Degradation of Synthetic Restoration Materials by Xerotolerant/Xerophilic Fungi Contaminating Canvas Paintings
Abstract
1. Introduction
2. Materials and Methods
2.1. Film Preparation and Artificial Aging
| Material | Form Used in This Study | Usage | Chemical Composition | Reference |
|---|---|---|---|---|
| Skin Glue | Solution (Samson Kamnik, Kamnik, Slovenia) | Consolidation, local sanation, gluing | Gelatin and other protein residues of collagen, keratin, or elastin | [1] |
| Lascaux Acrylic Glue 303 HV | Original form (Lascaux Colours & Restauro, Wangen-Brüttisellen, Switzerland) | Consolidation, local sanation, gluing | Thermoplastic copolymeric butyl-methacrylate dispersion, thickened with acrylic ester acid | [41] |
| Lascaux Acrylic Glue 498 HV | Original form (Lascaux Colours & Restauro, Wangen-Brüttisellen, Switzerland) | Consolidation, local sanation, gluing | Thermoplastic copolymeric butyl-methacrylate dispersion, thickened with acrylic ester acid | [42] |
| Acrylharz P550 | Original form (Lascaux Colours & Restauro, Wangen-Brüttisellen, Switzerland) | Consolidation | Organic solution of an acrylic resin on the basis of butyl methacrylate (diluted in white spirit) | [43] |
| Laropal A81 | Solution (Kremer Pigmente, Aichstetten, Germany) | Varnish, binder for pigments | Aldehyde resin-condensation product of urea and aliphatic aldehydes | [12,44] |
| BEVA 371 | Original form (CTS, Briosco, Italy) | Consolidation, local sanation, gluing | Ethylene vinyl acetate copolymer (Elvax 150), cyclohexanone resin (LaropalK80), ethylene vinyl acetate copolymer (A-C 400), phtalate ester of hydroabietyl acid (Cellolyn 21), and paraffinic hydrocarbons (Paraffin). Solvents make up 60% of the solution and are a combination of toluene and petroleum. | [45] |
| Regalrez 1094 | Solution (Kremer Pigmente, Aichstetten, Germany) | Varnish | Low-molecular-weight hydrocarbon resin, formed by a hydrogenated oligomer of styrene and alpha-methyl styrene. It is produced by polymerization and hydrogenation of pure monomeric hydrocarbons. | [14,44] |
2.2. Fungal Strains, Inocula Preparation, and Culture Conditions
2.3. Microscopic Analysis of Fungal Growth
2.4. FTIR-PAS Analysis
2.5. Extracellular Esterase Activity Assay
2.5.1. Cultivation Conditions
2.5.2. Preparation of Substrates and Measurement of Activity Using UV-VIS Spectrophotometry
2.5.3. Statistical Analyses
2.6. Transcriptome Sequencing of Aspergillus puulaauensis (EXF-7678) Exposed to Lascaux 498 HV and Regalrez 1094
2.6.1. Cultivation of the Fungus
2.6.2. RNA Isolation and Transcriptome Sequencing
2.6.3. Transcriptome Assembly and Analysis
3. Results
3.1. Microscopic Analysis of Fungal Growth
3.2. FTIR-PAS Analysis
3.3. Extracellular Esterase Activity Assay
3.3.1. Spectrophotometric Measurements of Esterase and Lipase Activity
3.3.2. Statistical Analysis of Esterase Assay
3.4. Transcriptome Sequencing of Aspergillus puulaauensis (EXF-7678) Exposed to Lascaux 498 HV and Regalrez 1094
4. Discussion
4.1. The Influence of Common Contaminants of Artistic Paintings, Facultatively Xerophilic Fungi of the Genera Aspergillus, Penicillium, and Cladosporium
4.2. Some Obligately Xerophilic Fungi with a Narrow Spectrum of Enzymatic Activities Can Chemically Modify Synthetic Materials
4.3. The Restoration Materials Most Susceptible to the Effects of Fungal Growth Are Laropal A81, Regalrez 1094, and Acrylic Dispersion Lascaux 498 HV
4.4. Analysis of the Transcriptome of Aspergillus puulaauensis Indicates Ability to Degrade Restoration Materials by Oxidases and Esterases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EXF | designation of fungal strains from the Ex Culture Collection of the Infrastructural Centre Mycosmo, MRIC UL, Slovenia (Department of Biology, Biotechnical Faculty, University of Ljubljana) |
| FTIR-PAS | Fourier-transform infrared photoacoustic spectroscopy |
| SEM | Scanning electron microscopy |
References
- Sterflinger, K. Fungi: Their Role in Deterioration of Cultural Heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Paiva de Carvalho, H.; Mesquita, N.; Trovão, J.; Fernández Rodríguez, S.; Pinheiro, A.C.; Gomes, V.; Alcoforado, A.; Gil, F.; Portugal, A. Fungal Contamination of Paintings and Wooden Sculptures inside the Storage Room of a Museum: Are Current Norms and Reference Values Adequate? J. Cult. Herit. 2018, 34, 268–276. [Google Scholar] [CrossRef]
- Zmeu, C.N.; Bosch-Roig, P. Risk Analysis of Biodeterioration in Contemporary Art Collections: The Poly-Material Challenge. J. Cult. Herit. 2022, 58, 33–48. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F.; Sanmartín, P. Interactions of Microorganisms and Synthetic Polymers in Cultural Heritage Conservation. Int. Biodeterior. Biodegrad. 2021, 163, 105282. [Google Scholar] [CrossRef]
- Cappitelli, F.; Sorlini, C. Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage. Appl. Environ. Microbiol. 2008, 74, 564–569. [Google Scholar] [CrossRef]
- Farmakalidis, H.V.; Douvas, A.M.; Karatasios, I.; Sotiropoulou, S.; Boyatzis, S.; Argitis, P.; Chryssoulakis, Y.; Kilikoglou, V. Accelerated Thermal Ageing of Acrylic Copolymers, Cyclohexanone-Based and Urea-Aldehyde Resins Used in Paintings Conservation. Mediterr. Archaeol. Archaeom. 2016, 16, 213–228. [Google Scholar] [CrossRef]
- Gaytán, I.; Burelo, M.; Loza-Tavera, H. Current Status on the Biodegradability of Acrylic Polymers: Microorganisms, Enzymes and Metabolic Pathways Involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, K.E.; Halloran, J.W.; Oliveira, A.; Kaviany, M. Chemistry of Removal of Ethylene Vinyl Acetate Binders. J. Mater. Sci. 1998, 33, 2795–2803. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Bitossi, G.; Osete-Cortina, L.; Yusá-Marco, D.J. Study of Ageing of Ketone Resins Used as Picture Varnishes by Pyrolysis–Silylation–Gas Chromatography–Mass Spectrometry. J. Anal. Appl. Pyrolysis 2009, 85, 470–479. [Google Scholar] [CrossRef]
- Adlan, N.A.; Sabri, S.; Masomian, M.; Ali, M.S.M.; Rahman, R.N.Z.R.A. Microbial Biodegradation of Paraffin Wax in Malaysian Crude Oil Mediated by Degradative Enzymes. Front. Microbiol. 2020, 11, 565608. [Google Scholar] [CrossRef]
- Bianco, L.; Avalle, M.; Scattina, A.; Croveri, P.; Pagliero, C.; Chiantore, O. A Study on Reversibility of BEVA®371 in the Lining of Paintings. J. Cult. Herit. 2015, 16, 479–485. [Google Scholar] [CrossRef]
- Laropal A81. Available online: https://www.kremer-pigmente.com/elements/resources/products/files/67204e.pdf (accessed on 30 November 2024).
- Fleischer, O.; Marutzky, R. Hydrolyse von Harnstoff-Formaldehyd-Harzen: Auflösung Des Spangefüges in Holzwerkstoffen Durch Hydrolytischen Abbau Der Leimfuge. Holz Als Roh-Und Werkst. 2000, 58, 295–300. [Google Scholar] [CrossRef]
- Regalrez 1094. Available online: https://www.kremer-pigmente.com/elements/resources/products/files/67280e.pdf (accessed on 30 November 2024).
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The Preservation Damage of Hydrophobic Polymer Coating Materials in Conservation of Stone Relics. Prog. Org. Coatings 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Campana, R.; Sabatini, L.; Giorgi, L.; Pettinari, G.; Valentini, L.; Gobbi, P. A Multidisciplinary Approach in Examining the Susceptibility to Microbial Attack of Polyacrylic and Polyurethane Resins Used in Art Restoration. Int. J. Mol. Sci. 2022, 23, 11725. [Google Scholar] [CrossRef] [PubMed]
- Cappitelli, F.; Vicini, S.; Piaggio, P.; Abbruscato, P.; Princi, E.; Casadevall, A.; Nosanchuk, J.D.; Zanardini, E. Investigation of Fungal Deterioration of Synthetic Paint Binders Using Vibrational Spectroscopic Techniques. Macromol. Biosci. 2005, 5, 49–57. [Google Scholar] [CrossRef]
- Cappitelli, F.; Nosanchuk, J.D.; Casadevall, A.; Toniolo, L.; Brusetti, L.; Florio, S.; Principi, P.; Borin, S.; Sorlini, C. Synthetic Consolidants Attacked by Melanin-Producing Fungi: Case Study of the Biodeterioration of Milan (Italy) Cathedral Marble Treated with Acrylics. Appl. Environ. Microbiol. 2007, 73, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Romero-Noguera, J.; Doménech Carbó, M.T.; Bitossi, G.; Bolívar Galiano, F.C.; López Miras, M.d.M.; Martín Sanchez, I.; Gimeno Adelantado, J.V.; Doménech Carbó, A.; de la Cruz Cañizares, J. Study on the Biodeterioration of Alkyd Ah Resin Used as a Binding Medium for Modern Paintings by Pyrolysis-Gas Chromatography-Mass Spectrometry and FTIR Spectroscopy. Arché 2008, 3, 157–162. [Google Scholar]
- Kigawa, R. Evaluation of Mould Resistance of Various Synthetic Resins Used in Conservation of Historic Sites. Sci. Conserv. Hozon kagaku 2005, 44, 149–156. [Google Scholar]
- Pinna, D.; Salvadori, O. Biological Growth on Italian Monuments Restored with Organic or Carbonatic Compounds. In Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage; Ciferri, O., Mastromei, G., Tiano, P., Eds.; Plenum Publishers: New York, NY, USA, 1999; pp. 149–154. [Google Scholar]
- Borgia, G.C.; Camaiti, M.; Cerri, F.; Fantazzini, P.; Piacenti, F. Hydrophobic Treatments for Stone Conservation—Influence of the Application Method on Penetration, Distribution and Efficiency. Stud. Conserv. 2003, 48, 217–226. [Google Scholar] [CrossRef]
- López-Miras, M.; Piñar, G.; Romero-Noguera, J.; Bolívar-Galiano, F.C.; Ettenauer, J.; Sterflinger, K.; Martín-Sánchez, I. Microbial Communities Adhering to the Obverse and Reverse Sides of an Oil Painting on Canvas: Identification and Evaluation of Their Biodegradative Potential. Aerobiologia 2013, 29, 301–314. [Google Scholar] [CrossRef]
- López-Miras, M.d.M.; Martín-Sánchez, I.; Yebra-Rodríguez, Á.; Romero-Noguera, J.; Bolívar-Galiano, F.; Ettenauer, J.; Sterflinger, K.; Piñar, G. Contribution of the Microbial Communities Detected on an Oil Painting on Canvas to Its Biodeterioration. PLoS ONE 2013, 8, e80198. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Miranda, A.S.; Doménech-Carbó, A.; Doménech-Carbó, M.T.; Osete-Cortina, L.; Bolívar-Galiano, F.; Martín-Sánchez, I. Analyzing Chemical Changes in Verdigris Pictorial Specimens upon Bacteria and Fungi Biodeterioration Using Voltammetry of Microparticles. Herit. Sci. 2017, 5, 8. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Ivanović, Ž.; Blagojević, J.; Vukojević, J.; Ljaljević Grbić, M. In Vitro Biodegradation Potential of Airborne Aspergilli and Penicillia. Sci. Nat. 2019, 106, 8. [Google Scholar] [CrossRef]
- Văcar, C.L.; Mircea, C.; Pârvu, M.; Podar, D. Diversity and Metabolic Activity of Fungi Causing Biodeterioration of Canvas Paintings. J. Fungi 2022, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.; Avdanina, D.; Shumikhin, K.; Simonenko, N.; Lyubavskaya, E.; Volkov, I.; Ivanov, V. Detection of Potential Biodeterioration Risks for Tempera Painting in 16th Century Exhibits from State Tretyakov Gallery. PLoS ONE 2020, 15, 1–20. [Google Scholar] [CrossRef]
- Kujović, A.; Gostinčar, C.; Kavkler, K.; Govedić, N.; Gunde-Cimerman, N.; Zalar, P. Degradation Potential of Xerophilic and Xerotolerant Fungi Contaminating Historic Canvas Paintings. J. Fungi 2024, 10, 76. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biodeterioration of Synthetic Materials—A Brief Review. Mater. Corros. 2010, 61, 986–992. [Google Scholar] [CrossRef]
- Scott, G. Polymer and the Environment; RSC Paperbacks: Cambridge, UK, 1999. [Google Scholar]
- Trovão, J.; Portugal, A. Current Knowledge on the Fungal Degradation Abilities Profiled through Biodeteriorative Plate Essays. Appl. Sci. 2021, 11, 4196. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the Past: “Find the Guilty Bug—Microorganisms Involved in the Biodeterioration of Archeological and Historical Artifacts”. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef]
- Kumar, S.; Priyanka; Kumar, U. Microbial Community Present on the Reverse Side of a Deteriorated Canvas BT. In Microbial Biotechnology Approaches to Monuments of Cultural Heritage; Yadav, A.N., Rastegari, A.A., Gupta, V.K., Yadav, N., Eds.; Springer: Singapore, 2020; pp. 1–12. ISBN 978-981-15-3401-0. [Google Scholar]
- Caneva, G.; Maggi, O.; Nugari, M.P.; Pietrini, A.M.; Piervittori, R.; Ricci, S.; Roccardi, A. The Biological Aerosol as a Factor of Biodeterioration BT. In Cultural Heritage and Aerobiology: Methods and Measurement Techniques for Biodeterioration Monitoring; Mandrioli, P., Caneva, G., Sabbioni, C., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 3–29. ISBN 978-94-017-0185-3. [Google Scholar]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Romero, S.M.; Giudicessi, S.L.; Vitale, R.G. Is the Fungus Aspergillus a Threat to Cultural Heritage? J. Cult. Herit. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Shchiparev, S.M.; Vlasov, D.Y. Formation of Organic Acids by Fungi Isolated from the Surface of Stone Monuments. Microbiology 2014, 83, 516–522. [Google Scholar] [CrossRef]
- Zalar, P.; Graf Hriberšek, D.; Gostinčar, C.; Breskvar, M.; Džeroski, S.; Matul, M.; Novak Babič, M.; Čremožnik Zupančič, J.; Kujović, A.; Gunde-Cimerman, N.; et al. Xerophilic Fungi Contaminating Historically Valuable Easel Paintings from Slovenia. Front. Microbiol. 2023, 14, 1258670. [Google Scholar] [CrossRef]
- ISO 11507:2007; Paints and Varnishes—Exposure of Coatings to Artificial Weathering—Exposure to Fluorescent UV Lamps and Water. International Organization for Standardization: Geneva, Switzerland, 2007.
- Lascaux Acrylic Glue 303 HV. Available online: https://www.kremer-pigmente.com/elements/resources/products/files/81000e.pdf (accessed on 30 November 2024).
- Lascaux Acrylic Glue 498 HV. Available online: https://www.kremer-pigmente.com/elements/resources/products/files/81002e.pdf (accessed on 30 November 2024).
- Acrylharz P550. Available online: https://shop.kremerpigments.com/elements/resources/products/files/81040e.pdf (accessed on 30 November 2024).
- Del Grosso, C.A.; Mosleh, Y.; Beerkens, L.; Poulis, J.A.; de la Rie, E.R. The Photostability and Peel Strength of Ethylene Butyl Acrylate Copolymer Blends for Use in Conservation of Cultural Heritage. J. Adhes. Sci. Technol. 2022, 36, 75–97. [Google Scholar] [CrossRef]
- Knight, E.; Berger, G. “Gustav Berger’s Original Formula®” Products For Art Conservation. Available online: https://deffner-johann.de/media/datasheets/2085001/EN/Technical Data Sheet_Beva Products_EN_DJ.pdf (accessed on 30 November 2024).
- Galloway, L.D.; Burgess, R. Applied Mycology and Bacteriology, 3rd ed.; Leonard Hill: London, UK, 1952. [Google Scholar]
- Hocking, A.D.; Pitt, J.I. Dichloran-Glycerol Medium for Enumeration of Xerophilic Fungi from Low-Moisture Foods. Appl. Environ. Microbiol. 1980, 39, 488–492. [Google Scholar] [CrossRef]
- Pardo, E.; Marín, S.; Sanchis, V.; Ramos, A.J. Impact of Relative Humidity and Temperature on Visible Fungal Growth and OTA Production of Ochratoxigenic Aspergillus Ochraceus Isolates on Grapes. Food Microbiol. 2005, 22, 383–389. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Gilham, D.; Lehner, R. Techniques to Measure Lipase and Esterase Activity in Vitro. Methods 2005, 36, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Lapides, J. Chromogenic Substrates: iv. Acyl Esters of p-Nitrophenol as Substrates for the Colorimetric Determination of Esterase. J. Biol. Chem. 1947, 170, 467–482. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Lu, J.; Rincon, N.; Wood, D.E.; Breitwieser, F.P.; Pockrandt, C.; Langmead, B.; Salzberg, S.L.; Steinegger, M. Metagenome Analysis Using the Kraken Software Suite. Nat. Protoc. 2022, 17, 2815–2839. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De Novo Transcript Sequence Reconstruction from RNA-Seq Using the Trinity Platform for Reference Generation and Analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Mirdita, M.; Steinegger, M.; Breitwieser, F.; Söding, J.; Levy Karin, E. Fast and Sensitive Taxonomic Assignment to Metagenomic Contigs. Bioinformatics 2021, 37, 3029–3031. [Google Scholar] [CrossRef] [PubMed]
- Mirdita, M.; Steinegger, M.; Söding, J. MMseqs2 Desktop and Local Web Server App for Fast, Interactive Sequence Searches. Bioinformatics 2019, 35, 2856–2858. [Google Scholar] [CrossRef] [PubMed]
- Steinegger, M.; Söding, J. Clustering Huge Protein Sequence Sets in Linear Time. Nat. Commun. 2018, 9, 2542. [Google Scholar] [CrossRef] [PubMed]
- Steinegger, M.; Söding, J. MMseqs2 Enables Sensitive Protein Sequence Searching for the Analysis of Massive Data Sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simão, F.A.; Zdobnov, E.M. BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.-H.; Davis, F.G.; et al. A Tissue-Mapped Axolotl De Novo Transcriptome Enables Identification of Limb Regeneration Factors. Cell Rep. 2017, 18, 762–776. [Google Scholar] [CrossRef]
- Powell, S.; Szklarczyk, D.; Trachana, K.; Roth, A.; Kuhn, M.; Muller, J.; Arnold, R.; Rattei, T.; Letunic, I.; Doerks, T.; et al. EggNOG v3.0: Orthologous Groups Covering 1133 Organisms at 41 Different Taxonomic Ranges. Nucleic Acids Res. 2012, 40, D284–D289. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.; Teufel, F.; Brunak, S.; von Heijne, G. SignalP: The Evolution of a Web Server BT. In Protein Bioinformatics; Lisacek, F., Ed.; Springer: New York, NY, USA, 2024; pp. 331–367. ISBN 978-1-0716-4007-4. [Google Scholar]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- NCBI BioProject. Available online: https://www.ncbi.nlm.nih.gov/bioproject (accessed on 15 December 2024).
- Ciferri, O. Microbial Degradation of Paintings. Appl. Environ. Microbiol. 1999, 65, 879–885. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and Nomenclature of the Genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Pancaldi, S.; Baldisserotto, C.; Petrucci, F.; Impallaria, A.; Volpe, L.; D’Accolti, M.; Soffritti, I.; Coccagna, M.; Sassu, G.; et al. Characterization of Biodegradation in a 17 Th Century Easel Painting and Potential for a Biological Approach. PLoS ONE 2018, 13, e0207630. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, O. Processes of Biodeterioration: General Mechanisms. In Plant Biology for Cultural Heritage. Biodeterioration and Conservation; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; Getty Publications: Los Angeles, CA, USA, 2008; ISBN 9780892369393. [Google Scholar]
- Lawrence, D.P.; Rotondo, F.; Gannibal, P.B. Biodiversity and Taxonomy of the Pleomorphic Genus Alternaria. Mycol. Prog. 2016, 15, 3. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but Different: The Expanding Realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74. [Google Scholar] [CrossRef]
- Abdel-kareem, O. Microbiological Testing to Assess the Susceptibility of Museum Textiles Conserved with Polymers to Fungal Deterioration. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center S.L.: Badajoz, Spain, 2016. [Google Scholar]
- Cappitelli, F.; Zanardini, E.; Sorlini, C. The Biodeterioration of Synthetic Resins Used in Conservation. Macromol. Biosci. 2004, 4, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer Biodegradation: Mechanisms and Estimation Techniques—A Review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- do Canto, V.P.; Thompson, C.E.; Netz, P.A. Polyurethanases: Three-Dimensional Structures and Molecular Dynamics Simulations of Enzymes That Degrade Polyurethane. J. Mol. Graph. Model. 2019, 89, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Łojewski, T.; Miśkowiec, P.; Missori, M.; Lubańska, A.; Proniewicz, L.M.; Łojewska, J. FTIR and UV/Vis as Methods for Evaluation of Oxidative Degradation of Model Paper: DFT Approach for Carbonyl Vibrations. Carbohydr. Polym. 2010, 82, 370–375. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A. Photostabilization of Poly(Vinyl Chloride)—Still on the Run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef]
- Milanesi, C.; Baldi, F.; Borin, S.; Brusetti, L.; Ciampolini, F.; Iacopini, F.; Cresti, M. Deterioration of Medieval Painting in the Chapel of the Holy Nail, Siena (Italy) Partially Treated with Paraloid B72. Int. Biodeterior. Biodegrad. 2009, 63, 844–850. [Google Scholar] [CrossRef]
- Dhar, K.; Dutta, S.; Anwar, M.N. Biodegradation of Petroleum Hydrocarbon by Indigenous Fungi Isolated from Ship Breaking Yards of Bangladesh. Int. Res. J. Biol. Sci. 2014, 3, 22–30. [Google Scholar]
- González-Abradelo, D.; Pérez-Llano, Y.; Peidro-Guzmán, H.; Sánchez-Carbente, M.d.R.; Folch-Mallol, J.L.; Aranda, E.; Vaidyanathan, V.K.; Cabana, H.; Gunde-Cimerman, N.; Batista-García, R.A. First Demonstration That Ascomycetous Halophilic Fungi (Aspergillus Sydowii and Aspergillus Destruens) Are Useful in Xenobiotic Mycoremediation under High Salinity Conditions. Bioresour. Technol. 2019, 279, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of Plastic Polymers by Fungi: A Brief Review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Peidro-Guzmán, H.; Pérez-Llano, Y.; González-Abradelo, D.; Fernández-López, M.G.; Dávila-Ramos, S.; Aranda, E.; Hernández, D.R.O.; García, A.O.; Lira-Ruan, V.; Pliego, O.R.; et al. Transcriptomic Analysis of Polyaromatic Hydrocarbon Degradation by the Halophilic Fungus Aspergillus Sydowii at Hypersaline Conditions. Environ. Microbiol. 2021, 23, 3435–3459. [Google Scholar] [CrossRef]
- Leitão, A.L. Potential of Penicillium Species in the Bioremediation Field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.R.; Campbell, J.R.; Thompson, L.; Walz, S.L.; Schultz, W.W. Biodegradation of a Colloidal Ester-Based Polyurethane by Soil Fungi. Int. Biodeterior. Biodegrad. 1994, 33, 103–113. [Google Scholar] [CrossRef]
- Cappitelli, F.; Principi, P.; Pedrazzani, R.; Toniolo, L.; Sorlini, C. Bacterial and Fungal Deterioration of the Milan Cathedral Marble Treated with Protective Synthetic Resins. Sci. Total Environ. 2007, 385, 172–181. [Google Scholar] [CrossRef]
- Birolli, W.G.; Santos, D.d.A.; Alvarenga, N.; Garcia, A.C.F.S.; Romão, L.P.C.; Porto, A.L.M. Biodegradation of Anthracene and Several PAHs by the Marine-Derived Fungus Cladosporium Sp. CBMAI 1237. Mar. Pollut. Bull. 2018, 129, 525–533. [Google Scholar] [CrossRef]
- Potin, O.; Veignie, E.; Rafin, C. Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Cladosporium Sphaerospermum Isolated from an Aged PAH Contaminated Soil. FEMS Microbiol. Ecol. 2004, 51, 71–78. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Bastholm, C.J.; Madsen, A.M.; Andersen, B.; Frisvad, J.C.; Richter, J. The Mysterious Mould Outbreak—A Comprehensive Fungal Colonisation in a Climate-Controlled Museum Repository Challenges the Environmental Guidelines for Heritage Collections. J. Cult. Herit. 2022, 55, 78–87. [Google Scholar] [CrossRef]
- Segers, F.J.J.; Meijer, M.; Houbraken, J.; Samson, R.A.; Wösten, H.A.B.; Dijksterhuis, J. Xerotolerant Cladosporium Sphaerospermum Are Predominant on Indoor Surfaces Compared to Other Cladosporium Species. PLoS ONE 2015, 10, e0145415. [Google Scholar] [CrossRef] [PubMed]
- Miliani, C.; Ombelli, M.; Morresi, A.; Romani, A. Spectroscopic Study of Acrylic Resins in Solid Matrices. Surf. Coatings Technol. 2002, 151–152, 276–280. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage-an Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Cappitelli, F.; Sorlini, C.; Pedemonte, E.; Princi, E.; Vicini, S. Effectiveness of Graft Synthetic Polymers in Preventing Biodeterioration of Cellulose-Based Materials. Macromol. Symp. 2006, 238, 84–91. [Google Scholar] [CrossRef]
- Zalewska, A.; Kowalik, J.; Tworek, M. Investigation of the Properties of a Water-Based Acrylic Dispersion Modified with an Ionic Liquid, Surfactant, and Thickener. Environ. Sci. Pollut. Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Mezzadri, P.; Valentini, F.; Capua, M.-C. Critical and Analytical Approaches in a Contemporary Mural Painting’ Retouching Process: The Key Study of Murals By Antonio Carena. In Proceedings of the 6th International Meeting on Retouching of Cultural Heritage, RECH6, Valencia, Spain, 4–6 November 2021; Editorial Universitat Politècnica de València: Valencia, Spain, 2023; pp. 25–33. [Google Scholar] [CrossRef]
- Arslanoglu, J.; Learner, T. The Evaluation of Laropal A81: Paraloid B-72 Polymer Blend Varnishes for Painted and Decorative Surfaces—Appearance and Practical Considerations. Conservator 2001, 25, 62–72. [Google Scholar] [CrossRef]
- Pieralli, I.; Salvini, A.; Angelin, E.M.; Pamplona, M.; Cocchetti, V.; Bartolozzi, G.; Picollo, M. A Formulation for a New Environmentally Friendly Varnish for Paintings. Coatings 2023, 13, 1566. [Google Scholar] [CrossRef]
- Bestetti, R. La Verniciatura Dei Manufatti Policromi: Dalle Vernici Tradizionali Alle Resine a Basso Peso Molecolare, 1st ed.; Collana I Talenti, il Prato: Padova, Italy, 2020. [Google Scholar]
- Proctor, R.; Whitten, J. Varnishing as Part of the Conservation Treatment of Easel Paintings. In Conservation of Easel Paintings, 1st ed.; Stoner, J.H., Rushfield, R.A., Eds.; Routledge Series in Conservation and Museology; Routledge: London, UK, 2012. [Google Scholar]
- Carvalho, C.R.; Cortina, L.O.; Araújo, M.E.; Pérez-Marín, E.; Maria, A.; Bailão, S. Study on the Impregnation of Laropal A81 To Consolidate the Wood Support in Easel Paintings. Int. J. Conserv. Sci. 2021, 12, 869–878. [Google Scholar]
- Clausi, M.; Crisci, G.M.; La Russa, M.F.; Malagodi, M.; Palermo, A.; Ruffolo, S.A. Protective Action against Fungal Growth of Two Consolidating Products Applied to Wood. J. Cult. Herit. 2011, 12, 28–33. [Google Scholar] [CrossRef]
- Bonaduce, I.; Colombini, M.P.; Degano, I.; Di Girolamo, F.; La Nasa, J.; Modugno, F.; Orsini, S. Mass Spectrometric Techniques for Characterizing Low-Molecular-Weight Resins Used as Paint Varnishes. Anal. Bioanal. Chem. 2013, 405, 1047–1065. [Google Scholar] [CrossRef]
- Berger, G.A. Formulating Adhesives For The Conservation Of Paintings. Stud. Conserv. 1972, 17, 613–629. [Google Scholar] [CrossRef]
- Salman, A.; Tsror, L.; Pomerantz, A.; Moreh, R.; Mordechai, S.; Huleihel, M. FTIR Spectroscopy for Detection and Identification of Fungal Phytopathogenes. Spectroscopy 2010, 24, 723489. [Google Scholar] [CrossRef]
- Kavkler, K.; Humar, M.; Kržišnik, D.; Turk, M.; Tavzes, Č.; Gostinčar, C.; Džeroski, S.; Popov, S.; Penko, A.; Gunde-Cimerman, N.; et al. A Multidisciplinary Study of Biodeteriorated Celje Ceiling, a Tempera Painting on Canvas. Int. Biodeterior. Biodegrad. 2022, 170, 105389. [Google Scholar] [CrossRef]
- Chen, C.-C.; Dai, L.; Ma, L.; Guo, R.-T. Enzymatic Degradation of Plant Biomass and Synthetic Polymers. Nat. Rev. Chem. 2020, 4, 114–126. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Popova, T.N.; Agarkov, A.A.; Pinheiro de Carvalho, M.Â.A.; Rjevskiy, S.G. Expression of Glutathione Peroxidase and Glutathione Reductase and Level of Free Radical Processes under Toxic Hepatitis in Rats. J. Toxicol. 2013, 2013, 870628. [Google Scholar] [CrossRef] [PubMed]
- Iiyoshi, Y.; Tsutsumi, Y.; Nishida, T. Polyethylene Degradation by Lignin-Degrading Fungi and Manganese Peroxidase. J. Wood Sci. 1998, 44, 222–229. [Google Scholar] [CrossRef]
- Khatoon, N.; Jamal, A.; Ali, M.I. Lignin Peroxidase Isoenzyme: A Novel Approach to Biodegrade the Toxic Synthetic Polymer Waste. Environ. Technol. 2019, 40, 1366–1375. [Google Scholar] [CrossRef]
- Kleeberg, I.; Welzel, K.; Vandenheuvel, J.; Müller, R.-J.; Deckwer, W.-D. Characterization of a New Extracellular Hydrolase from Thermobifida Fusca Degrading Aliphatic-Aromatic Copolyesters. Biomacromolecules 2005, 6, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, D.; Herrero Acero, E.; Greimel, K.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Rodriguez, R.D.; Steinkellner, G.; Gruber, K.; Schwab, H.; et al. A New Esterase from Thermobifida halotolerans Hydrolyses Polyethylene Terephthalate (PET) and Polylactic Acid (PLA). Polymers 2012, 4, 617–629. [Google Scholar] [CrossRef]
- Eberl, A.; Heumann, S.; Brückner, T.; Araujo, R.; Cavaco-Paulo, A.; Kaufmann, F.; Kroutil, W.; Guebitz, G.M. Enzymatic Surface Hydrolysis of Poly(Ethylene Terephthalate) and Bis(Benzoyloxyethyl) Terephthalate by Lipase and Cutinase in the Presence of Surface Active Molecules. J. Biotechnol. 2009, 143, 207–212. [Google Scholar] [CrossRef]
- Perez-Garcia, P.; Chow, J.; Costanzi, E.; Gurschke, M.; Dittrich, J.; Dierkes, R.F.; Molitor, R.; Applegate, V.; Feuerriegel, G.; Tete, P.; et al. An Archaeal Lid-Containing Feruloyl Esterase Degrades Polyethylene Terephthalate. Commun. Chem. 2023, 6, 193. [Google Scholar] [CrossRef]
- Gricajeva, A.; Nadda, A.K.; Gudiukaite, R. Insights into Polyester Plastic Biodegradation by Carboxyl Ester Hydrolases. J. Chem. Technol. Biotechnol. 2022, 97, 359–380. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef]
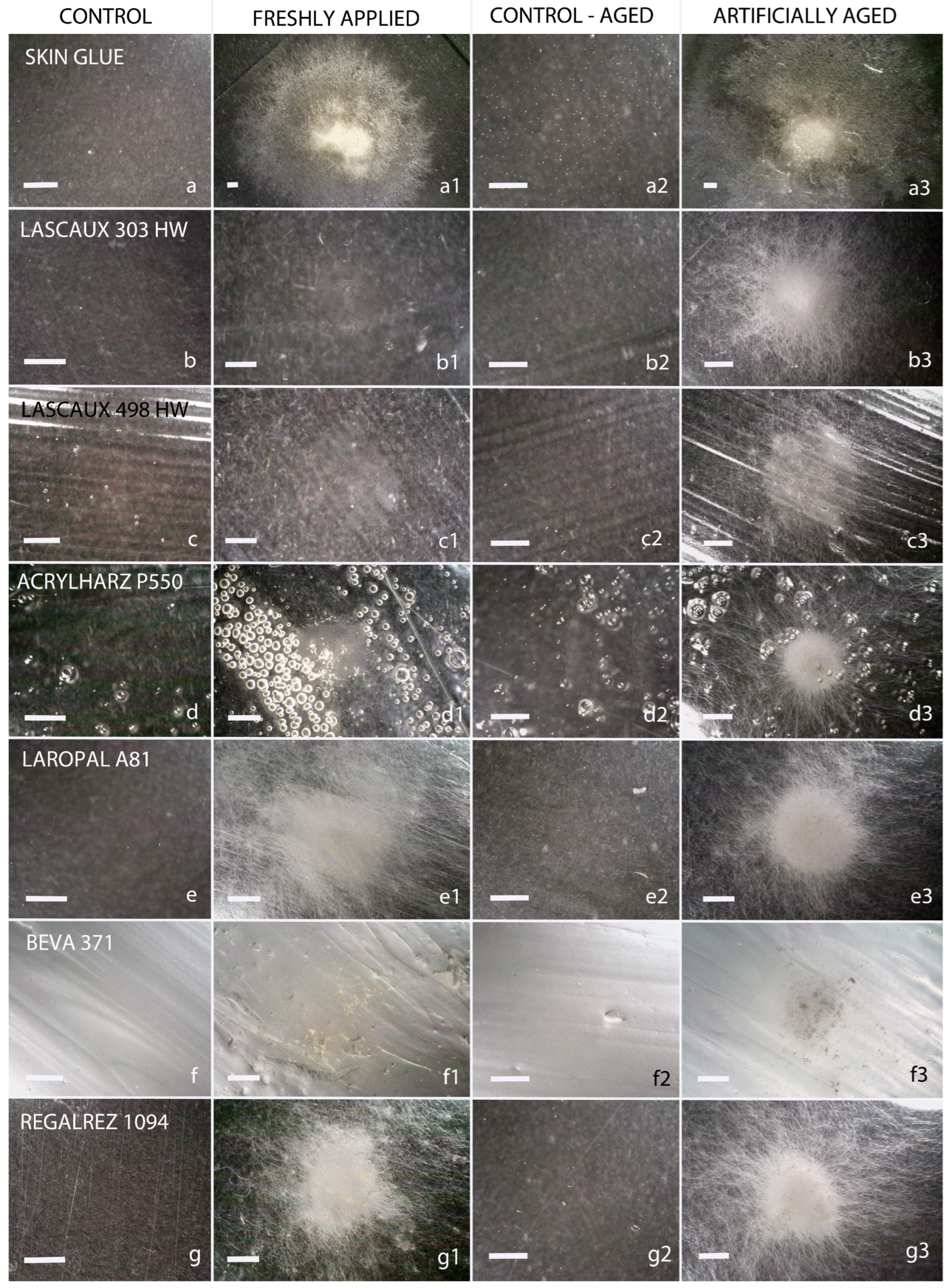
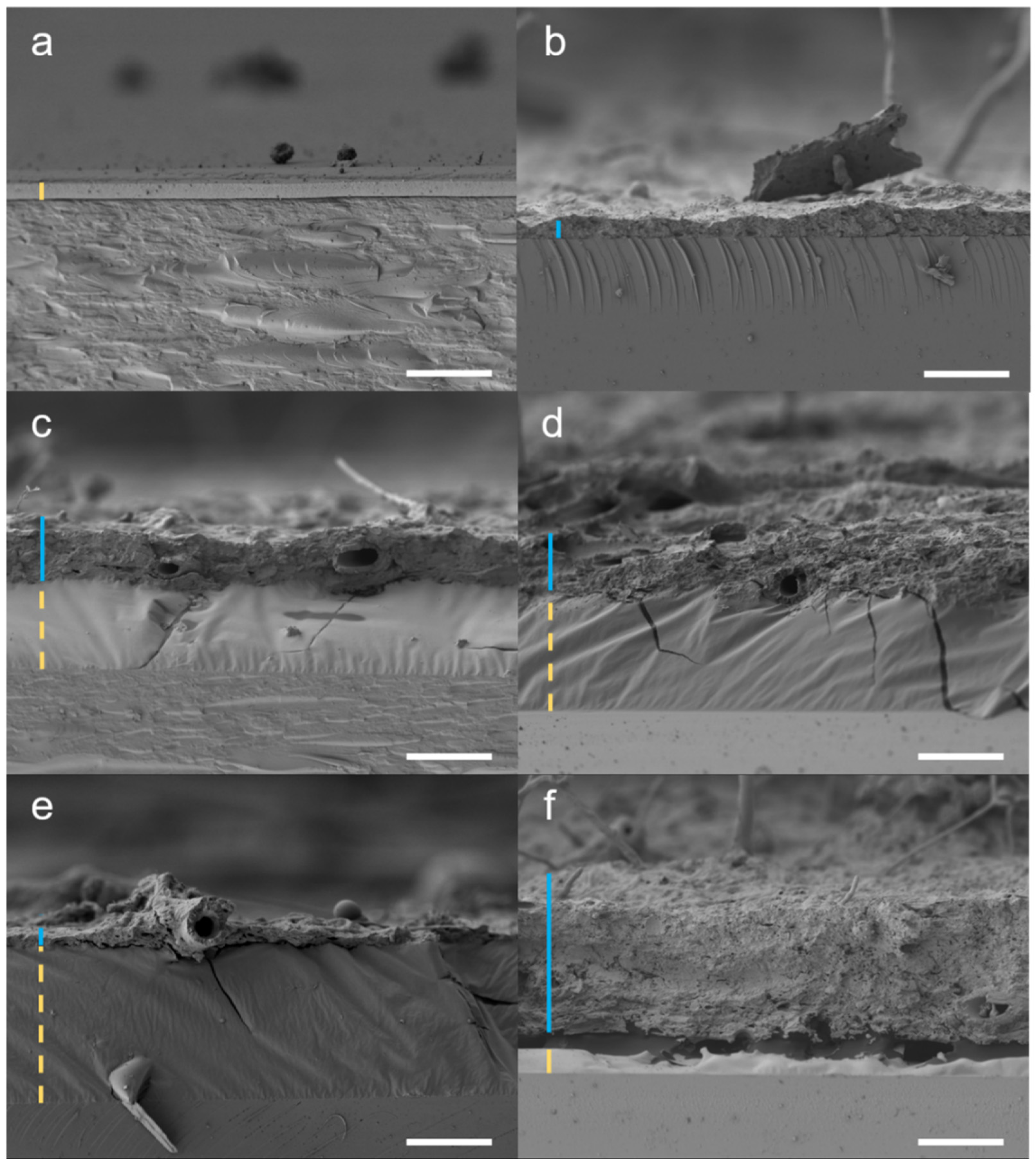


| Skin Glue | Lascaux 303 | Lascaux 498 | Acrylharz P550 | Laropal A81 | Beva 371 | Regalrez 1094 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EXF- a | Species | N | A | N | A | N | A | N | A | N | A | N | A | N | A |
| 10360 | Aspergillus destruens | +++ | +++ | ++ | ++ | + | +++ | + | + | + | + | + | + | + | + |
| 10353 | Aspergillus magnivesiculatus | +++ | +++ | − | ++ | + | + | + | + | + | + | + | + | + | + |
| 14316 | Aspergillus pseudoglaucus | ++ | +++ | − | + | − | + | + | + | + | ++ | − | + | + | ++ |
| 7678 | Aspergillus puulaauensis | +++ | +++ | − | + | − | + | ++ | ++ | + | ++ | + | + | ++ | ++ |
| 15210 | Aspergillus vitricola | +++ | +++ | − | + | − | ++ | + | + | + | + | + | + | + | + |
| 14317 | Aureobasidium pullulans | ++ | ++ | − | − | − | − | − | − | − | − | − | − | − | − |
| 10556 | Beauveria pseudobassiana | ++ | +++ | − | + | − | + | − | + | + | + | + | + | + | + |
| 7690 | Chaetomium globosum | +++ | + | − | − | − | + | − | − | − | + | − | − | − | − |
| 14315 | Cladosporium cladosporioides | ++ | +++ | − | + | − | − | − | + | + | + | + | + | + | + |
| 10663 | Parengyodontium album | ++ | +++ | − | − | − | + | − | − | − | + | − | − | − | + |
| 10495 | Penicillium chrysogenum | +++ | +++ | − | + | + | − | + | + | + | + | + | + | + | + |
| 15064 | Penicillium corylophilum | ++ | ++ | − | ++ | − | ++ | + | − | + | + | + | ++ | + | + |
| 10120 | Wallemia sp. (aff. muriae) | +++ | +++ | − | − | − | − | − | − | − | − | − | − | − | − |
| 10342 | Wallemia canadensis | ++ | ++ | − | − | − | − | + | + | + | + | − | − | + | + |
| UP (Lascaux 498 HV) | DOWN (Lascaux 498 HV) | UP (Regalrez 1094) | DOWN (Regalrez 1094) | |
|---|---|---|---|---|
| Esterase | 9 | 16 | 2 | 3 |
| Esterase SP | 4 | 11 | 1 | 3 |
| Oxidase | 15 | 22 | 1 | 3 |
| Oxidase SP | 2 | 6 | 0 | 2 |
| Oxidoreductase | 12 | 16 | 1 | 4 |
| Oxidoreductase SP | 1 | 3 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujović, A.; Kavkler, K.; Wilson-Hernandez, M.A.; Vittori, M.; Zidar, L.; Gostinčar, C.; Sepčić, K.; Pérez-Llano, Y.; Batista-García, R.A.; Gunde-Cimerman, N.; et al. Degradation of Synthetic Restoration Materials by Xerotolerant/Xerophilic Fungi Contaminating Canvas Paintings. J. Fungi 2025, 11, 568. https://doi.org/10.3390/jof11080568
Kujović A, Kavkler K, Wilson-Hernandez MA, Vittori M, Zidar L, Gostinčar C, Sepčić K, Pérez-Llano Y, Batista-García RA, Gunde-Cimerman N, et al. Degradation of Synthetic Restoration Materials by Xerotolerant/Xerophilic Fungi Contaminating Canvas Paintings. Journal of Fungi. 2025; 11(8):568. https://doi.org/10.3390/jof11080568
Chicago/Turabian StyleKujović, Amela, Katja Kavkler, Michel Alexander Wilson-Hernandez, Miloš Vittori, Luen Zidar, Cene Gostinčar, Kristina Sepčić, Yordanis Pérez-Llano, Ramón Alberto Batista-García, Nina Gunde-Cimerman, and et al. 2025. "Degradation of Synthetic Restoration Materials by Xerotolerant/Xerophilic Fungi Contaminating Canvas Paintings" Journal of Fungi 11, no. 8: 568. https://doi.org/10.3390/jof11080568
APA StyleKujović, A., Kavkler, K., Wilson-Hernandez, M. A., Vittori, M., Zidar, L., Gostinčar, C., Sepčić, K., Pérez-Llano, Y., Batista-García, R. A., Gunde-Cimerman, N., & Zalar, P. (2025). Degradation of Synthetic Restoration Materials by Xerotolerant/Xerophilic Fungi Contaminating Canvas Paintings. Journal of Fungi, 11(8), 568. https://doi.org/10.3390/jof11080568










