The Novel Disease Vicia unijuga Caused by Colletotrichum tofieldiae in China: Implications for Host Growth, Photosynthesis, and Nutritional Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation
2.2. Morphological Characterization
2.3. DNA Extraction, PCR Amplification, Sequencing, and Phylogenetic Analysis
2.4. Koch’s Postulates and Host Range
2.5. Biological Characteristics of Isolated Fungi
2.6. Influence of the Isolate LYZ0664 on the Growth Indicators of V. unijuga
2.7. Influence of the Isolate LYZ0664 on the Photosynthesis Indicators of V. unijuga
2.8. Influence of the Isolate LYZ0664 on the Nutritional Quality of V. unijuga
2.9. Data Analysis
3. Results
3.1. Disease Survey and Strain Isolation
3.2. Morphological Characterization of LYZ0664
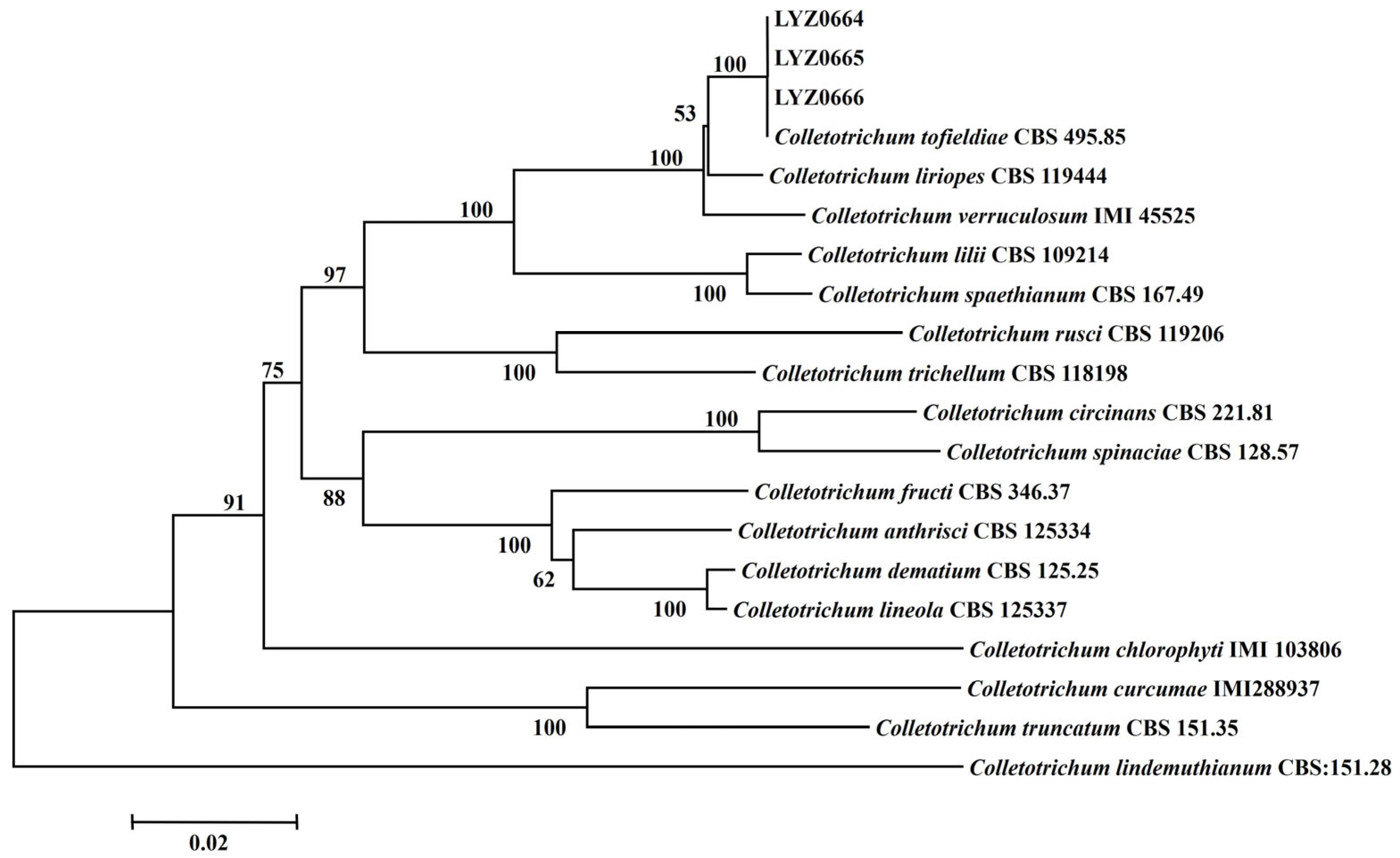
3.3. Phylogenetic Analysis
3.4. Koch’s Postulates and Host Range of LYZ0664
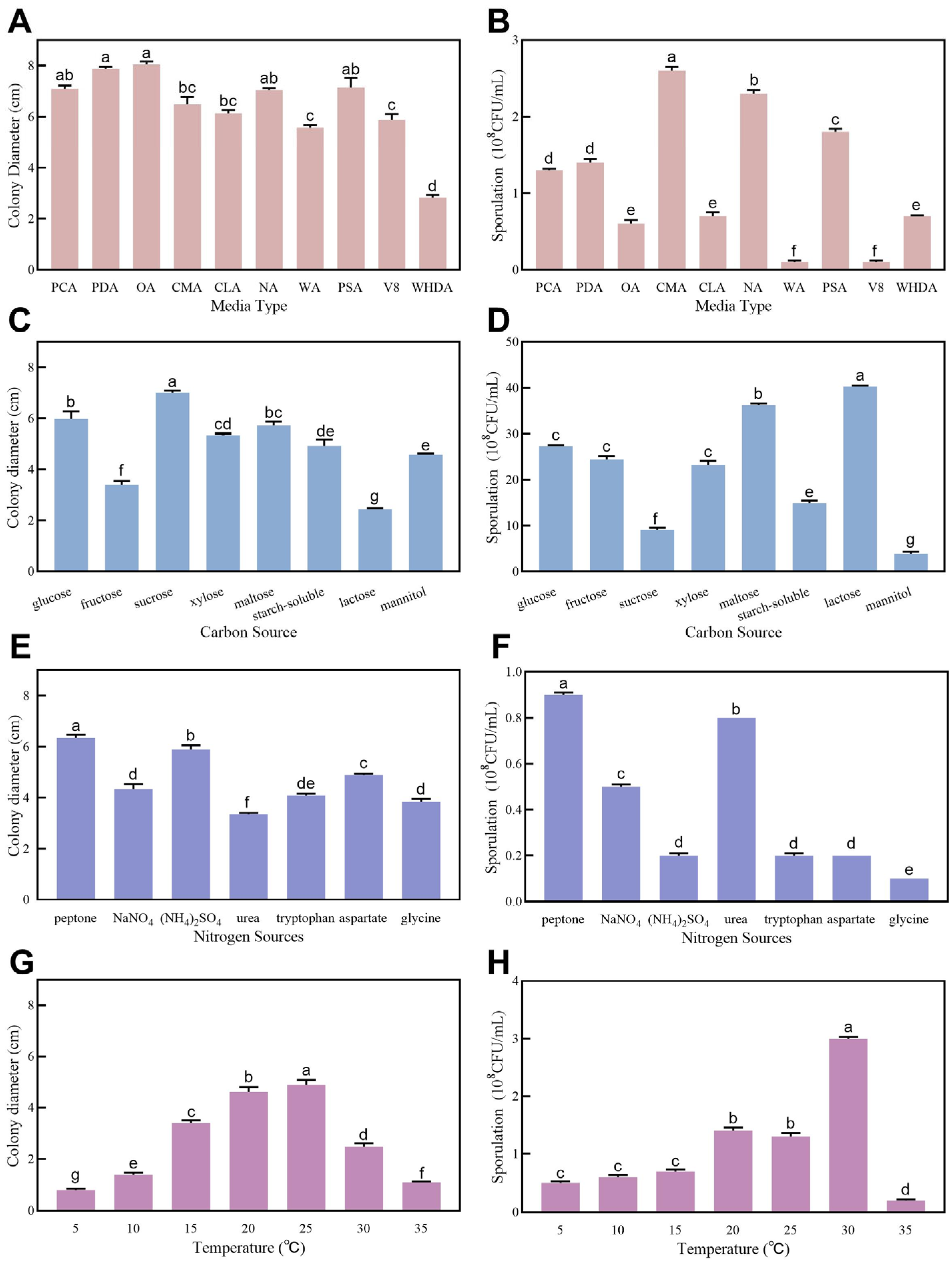
3.5. Biological Characteristics of LYZ0664
3.6. Influence of LYZ0664 on Growth Indicators of V. unijuga
3.7. Influence of LYZ0664 on Photosynthesis Indicators of V. unijuga
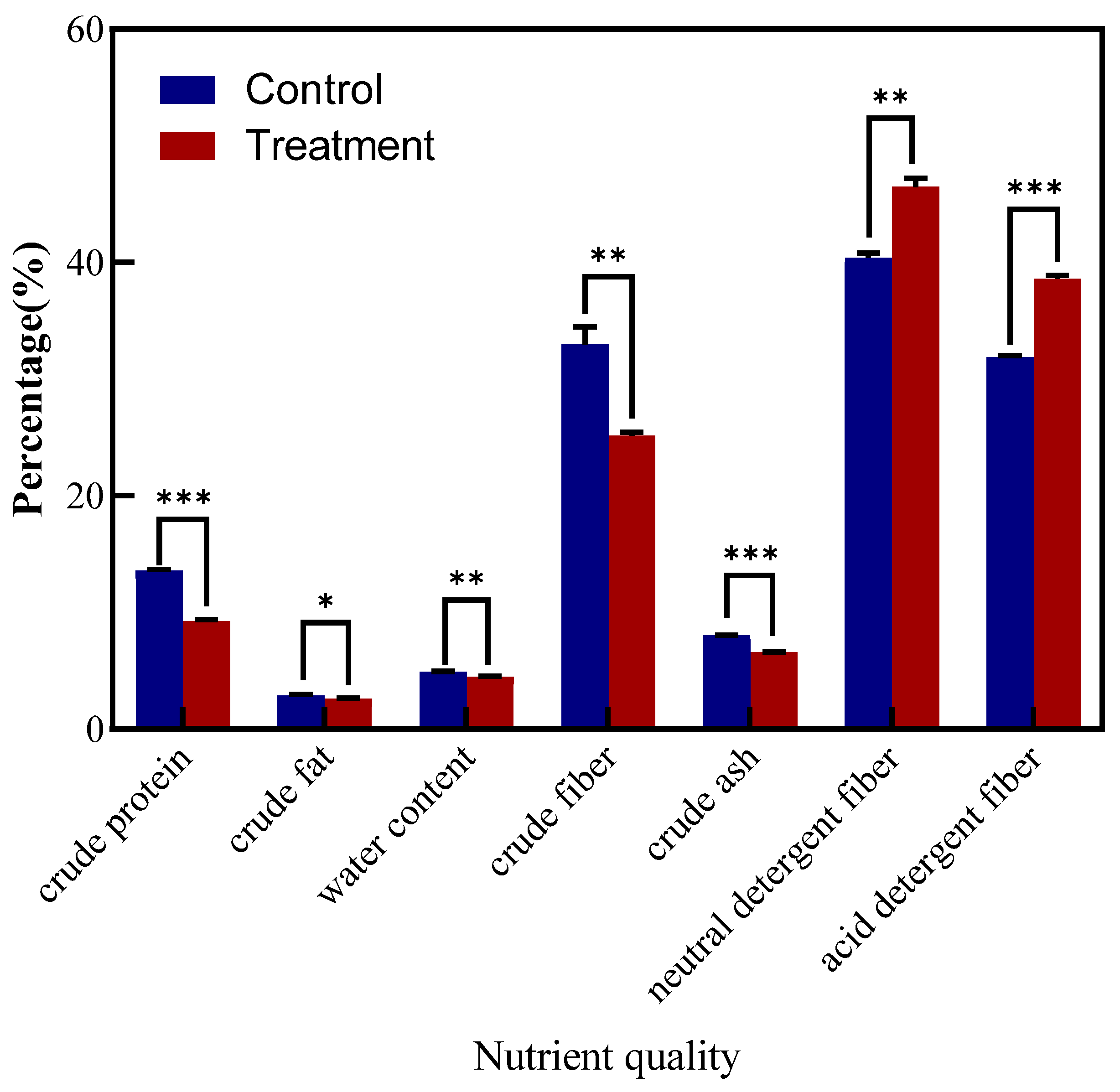
3.8. Influence of LYZ0664 on Nutritional Quality of V. unijuga
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Board, C.F.E. Flora of China. Science Press: Beijing, China, 2006. [Google Scholar]
- Mao, Z.X.; Fu, H.; Niu, D.C.; Nie, B.; Chen, H. Nutrient variation and forage evaluation of Vicia unijuga in alpine grassland. Acta Prataculturae Sin. 2015, 24, 27–233. [Google Scholar]
- Watson, A.J. Foreign Bacterial and Fungus Diseases of Food, Forage, and Fiber Crops: An Annotated List; Department of Agriculture: Washington, DC, USA, 1971. [Google Scholar]
- Tai, F.L. Sylloge Fungorum Sinicorum; Science Press: Beijing, China, 1979. [Google Scholar]
- List of Plant Diseases in Korea. Available online: https://genebank.rda.go.kr/english/plntDissInfoMain.do (accessed on 16 November 2024).
- Zheng, R.Y.; Yu, Y.N. Flora Fungorum Sinicorum; Science Press: Beijing, China, 1987. [Google Scholar]
- Andrianova, T.V.; Minter, D.W. New species of Bartalinia and Septoriella from the Altai Mountains. Mycotaxon 2007, 101, 297–313. [Google Scholar]
- Cao, Z.M.; Li, Z.Q. Rust Fungi of Qinling Mountains; China Forestry Publishing House: Beijing, China, 1999. [Google Scholar]
- Guyot, A.L. Les Rouilles des Legumineuses Fourrageres et Spontanees; Editions Paul Lechevalier: Paris, France, 1957. [Google Scholar]
- Zhuang, J.Y. Flora Fungorum Sinicorum; Science Press: Beijing, China, 2005. [Google Scholar]
- USDA Fungus Databases. Available online: https://fungi.ars.usda.gov/ (accessed on 16 November 2024).
- Liu, F.; Ma, Z.Y.; Hou, L.W.; Diao, Y.Z.; Wu, W.P.; Damm, U.; Song, S.; Cai, L. Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud Mycol. 2022, 101, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P.; Baroncelli, R. Hosts of Colletotrichum. Mycosphere 2023, 14, 158–261. [Google Scholar] [CrossRef]
- Rodriguez, R.; Redman, R. More than 400 milyears of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef]
- Liu, X.B.; Li, B.X.; Cai, J.M.; Zheng, X.L.; Feng, Y.L.; Huang, G.X. Colletotrichum species causing anthracnose of rubber trees in China. Sci. Rep. 2018, 8, 10435. [Google Scholar] [CrossRef]
- Shivas, R.G.; Tan, Y.P.; Edwards, J.; Dinh, Q.; Maxwell, A.; Andjic, V.; Liberato, J.R.; Anderson, C.; Beasley, D.R.; Bransgrove, K.; et al. Colletotrichum species in Australia. Australas Plant Pathol. 2016, 45, 447–464. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, T.Y.; Nan, Z.B. First report of anthracnose caused by Colletotrichum spinaciae on Vicia sativa in China. Plant Dis. 2019, 103, 2138. [Google Scholar] [CrossRef]
- Xu, S.; Li, Y.Z. First report of common vetch anthracnose caused by Colletotrichum lentis in China. Plant Dis. 2015, 99, 1859. [Google Scholar] [CrossRef]
- Xu, S.; Christensen, M.J.; Li, Y.Z. Pathogenicity and characterization of Colletotrichum lentis: A causal agent of anthracnose in common vetch (Vicia sativa). Eur. J. Plant Pathol. 2017, 149, 719–731. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum: Current status and future directions. Stud Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.; Waller, J.M.; Abang, M.M.; Zang, J.C.; Yang, Y.L.; Phouliyong, S.; Prihastuti, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Johnston, P.R.; Weir, B.S.; Tan, Y.P.; Shivas, R.G.; Crous, P.W. The Colletotrichum boninense species complex. Stud Mycol. 2012, 73, 1–36. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Liu, F.; Barreto, R.W.; Guatimosim, E.; Crous, P.W. The Colletotrichum orbiculare species complex: Important pathogens of field crops and weeds. Fungal Divers. 2013, 61, 29–59. [Google Scholar] [CrossRef]
- Norman, H.C.; Humphries, A.W.; Hulm, E.; Young, P.; Vercoe, P.E. Productivity and nutritional value of 20 species of perennial legumes in a low-rainfall Mediterranean-type environment in southern Australia. Grass Forage Sci. 2021, 76, 134–158. [Google Scholar] [CrossRef]
- Tang, W.; Coulter, J.A.; Hu, X.W.; Han, Y.H.; Wang, Y.R.; Zhang, Z.X.; Li, R.; Nan, Z.B. Plastic-film mulch improves Vicia unijuga seed yield and yield components under subalpine climate conditions. Arch Agron Soil Sci. 2020, 66, 1171–1187. [Google Scholar] [CrossRef]
- Tang, W.; Christensen, M.J.; Nan, Z.B. Contributions of soil temperature and moisture drivers to variations in perennial vetch (Vicia unijuga) productivity potential in the Qinghai-Tibetan Plateau region of China. J. Agric Sci. 2019, 157, 150–168. [Google Scholar] [CrossRef]
- Yang, Y.L.; Liu, Z.Y.; Cai, L.; Hyde, K.D.; Yu, Z.N.; McKenzie, E.H.C. Colletotrichum anthracnose of Amaryllidaceae. Fungal Divers. 2009, 39, 123–146. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pcr Protocols. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Lousie, G.N.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Guerber, J.C.; Liu, B.; Correll, J.C.; Johnston, P.R. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 2003, 95, 872–895. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Groenewald, J.Z.; Risede, J.; Hyweljones, N. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud Mycol. 2004, 50, 415–430. [Google Scholar]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009, 37, 45–87. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kuhner, M.; Felsenstein, J. A simulation comparison of phylogeny algorithms under equal and unequal evolutionary rates. Mol. Biol. Evol. 1994, 11, 459–468. [Google Scholar] [CrossRef]
- Liu, L.P.; Liu, Y.N.; Yang, L.Y.; Lu, B.H.; Yang, L.N.; Wang, X.; Li, Y.; Gao, J.; Hsiang, T. First report of anthracnose caused by Colletotrichum destructivum on curly dock in China. Plant Dis. 2017, 101, 256. [Google Scholar] [CrossRef]
- Li, Y.Z.; Nan, Z.B. The Methods of Diagnose, Investigation and Loss Evaluation for Forage Disease; Phoenix Science Press: Nanjing, China, 2015. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 1999. [Google Scholar]
- Patouillard, N.T. Champignons parasites des phanérogames exotiques. Rev. Mycol. 1886, 8, 80–85. [Google Scholar]
- Wollenweber, H.W.; Hochapfel, H. Beiträge zur Kenntnis parasitärer und saprophytischer Pilze: VI. Vermicularia, Colletotrichum, Gloeosporium, Glomerella und ihre Beziehung zur Fruchtfäule. Z. Parasitenkd 1949, 14, 181–268. [Google Scholar]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud Mycol. 2019, 92, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Hacquard, S.; Kracher, B.; Hiruma, K.; Münch, P.C.; Garrido-Oter, R.; Thon, M.R.; Weimann, A.; Damm, U.; Dallery, J.F.; Hainaut, M.; et al. Survival trade-offs in plant roots during colonization by closely related beneficial and pathogenic fungi. Nat Commun. 2016, 7, 11362. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Moriwaki, J.; Kaneko, S. Anthracnose fungi with curved conidia, Colletotrichum spp. belonging to ribosomal groups 9-13, and their host ranges in Japan. JARQ. 2015, 49, 351–362. [Google Scholar] [CrossRef]
- Zhang, W.; Damm, U.; Crous, P.W.; Groenewald, J.Z.; Niu, X.L.; Lin, J.M.; Li, Y.T. Anthracnose disease of carpetgrass (Axonopus compressus) caused by Colletotrichum hainanense sp. nov. Plant Dis. 2020, 104, 1744–1750. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; McKenzie, E.H.C.; Yang, Y.L.; Zhang, J.Z.; Prihastuti, H. Colletotrichum: A catalogue of confusion. Fungal Divers. 2009, 39, 1–17. [Google Scholar]
- Yang, H.C.; Haudenshield, J.S.; Hartman, G.L. Colletotrichum incanum sp. nov., a curved-conidial species causing soybean anthracnose in USA. Mycologia 2014, 106, 32–42. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Groenewald, J.Z.; Polizzi, G.; Crous, P.W. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 2017, 39, 32–50. [Google Scholar] [CrossRef]
- Bai, Z.H.; Ma, W.Q.; Ma, L.; Velthof, G.L.; Wei, Z.B.; Havlík, P.; Oenema, O.; Lee, M.R.F.; Zhang, F.S. China’s livestock transition: Driving forces, impacts, and consequence. Sci. Adv. 2018, 4, eaar8534. [Google Scholar] [CrossRef]
- Thomas, D.T.; Moore, A.D.; Bell, L.W.; Webb, N.P. Ground cover, erosion risk and production implications of targeted management practices in Australian mixed farming systems: Lessons from the Grain and Graze program. Agric. Syst. 2018, 162, 123–135. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.C.; Ma, L. Economic, energy and environmental consequences of shifting from maize-wheat to forage rotation in the North China Plain. J. Clean Prod. 2021, 328, 129760. [Google Scholar] [CrossRef]
- Zhou, F.F.; Matthew, C.; Yang, P.F.; Hnag, Y.F.; Nie, B.; Nan, Z.B. Leaf morphology, functional trait and altitude response in perennial vetch (Vicia unijuga A. Braun), alfalfa (Medicago sativa L.) and sainfoin (Onobrychis viciifolia Scop.). Planta 2023, 257, 75. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, R.; Zhang, Z.; Baskin, C.C.; Nan, Z.B. Mulching affects seed set, provisioning, and offspring performance of Vicia unijuga (Fabaceae). Agron. J. 2019, 111, 1341–1357. [Google Scholar] [CrossRef]
- Hiruma, K.; Gerlach, N.; Sacristán, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramírez, D.; Bucher, M.; O’Connell, R.J.; et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef]
- Morcillo, R.J.; Singh, S.K.; He, D.X.; An, G.; Vílchez, J.I.; Tang, K.; Yuan, F.T.; Sun, Y.Z.; Shao, C.Y.; Zhang, S.; et al. Rhizobacterium-derived diacetyl modulates plant immunity in a phosphate-dependent manner. EMBO J. 2020, 39, e102602. [Google Scholar] [CrossRef]
- Zimmermann, S.E.; Blau, S.; Frerigmann, H.; Krueger, S. The phosphorylated pathway of serine biosynthesis is crucial for indolic glucosinolate biosynthesis and plant growth promotion conferred by the root endophyte Colletotrichum tofieldiae. Plant Mol. Biol. 2021, 107, 85–100. [Google Scholar] [CrossRef]
- Hiruma, K.; Aoki, S.; Takino, J.; Higa, T.; Utami, Y.D.; Shiina, A.; Okamoto, M.; Nakamura, M.; Kawamura, N.; Ohmori, Y.; et al. A fungal sesquiterpene biosynthesis gene cluster critical for mutualist-pathogen transition in Colletotrichum tofieldiae. Nat Commun. 2023, 14, 5288. [Google Scholar] [CrossRef]
- Díaz-González, S.; Marín, P.; Sánchez, R.; Arribas, C.; Kruse, J.; González-Melendi, P.; Brunner, F.; Sacristán, S. Mutualistic fungal endophyte Colletotrichum tofieldiae Ct0861 colonizes and increases growth and yield of maize and tomato plants. Agronomy 2020, 10, 1493. [Google Scholar] [CrossRef]
- Ryu, Y.; Berry, J.A.; Baldocchi, D.D. What is global photosynthesis? History, uncertainties and opportunities. Remote Sens Environ. 2019, 223, 95–114. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef]
- Liddle, R.L.; Akinsanmi, O.A.; Galea, V.J. Non-host specificity of Botryosphaeriaceae on macadamia and blueberry. Australas. Plant Pathol. 2019, 48, 65. [Google Scholar] [CrossRef]
- Mengistu, G.; Shimelis, H.; Laing, M.; Lule, D. Assessment of farmers’perceptions of production constraints, and their trait preferences of sorghum in western Ethiopia: Implications for anthracnose resistance breeding. Acta Agric. Scand. B 2019, 69, 241–249. [Google Scholar]
- Ridzuan, R.; Rafii, M.Y.; Ismail, S.I.; Mohammad, Y.M.; Miah, G.; Usman, M. Breeding for anthracnose disease resistance in chili: Progress and prospects. Int. J. Mol. Sci. 2018, 19, 3122. [Google Scholar] [CrossRef] [PubMed]
- Tuluhong, M.; Mu, M.Q.; Wang, S.N.; Li, Y.G.; Cui, G.W. Identification and characterization of Colletotrichum truncatum and C. destructivum causing stem rot of white clover in China. Plant Dis. 2024, 108, 1236–1245. [Google Scholar] [CrossRef] [PubMed]



| Forage Species | Incubation Period (d) | Reseparation Rate (%) | Incidence Rate (%) (Mean ± SE) | Disease Index (Mean ± SE) |
|---|---|---|---|---|
| Vicia unijuga | 4 | 65.00 | 60.00 ± 10.18 ab | 50.00 ± 2.89 a |
| Medicago sativa | 6 | 60.00 | 28.89 ± 4.44 b | 15.00 ± 5.00 bc |
| Onobrychis viciifoila | 4 | 53.33 | 40.00 ± 7.69 ab | 20.00 ± 6.67 bc |
| Vicia sativa | 3 | 76.00 | 73.33 ± 13.87 a | 57.22 ± 15.48 a |
| Astragalus adsurgens | 5 | 60.00 | 40.00 ± 11.55 ab | 15.00 ± 8.66 bc |
| Trifolium pratense | 3 | 80.00 | 60.00 ± 20.00 ab | 28.33 ± 8.33 b |
| Trifolium repens | 3 | 53.33 | 26.67 ± 6.67 b | 6.67 ± 1.67 c |
| Measurement Index | Control | Treatment | Biomass Loss Rate (%) |
|---|---|---|---|
| Plant height (cm) | 14.60 ± 0.81 | 10.8 ± 0.73 | 26.0 |
| Root length (cm) | 9.60 ± 0.40 | 8.20 ± 0.58 | 14.6 |
| Aboveground fresh weights (g) | 0.19 ± 0.02 | 0.13 ± 0.01 | 33.7 |
| Aboveground dry weights (g) | 0.05 ± 0.00 | 0.04 ± 0.00 | 19.1 |
| Underground fresh weights (g) | 0.24 ± 0.03 | 0.14 ± 0.02 | 39.0 |
| Underground dry weights (g) | 0.06 ± 0.00 | 0.02 ± 0.00 | 61.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-T.; Li, H.; Li, Y.-Z. The Novel Disease Vicia unijuga Caused by Colletotrichum tofieldiae in China: Implications for Host Growth, Photosynthesis, and Nutritional Quality. J. Fungi 2025, 11, 567. https://doi.org/10.3390/jof11080567
Wang T-T, Li H, Li Y-Z. The Novel Disease Vicia unijuga Caused by Colletotrichum tofieldiae in China: Implications for Host Growth, Photosynthesis, and Nutritional Quality. Journal of Fungi. 2025; 11(8):567. https://doi.org/10.3390/jof11080567
Chicago/Turabian StyleWang, Tong-Tong, Hang Li, and Yan-Zhong Li. 2025. "The Novel Disease Vicia unijuga Caused by Colletotrichum tofieldiae in China: Implications for Host Growth, Photosynthesis, and Nutritional Quality" Journal of Fungi 11, no. 8: 567. https://doi.org/10.3390/jof11080567
APA StyleWang, T.-T., Li, H., & Li, Y.-Z. (2025). The Novel Disease Vicia unijuga Caused by Colletotrichum tofieldiae in China: Implications for Host Growth, Photosynthesis, and Nutritional Quality. Journal of Fungi, 11(8), 567. https://doi.org/10.3390/jof11080567





