Human Brain Organoids: A New Model to Study Cryptococcus neoformans Neurotropism
Abstract
1. Introduction
2. Results
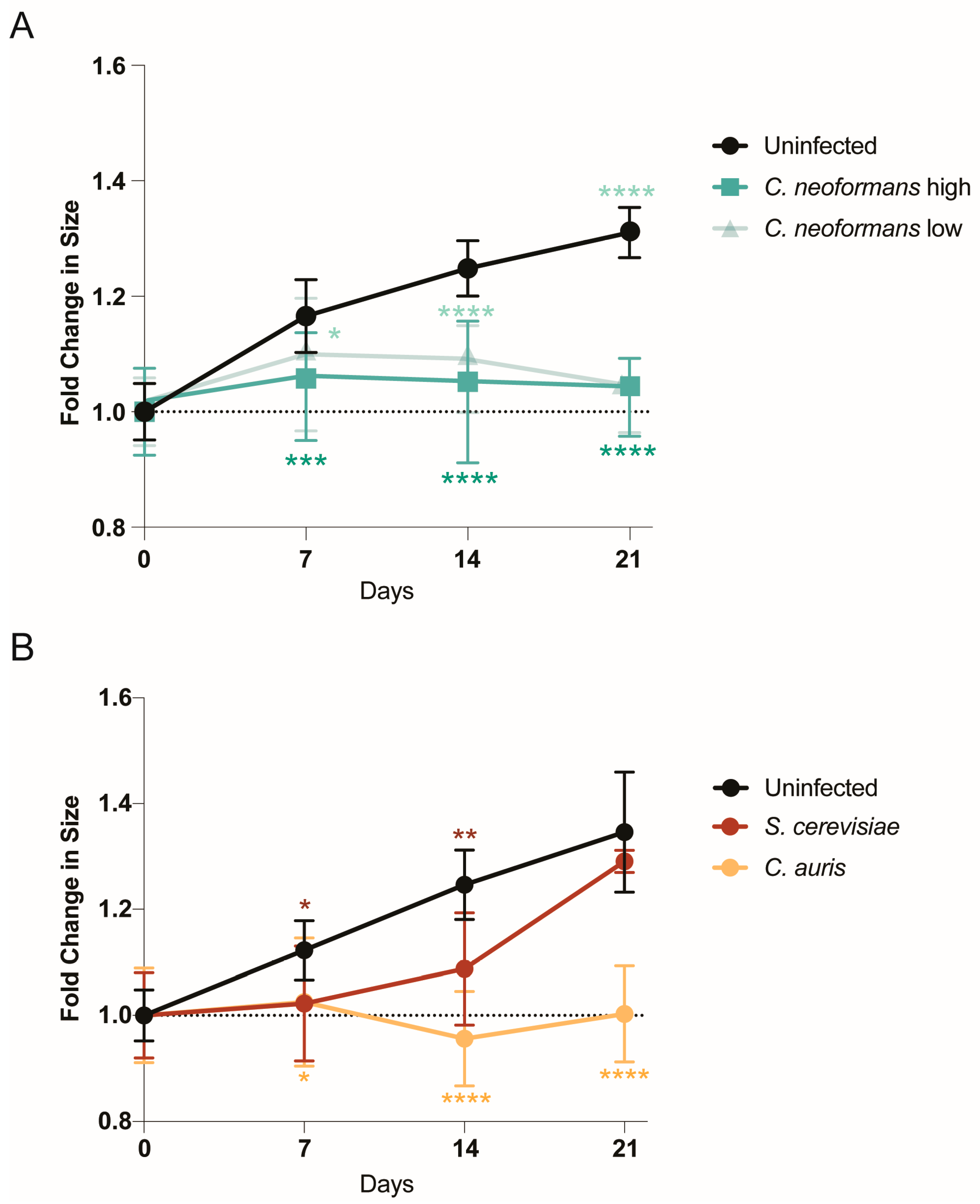
2.1. Organoids Display Growth Defects in Response to Pathogenic Fungi
2.2. C. neoformans Effectively Penetrates Organoid Tissues
2.3. C. neoformans Infection Disrupts Organoids’ Cellular Architecture
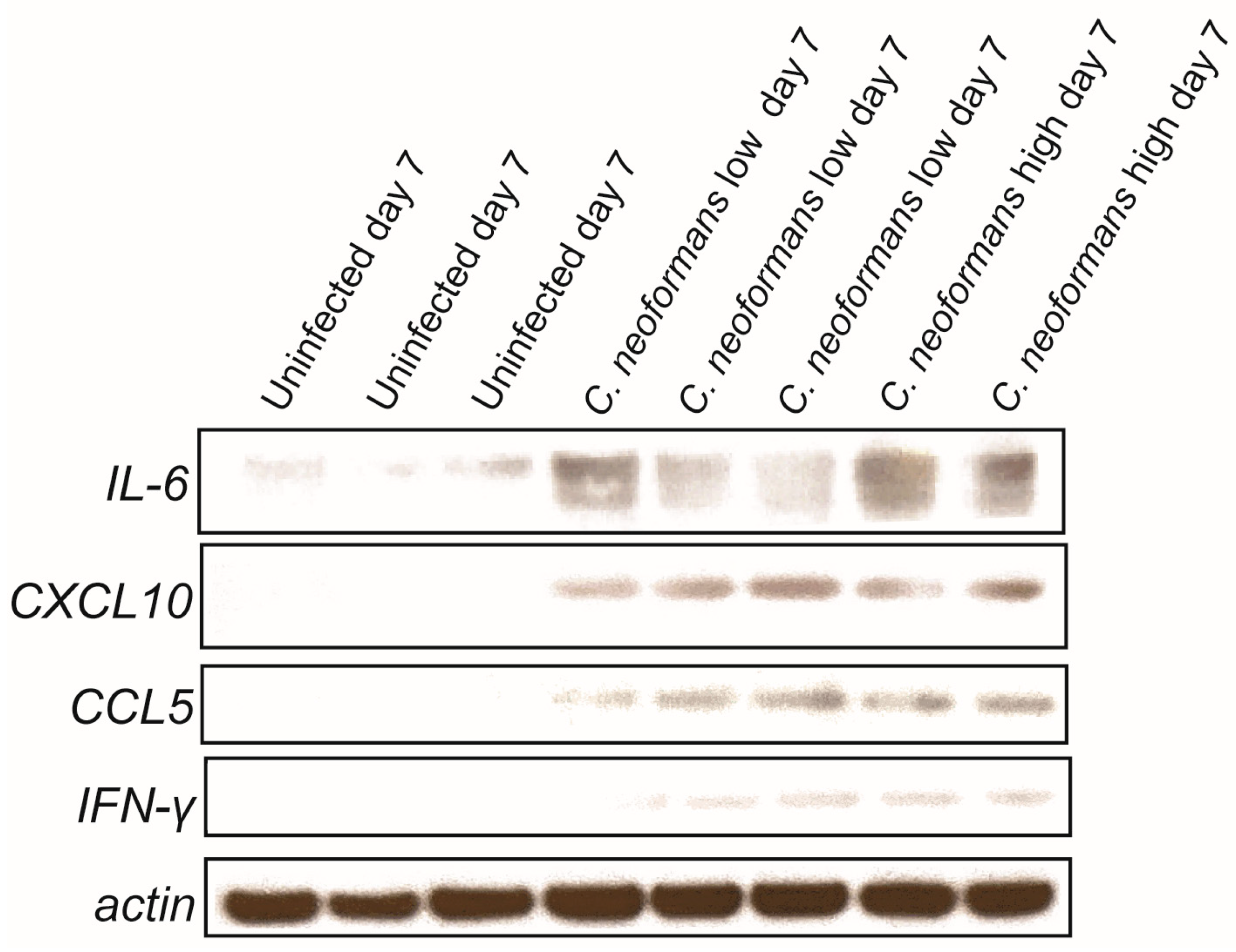
2.4. Cryptococcal Infection Induces Cytokine Induction in Brain Organoids
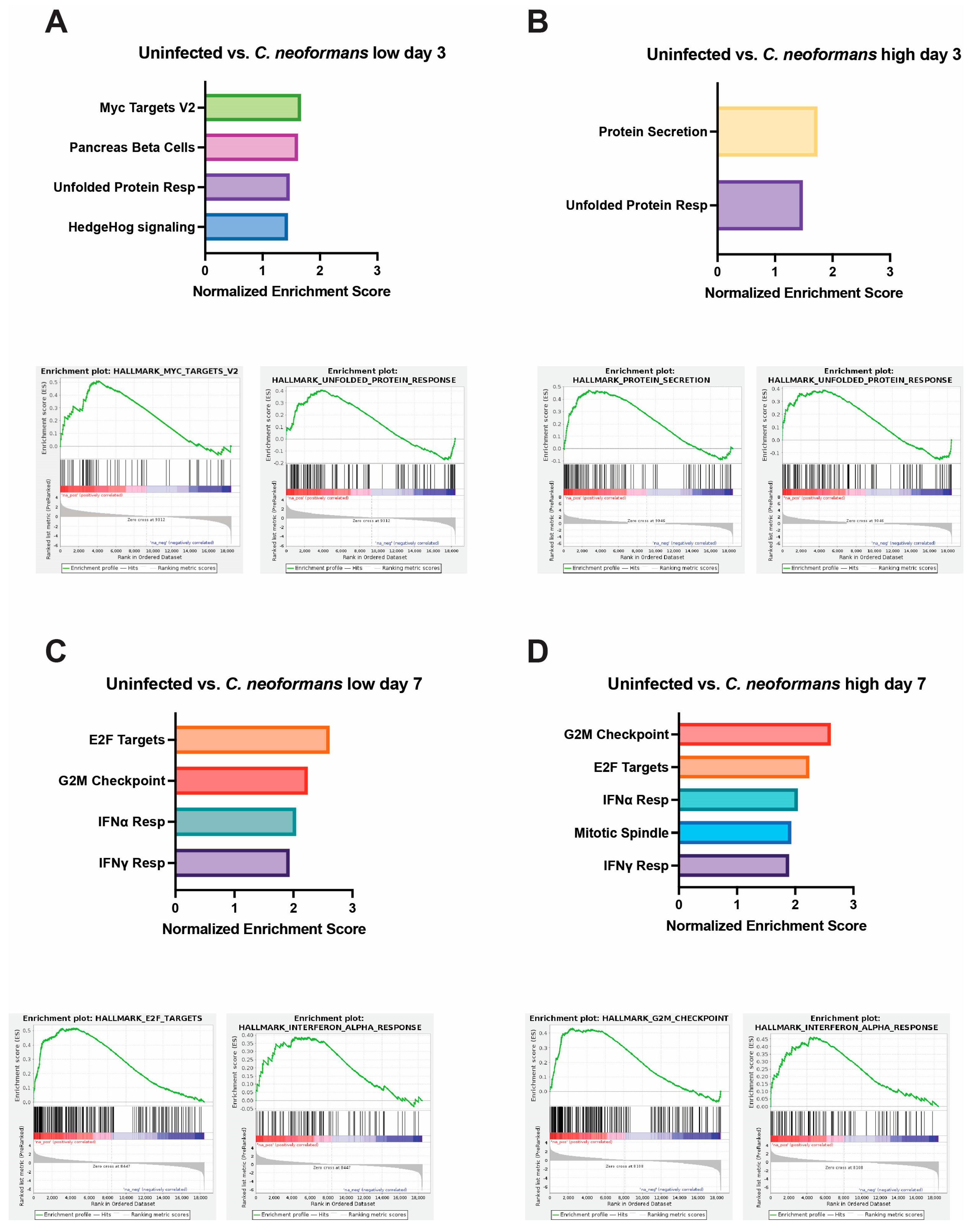
2.5. Organoid Transcriptional Response to Fungal Infection
3. Discussion
4. Methods
4.1. Organoid Generation from Human Embryonic Stem Cells (hESCs)
4.2. Fungal Infection of Organoids
4.3. Growth Measurements
4.4. Organoid Collection for Staining, Embedding, and Sectioning
4.5. Immunofluorescence Staining
4.6. Fungal Periodic Acid–Schiff Stain
4.7. Immunoblot Analysis
4.8. RNA Isolation and Sequencing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Bratton, E.W.; El Husseini, N.; Chastain, C.A.; Lee, M.S.; Poole, C.; Stürmer, T.; Juliano, J.J.; Weber, D.J.; Perfect, J.R.; Scheurer, M. Comparison and Temporal Trends of Three Groups with Cryptococcosis: HIV-Infected, Solid Organ Transplant, and HIV-Negative/Non-Transplant. PLoS ONE 2012, 7, e43582. [Google Scholar] [CrossRef]
- Sabiiti, W.; May, R.C. Mechanisms of Infection by the Human Fungal Pathogen Cryptococcus neoformans. Future Microbiol. 2012, 7, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A. Cryptococci at the brain gate: Break and enter or use a Trojan horse? J. Clin. Investig. 2010, 120, 1389. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Stins, M.F.; McCaffery, M.J.; Miller, G.F.; Pare, D.R.; Dam, T.; Paul-Satyasee, M.; Kim, K.S.; Kwon-Chung, K.J. Cryptococcal Yeast Cells Invade the Central Nervous System via Transcellular Penetration of the Blood-Brain Barrier. Infect. Immun. 2004, 72, 4985. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Tirado, F.H.; Klein, R.S.; Doering, T.L. An In Vitro Brain Endothelial Model for Studies of Cryptococcal Transmigration into the Central Nervous System. Curr. Protoc. Microbiol. 2019, 53, e78. [Google Scholar] [CrossRef] [PubMed]
- Normile, T.G.; Bryan, A.M.; Del Poeta, M. Animal Models of Cryptococcus neoformans in Identifying Immune Parameters Associated With Primary Infection and Reactivation of Latent Infection. Front. Immunol. 2020, 11, 581750. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Dickson, D.W.; Casadevall, A. Pathology of cryptococcal meningoencephalitis: Analysis of 27 patients with pathogenetic implications. Hum. Pathol. 1996, 27, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Wensink, G.E.; Elias, S.G.; Mullenders, J.; Koopman, M.; Boj, S.F.; Kranenburg, O.W.; Roodhart, J.M.L. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 2021, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tiwari, S.K.; Lichinchi, G.; Qin, Y.; Patil, V.S.; Eroshkin, A.M.; Rana, T.M. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 2016, 19, 258. [Google Scholar] [CrossRef] [PubMed]
- Halioti, A.; Vrettou, C.S.; Neromyliotis, E.; Gavrielatou, E.; Sarri, A.; Psaroudaki, Z.; Magira, E.E. Cerebrospinal Drain Infection by Candida auris: A Case Report and Review of the Literature. J. Fungi 2024, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Reguera-Gomez, M.; Munzen, M.E.; Hamed, M.F.; Charles-Niño, C.L.; Martinez, L.R. IL-6 deficiency accelerates cerebral cryptococcosis and alters glial cell responses. J. Neuroinflamm. 2024, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, G.; Ma, J.; Zhou, L.; Zhang, Q.; Gao, L. Lack of IL-6 increases blood–brain barrier permeability in fungal meningitis. J. Biosci. 2015, 40, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Meintjes, G.; Bicanic, T.; Buffa, V.; Hogan, L.; Mo, S.; Tomlinson, G.; Kropf, P.; Noursadeghi, M.; Harrison, T.S.; et al. Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis. PLoS Pathog. 2015, 11, e1004754. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; McNeil, L.K. Dissemination of C. neoformans to the central nervous system: Role of chemokines, Th1 immunity and leukocyte recruitment. J. Neurovirol. 1999, 5, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Hansakon, A.; Jeerawattanawart, S.; Pattanapanyasat, K.; Angkasekwinai, P. IL-25 Receptor Signaling Modulates Host Defense against Cryptococcus neoformans Infection. J. Immunol. 2020, 205, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gault, R.A.; Kozel, T.R.; Murphy, W.J. Protection from Direct Cerebral Cryptococcus Infection by Interferon-γ-Dependent Activation of Microglial Cells. J. Immunol. 2007, 178, 5753–5761. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Dickson, D.W.; Brosnan, C.F.; Casadevall, A. Human astrocytes inhibit Cryptococcus neoformans growth by a nitric oxide-mediated mechanism. J. Exp. Med. 1994, 180, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Uicker, W.C.; Doyle, H.A.; McCracken, J.P.; Langlois, M.; Buchanan, K.L. Cytokine and chemokine expression in the central nervous system associated with protective cell-mediated immunity against Cryptococcus neoformans. Med. Mycol. 2005, 43, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Neal, L.M.; Xing, E.; Xu, J.; Kolbe, J.L.; Osterholzer, J.J.; Segal, B.M.; Williamson, P.R.; Olszewski, M.A.; Dromer, F. Cd4+ T cells orchestrate lethal immune pathology despite fungal clearance during Cryptococcus neoformans meningoencephalitis. mBio 2017, 8, e01415-17. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Neal, L.M.; Ganguly, A.; Kolbe, J.L.; Hargarten, J.C.; Elsegeiny, W.; Hollingsworth, C.; He, X.; Ivey, M.; Lopez, R.; et al. Chemokine receptor CXCR3 is required for lethal brain pathology but not pathogen clearance during cryptococcal meningoencephalitis. Sci. Adv. 2020, 6, eaba2502. [Google Scholar] [CrossRef] [PubMed]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Yang, S.M.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545, 48. [Google Scholar] [CrossRef] [PubMed]
- Juan-Mateu, J.; Rech, T.H.; Villate, O.; Lizarraga-Mollinedo, E.; Wendt, A.; Turatsinze, J.V.; Brondani, L.A.; Nardelli, T.R.; Nogueira, T.C.; Esguerra, J.L.; et al. Neuron-enriched RNA-binding Proteins Regulate Pancreatic Beta Cell Function and Survival. J. Biol. Chem. 2017, 292, 3466–3480. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Blanco, R. N-myc and c-myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Mol. Brain Res. 2000, 77, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Casadesus, G.; Nunomura, A.; Zhu, X.; Castellani, R.J.; Richardson, S.L.; Perry, G.; Felsher, D.W.; Petersen, R.B.; Smith, M.A. The Neuronal Expression of MYC Causes a Neurodegenerative Phenotype in a Novel Transgenic Mouse. Am. J. Pathol. 2009, 174, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Kudo, W.; Zhu, X.; Smith, M.A.; Lee, H.G. Early induction of c-Myc is associated with neuronal cell death. Neurosci. Lett. 2011, 505, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, T.C.; Juillard, P.G.; Djordjevic, J.T.; Kaufman-Francis, K.; Dietmann, A.; Milonig, A.; Combes, V.; Grau, G.E. Cryptococcal transmigration across a model brain blood-barrier: Evidence of the Trojan horse mechanism and differences between Cryptococcus neoformans var. grubii strain H99 and Cryptococcus gattii strain R265. Microbes Infect. 2016, 18, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Weksler, B.; Romero, I.; Couraud, P.O.; Gelli, A. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot. Cell 2009, 8, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, R.D.; Putoczki, T.L.; Griffin, M.D.W. Structural Understanding of Interleukin 6 Family Cytokine Signaling and Targeted Therapies: Focus on Interleukin 11. Front. Immunol. 2020, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, S.; Miyamoto, T. Elevated levels of interleukin-6 in cerebrospinal fluid from patients with systemic lupus erythematosus and central nervous system invol vement. Arthritis Rheum. 1990, 33, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, M.A.; Sidén, Å.; Persson, M.A.A.; Norkrans, G.; Hagberg, L.; Chiodi, F. Cerebrospinal fluid interleukin-6 activity in HIV infection and inflammatory and noninflammatory diseases of the nervous system. Clin. Immunol. Immunopathol. 1990, 57, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S.; Lin, E.; Zhang, B.; Luster, A.D.; Tollett, J.; Samuel, M.A.; Engle, M.; Diamond, M.S. Neuronal CXCL10 Directs CD8+ T-Cell Recruitment and Control of West Nile Virus Encephalitis. J. Virol. 2005, 79, 11457. [Google Scholar] [CrossRef] [PubMed]
- Petrisko, T.J.; Bloemer, J.; Pinky, P.D.; Srinivas, S.; Heslin, R.T.; Du, Y.; Setti, S.E.; Hong, H.; Suppiramaniam, V.; Konat, G.W.; et al. Neuronal CXCL10/CXCR3 Axis Mediates the Induction of Cerebral Hyperexcitability by Peripheral Viral Challenge. Front. Neurosci. 2020, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Strickland, A.; Shi, M. IFN-γ signaling is essential for the clearance of C. neoformans in the brain and survival of the infected mice. J. Immunol. 2020, 204, 231.2. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Brouwer, A.E.; Wuthiekanun, V.; Jaffar, S.; Shattock, R.; Irving, D.; Sheldon, J.; Chierakul, W.; Peacock, S.; Day, N.; et al. IFN-γ at the Site of Infection Determines Rate of Clearance of Infection in Cryptococcal Meningitis. J. Immunol. 2005, 174, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xu, Y.; Guo, P.; Chen, Y.J.; Zhou, J.; Xia, M.; Tan, B.; Liu, X.; Feng, H.; Chen, Y. CCL5/CCR5-mediated peripheral inflammation exacerbates blood–brain barrier disruption after intracerebral hemorrhage in mice. J. Transl. Med. 2023, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, A. CCL5-glutamate cross-talk in astrocyte-neuron communication in multiple sclerosis. Front. Immunol. 2017, 8, 285787. [Google Scholar] [CrossRef] [PubMed]
- Stawowczyk, M.; Naseem, S.; Montoya, V.; Baker, D.P.; Konopka, J.; Reich, N.C. Pathogenic effects of IFIT2 and interferon-β during fatal systemic Candida albicans infection. mBio 2018, 9, e00365-18. [Google Scholar] [CrossRef] [PubMed]
- Del Fresno, C.; Soulat, D.; Roth, S.; Blazek, K.; Udalova, I.; Sancho, D.; Ruland, J.; Ardavín, C. Interferon-β Production via Dectin-1-Syk-IRF5 Signaling in Dendritic Cells Is Crucial for Immunity to C. albicans. Immunity 2013, 38, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Majer, O.; Bourgeois, C.; Zwolanek, F.; Lassnig, C.; Kerjaschki, D.; Mack, M.; Müller, M.; Kuchler, K.; Klein, B.S. Type I Interferons Promote Fatal Immunopathology by Regulating Inflammatory Monocytes and Neutrophils during Candida Infections. PLoS Pathog. 2012, 8, e1002811. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, V.; Dutta, O.; McElrath, C.; Du, P.; Chang, Y.J.; Cicciarelli, B.; Pitler, A.; Whitehead, I.; Obar, J.J.; Durbin, J.E.; et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci. Immunol. 2017, 2, eaan5357. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.P.; Ng, A.; Kumar, V.; Johnson, M.D.; Plantinga, T.S.; Van Diemen, C.; Arts, P.; Verwiel, E.T.P.; Gresnigt, M.S.; Fransen, K.; et al. Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans. Nat. Commun. 2013, 4, 1342. [Google Scholar] [CrossRef] [PubMed]
- Pekmezovic, M.; Hovhannisyan, H.; Gresnigt, M.S.; Iracane, E.; Oliveira-Pacheco, J.; Siscar-Lewin, S.; Seemann, E.; Qualmann, B.; Kalkreuter, T.; Müller, S.; et al. Candida pathogens induce protective mitochondria-associated type I interferon signalling and a damage-driven response in vaginal epithelial cells. Nat. Microbiol. 2021, 6, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.B.; Kwaku, G.; Reardon, C.; Khan, N.; Zamith-Miranda, D.; Zarnowski, R.; Tam, J.M.; Bohaen, C.K.; Richey, L.; Mosallanejad, K.; et al. Candida albicans Extracellular Vesicles Trigger Type I IFN Signaling via cGAS and STING. Nat. Microbiol. 2024, 9, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Guo, H.; Wang, L.; Zhang, Y.; Sun, L.; Dai, C.; Wu, X. The cGAS-STING signaling pathway contributes to the inflammatory response and autophagy in Aspergillus fumigatus keratitis. Exp. Eye Res. 2021, 202, 108366. [Google Scholar] [CrossRef] [PubMed]
- Kwaku, G.N.; Jensen, K.N.; Simaku, P.; Floyd, D.J.; Saelens, J.W.; Reardon, C.M.; Ward, R.A.; Basham, K.J.; Hepworth, O.W.; Vyas, T.D.; et al. Extracellular vesicles from diverse fungal pathogens induce species-specific and endocytosis-dependent immunomodulation. PLoS Pathog. 2025, 21, e1012879. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Muffat, J.; Omer, A.; Bosch, I.; Lancaster, M.A.; Sur, M.; Gehrke, L.; Knoblich, J.A.; Jaenisch, R. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 2016, 20, 385. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harding, A.T.; Gehrke, L.; Vyas, J.M.; Harding, H.B. Human Brain Organoids: A New Model to Study Cryptococcus neoformans Neurotropism. J. Fungi 2025, 11, 539. https://doi.org/10.3390/jof11070539
Harding AT, Gehrke L, Vyas JM, Harding HB. Human Brain Organoids: A New Model to Study Cryptococcus neoformans Neurotropism. Journal of Fungi. 2025; 11(7):539. https://doi.org/10.3390/jof11070539
Chicago/Turabian StyleHarding, Alfred T., Lee Gehrke, Jatin M. Vyas, and Hannah Brown Harding. 2025. "Human Brain Organoids: A New Model to Study Cryptococcus neoformans Neurotropism" Journal of Fungi 11, no. 7: 539. https://doi.org/10.3390/jof11070539
APA StyleHarding, A. T., Gehrke, L., Vyas, J. M., & Harding, H. B. (2025). Human Brain Organoids: A New Model to Study Cryptococcus neoformans Neurotropism. Journal of Fungi, 11(7), 539. https://doi.org/10.3390/jof11070539






