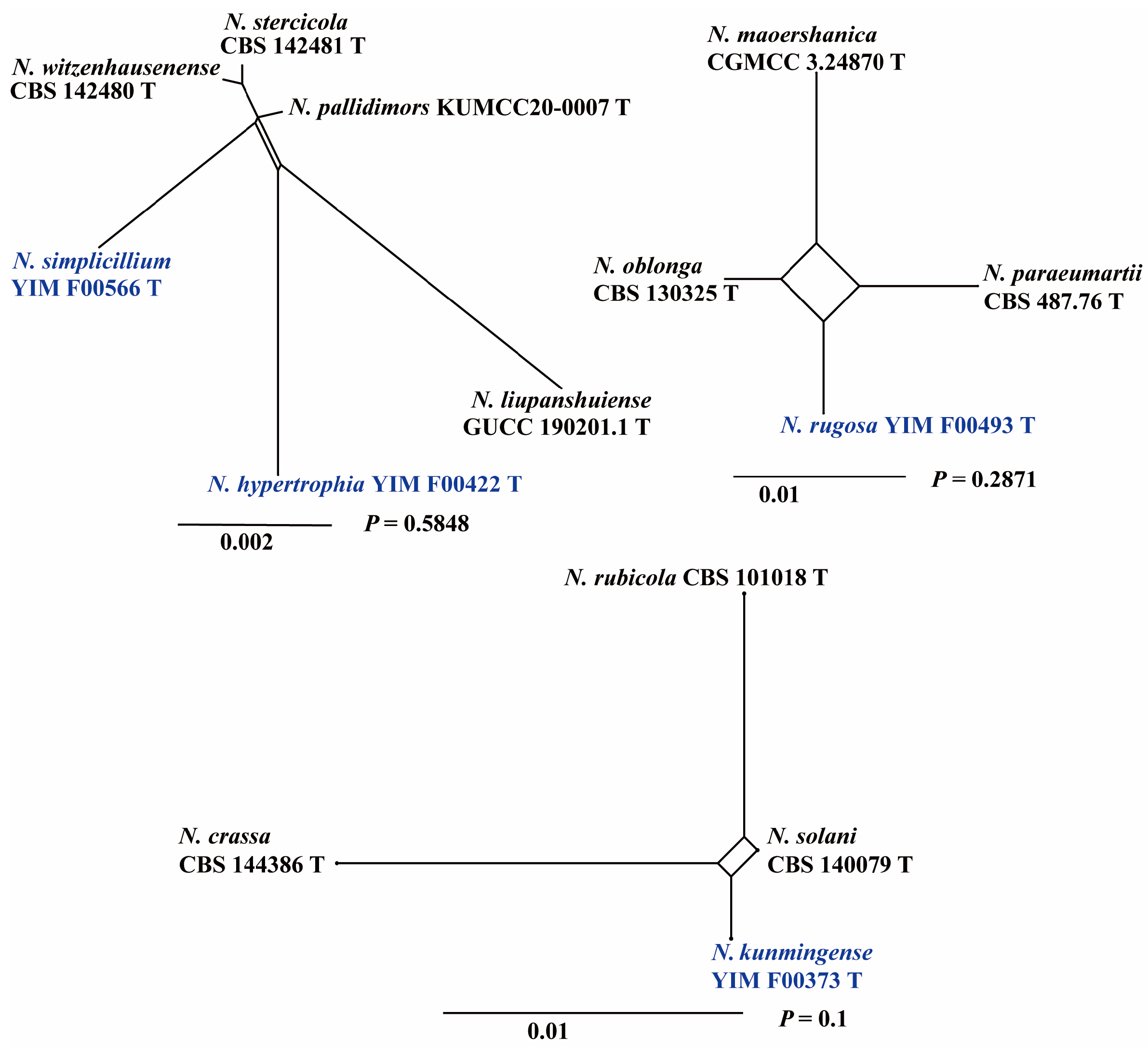
2.4. Genealogical Concordance Phylogenetic Species Recognition Analysis
2.5. Morphological Observation
3. Results
3.1. Multi-Gene Phylogeny
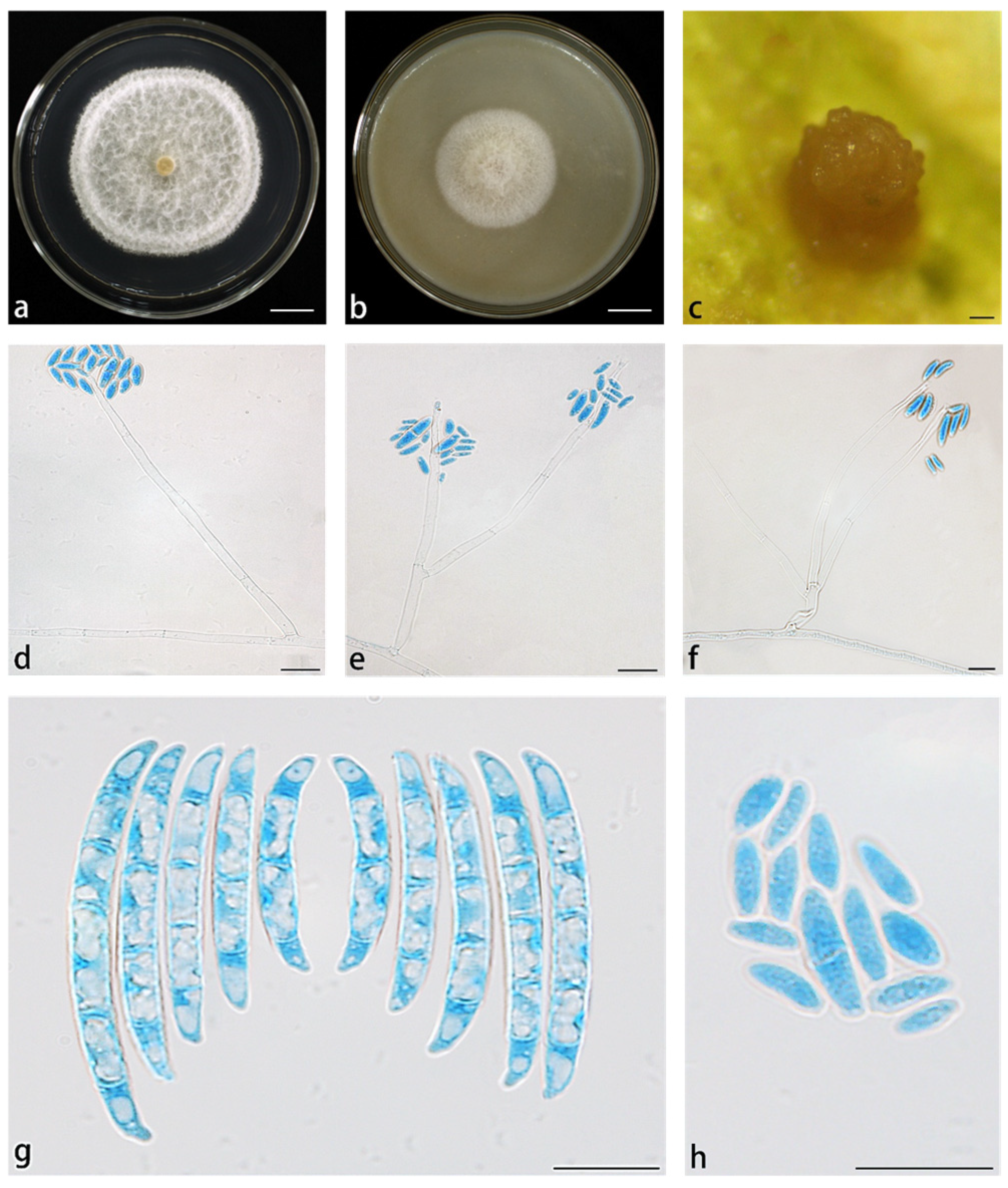
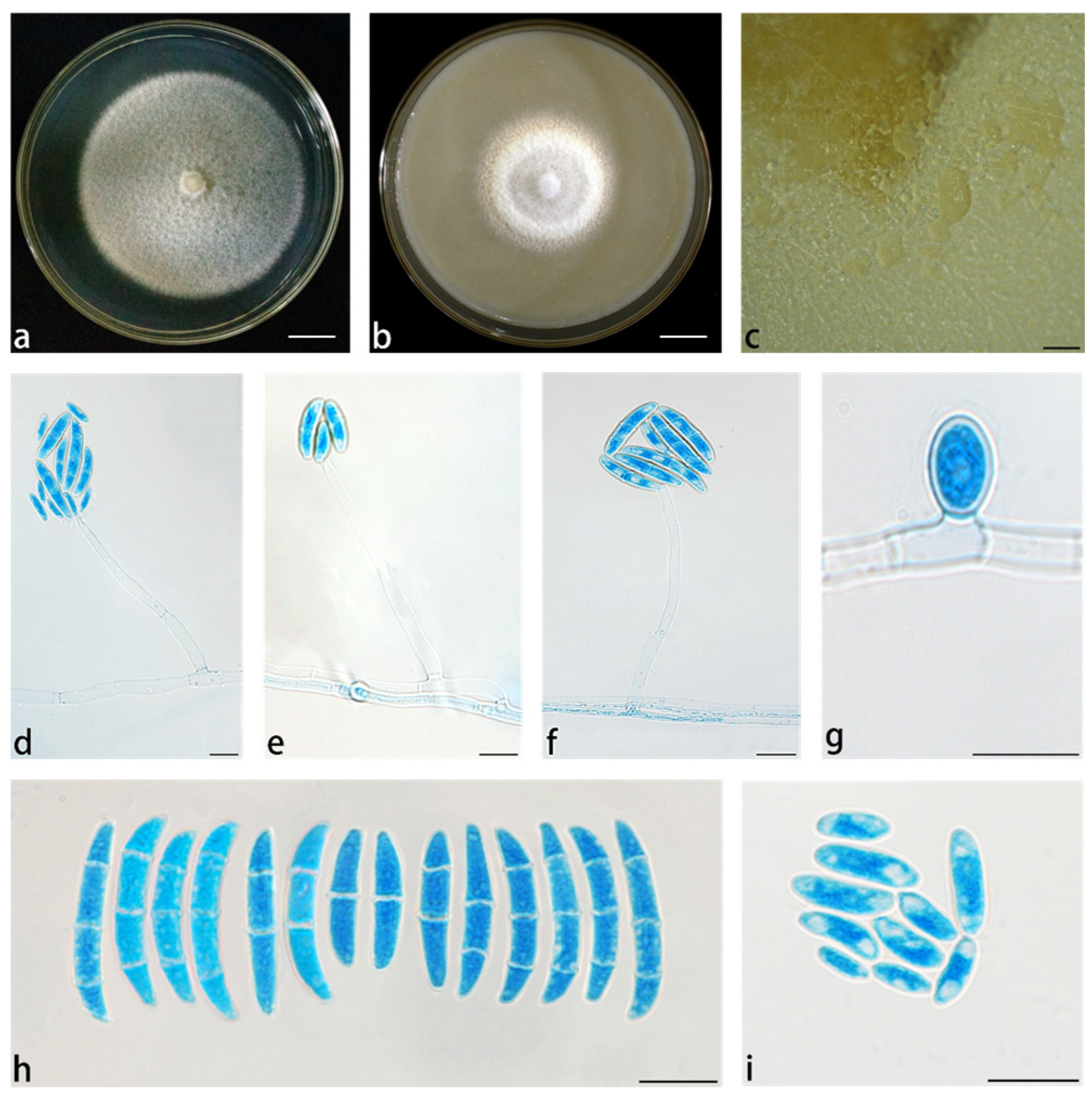
3.2. Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.H.; Zhang, C.H.; Yanga, L.L.; Laranjo, J.G. Production of dragon’s blood in Dracaena cochinchinensis plants by inoculation of Fusarium proliferatum. Plant Sci. 2011, 180, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.F.; Ma, P.; Wang, X.H.; Zhang, L.Q.; Li, Q.D.; Wang, J.L.; Cheng, Z.Y.; Yang, C.G. The studies of fungal population and relationship between fungi and forming of Dragon’s blood resin in Dracaena cochinchinensis. Acta Bot. Yunanica 1995, 17, 79–82. [Google Scholar]
- Wang, X.H.; Zhang, C.H.; Yang, L.L.; Yang, X.H.; Lou, J.D.; Cao, Q.E.; Laranjo, J.G. Enhanced dragon’s blood production in Dracaena cochinchinensis by elicitation of Fusarium oxysporum strains. J. Med. Plants Res. 2010, 4, 2633–2640. [Google Scholar]
- Wang, X.H.; Gong, M.; Tang, L.; Zheng, S.; Lou, J.D.; Ou, L.; Gomes-Laranjo, J.; Zhang, C. Cloning, bioinformatics and the enzyme activity analyses of a phenylalanine ammonia-lyase gene involved in dragon’s blood biosynthesis in Dracaena cambodiana. Mol. Biol. Rep. 2013, 40, 97–107. [Google Scholar] [CrossRef]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gene, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100–116. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Lombard, L.; Crous, P.W. Back to the roots: A reappraisal of Neocosmospora. Persoonia 2019, 43, 90–185. [Google Scholar] [CrossRef]
- Carpenter, C.W. Some potato tuber-rots caused by species of Fusarium. J. Agric. Res. 1915, 5, 183–209. [Google Scholar]
- Balmas, V.; Scherm, B.; Marcello, A.; Beyer, M.; Hoffmann, L.; Migheli, Q.; Pasquali, M. Fusarium species and chemotypes associated with Fusarium head blight and Fusarium root rot on wheat in Sardinia. Plant Pathol. 2015, 64, 972–979. [Google Scholar] [CrossRef]
- Porter, L.D.; Pasche, J.S.; Chen, W.; Harveson, R.M. Isolation, identification, storage, pathogenicity tests, hosts, and geographic range of Fusarium solani f. sp. pisi causing Fusarium root rot of pea. Plant Health Prog. 2015, 16, 136–145. [Google Scholar] [CrossRef]
- Federico, G.R.; Maria, M.R.; Marcela, F.; Sofía, N.C.; Adriana, M.T. Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Prot. 2007, 26, 549–555. [Google Scholar]
- McClure, T.T. Fusarium foot rot of sweet-potato sprouts. Phytopathology 1951, 41, 72–77. [Google Scholar]
- Nirenberg, H.I. A simplified method for identifying Fusarium spp. occurring on wheat. Can. J. Bot. 1981, 59, 1599–1609. [Google Scholar] [CrossRef]
- O’Donnell, K.; Al-Hatmi, A.M.; Aoki, T.; Brankovics, B.; Cano-Lira, J.F.; Coleman, J.J.; De Hoog, G.S.; Di Pietro, A.; Frandsen, R.J.; Geiser, D.M.J.M. No to Neocosmospora: Phylogenomic and practical peasons for continued inclusion of the Fusarium solani species complex in the genus Fusarium. mSphere 2020, 5, e00810-20. [Google Scholar] [CrossRef]
- Chowdhury, N.S.; Sohrab, M.H.; Rana, M.S.; Hasan, C.M.; Jamshidi, S.; Rahman, K.M. Cytotoxic naphthoquinone and azaanthraquinone derivatives from an endophytic Fusarium solani. J. Nat. Prod. 2017, 80, 1173–1177. [Google Scholar] [CrossRef]
- Khan, N.; Afroz, F.; Begum, M.N.; Roy Rony, S.; Sharmin, S.; Moni, F.; Mahmood Hasan, C.; Shaha, K.; Sohrab, M.H. Endophytic Fusarium solani: A rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol. Rep. 2018, 5, 970–976. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, H.; Wang, H.; Zong, M.; Lou, W. Immune enhancement activity of a novel polysaccharide produced by dendrobium officinale endophytic fungus Fusarium solani DO7. J. Funct. Foods 2019, 53, 266–275. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mahmoud, B.K.; Millán-Aguiñaga, N.; Abdelmohsen, U.R.; Fouad, M.A. The endophytic Fusarium strains: A treasure trove of natural products. RSC Adv. 2023, 13, 1339–1369. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.; Klomchit, A.; Calabon, M.S.; Chomnunti, P.; Worabandit, S. Novel hypocrealean fungi from Rhizophoraceae, Neocosmospora mangrovei sp. nov. and Neocosmospora ceriopis sp. nov., and their antifungal activity against anthracnose pathogen Colletotrichum spp. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Klomchit, A.; Calabon, M.S.; Worabandit, S.; Weaver, J.A.; Karima, E.M.; Alberti, F.; Greco, C.; Mahanil, S. Unveiling novel Neocosmospora species from Thai mangroves as potent biocontrol agents against Colletotrichum species. J. Appl. Microbiol. 2024, 135, lxae114. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Jiang, Z.; Zhang, X.; Wei, S.; Wu, Y.; Gan, X.; Wang, Y.; Xie, X. Two strains Neocosmospora stercicola (Sordariomycetes, Nectriaceae) with high nematicidal activity, isolated from the cysts of Globodera sp. (Heteroderidae) in China. Biodivers. Data J. 2023, 11, e100684. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Sandoval-Denis, M.; Costa, M.M.; Groenewald, J.Z.; van Iperen, A.L.; Starink-Willemse, M.; Hernández-Restrepo, M.; Kandemir, H.; Ulaszewski, B.; de Boer, W.; et al. Fusarium and allied fusarioid taxa (FUSA). 1. Fungal Syst. Evol. 2022, 9, 161–200. [Google Scholar] [CrossRef]
- Zeng, Z.Q.; Zhuang, W.Y. New Species of Neocosmospora (Ascomycota) from China as evidenced by morphological and molecular data. Life 2023, 13, 1515. [Google Scholar] [CrossRef]
- Bolboli, Z.; Mostowfizadeh-Ghalamfarsa, R.; Sandoval-Denis, M.; Jafari, M.; Crous, P.W. Neocosmospora caricae sp. nov. and N. metavorans, two new stem and trunk canker pathogens on Ficus carica in Iran. Mycol. Prog. 2022, 21, 89. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Groenewald, J.Z.; Crous, P.W. Neocosmospora lechatii (Nectriaceae) a new species from French Guiana. Ascomycete.Org 2022, 14, 165–171. [Google Scholar]
- Zhang, H.; Zeng, Y.; Wei, T.P.; Jiang, Y.L.; Zeng, X.Y. Endophytic Fusarium and allied fungi from Rosa roxburghii in China. Mycosphere 2023, 14, 2092–2207. [Google Scholar] [CrossRef]
- Herron, D.A.; Wingfield, M.J.; Wingfield, B.D.; Rodas, C.A.; Marincowitz, S.; Steenkamp, E.T. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud. Mycol. 2015, 80, 131–150. [Google Scholar]
- Wang, Y.B.; Wang, Y.; Fan, Q.; Duan, D.E.; Zhang, G.D.; Dai, R.Q.; Dai, Y.D.; Zeng, W.B.; Chen, Z.H.; Li, D.D.; et al. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): New taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers. 2020, 103, 1–46. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 38, 315–322. [Google Scholar]
- O’Donnell, K.; Kistler, C.H.; Cilgenik, E.; Ploetz, R. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Ploetz, R. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Reeb, V.; Lutzoni, F.; Roux, C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet. Evol. 2004, 32, 1036–1060. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Sun, B.L. Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 4599–4603. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software v. 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.E.; Zoller, S.; Lutzoni, F. Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chainMonte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 2003, 20, 255–266. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree visualization by one table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, 587–589. [Google Scholar] [CrossRef]
- Bruen, T.C.; Philippe, H.; Bryant, D. A simple and robust statistical test for detecting the presence of recombination. Genetics 2006, 172, 2665–2681. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Fisher, N.L.; Burgess, L.W.; Toussoun, T.A.; Nelson, P.E. Carnation leaves as a substrate and for preserving cultures of Fusarium species. Am. Phytopathol. Soc. 1982, 72, 151–153. [Google Scholar] [CrossRef]
- Nirenberg, H.I.; O′Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef]
- Gräfenhan, T.; Schroers, H.J.; Nirenberg, H.I.; Seifert, K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Schroers, H.J.; Gräfenhan, T.; Nirenberg, H.I.; Seifert, K.A. A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Stud. Mycol. 2011, 68, 115–138. [Google Scholar] [CrossRef]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Crous, P.W. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Persoonia 2018, 41, 109–129. [Google Scholar] [CrossRef]
- Geiser, D.M.; Aoki, T.; Bacon, C.W.; Baker, S.E.; Bhattacharyya, M.K.; Brandt, M.E.; Brown, D.W.; Burgess, L.W.; Chulze, S.; Coleman, J.J.; et al. One fungus, one name: Defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 2013, 103, 400–408. [Google Scholar] [CrossRef]
- Geiser, D.M.; Al-Hatmi, A.M.S.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.; Blomquist, C.L.; Bowden, R.L.; et al. Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani species complex. Phytopathology 2021, 111, 1064–1079. [Google Scholar] [CrossRef]
- Riaz, M.; Akhtar, N.; Msimbira, L.A.; Antar, M.; Ashraf, S.; Khan, S.N.; Smith, D.L. Neocosmospora rubicola, a stem rot disease in potato: Characterization, distribution and management. Front. Microbiol. 2022, 13, 953097. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, S.C.; Yang, D.Y.; Seigi Karasaki, S.; Tibpromma, S.; Hyde, K.D.; Lumyong, S.; Xu, J.C.; Sheng, J.; Mortimera, P.E. Discovery of novel fungal species and pathogens on bat carcasses in a cave in Yunnan province, China. Emerg. Microbes Infect. 2020, 9, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef]
- Liu, F.; Hou, L.; Raza, M.; Cai, L. Pestalotiopsis and allied genera from Camellia, with description of 11 new species from China. Sci. Rep. 2017, 7, 866. [Google Scholar] [CrossRef] [PubMed]
- Norphanphoun, C.; Jayawardena, R.S.; Chen, Y.; Wen, T.C.; Meepol, W.; Hyde, K.D. Morphological and phylogenetic characterization of novel pestalotioid species associated with mangroves in Thailand. Mycosphere 2019, 10, 531–578. [Google Scholar] [CrossRef]
- Peng, C.; Crous, P.W.; Jiang, N.; Fan, X.L.; Liang, Y.M.; Tian, C.M. Diversity of Sporocadaceae (pestalotioid fungi) from Rosa in China. Persoonia 2022, 49, 201–260. [Google Scholar] [CrossRef]



| Gene | Primer | 5′–3′ Sequence | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| ITS | ITS1 | TCCGTAGGTGAACCTGCGG | 55 | [29] |
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| nrLSU | LR5 | ATCCTGAGGGAAACTTC | 52 | [30] |
| LR0R | GTACCCGCTGAACTTAAGC | |||
| tef1 | EF1 | ATGGGTAAGGARGACAAGAC | 57 | [31] |
| EF2 | GGARGTACCAGTSATCATG | |||
| rpb1 | RPB1-Fa | CAYAARGARTCYATGATGGGWC | 58 (5 cycles) → 57 (5) → 56 (35) | [32] |
| RPB1-G2R | GTCATYTGDGTDGCDGGYTCDCC | |||
| rpb2 | RPB2-5f2 | GGGGWGAYCAGAAGAAGGC | 59 | [33] |
| RPB2-7cr | CCCATRGCTTGYTTRCCCAT | |||
| RPB2-7cf | ATGGGYAARCAAGCYATGGG | 58 | [34] | |
| RPB2-11ar | GCRTGGATCTTRTCRTCSACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Fan, Q.; Yang, Z.-S.; Wang, Y.-B.; Wang, X.-H.; Zeng, W.-B. Molecular and Morphological Evidence Reveals Four New Neocosmospora Species from Dragon Trees in Yunnan Province, China. J. Fungi 2025, 11, 571. https://doi.org/10.3390/jof11080571
Jia M, Fan Q, Yang Z-S, Wang Y-B, Wang X-H, Zeng W-B. Molecular and Morphological Evidence Reveals Four New Neocosmospora Species from Dragon Trees in Yunnan Province, China. Journal of Fungi. 2025; 11(8):571. https://doi.org/10.3390/jof11080571
Chicago/Turabian StyleJia, Mei, Qi Fan, Zu-Shun Yang, Yuan-Bing Wang, Xing-Hong Wang, and Wen-Bo Zeng. 2025. "Molecular and Morphological Evidence Reveals Four New Neocosmospora Species from Dragon Trees in Yunnan Province, China" Journal of Fungi 11, no. 8: 571. https://doi.org/10.3390/jof11080571
APA StyleJia, M., Fan, Q., Yang, Z.-S., Wang, Y.-B., Wang, X.-H., & Zeng, W.-B. (2025). Molecular and Morphological Evidence Reveals Four New Neocosmospora Species from Dragon Trees in Yunnan Province, China. Journal of Fungi, 11(8), 571. https://doi.org/10.3390/jof11080571







