Biocontrol Potential of Native Trichoderma Strains Toward Soil-Borne Phytopathogenic and Saprotrophic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Cultivation Conditions
2.2. Molecular Identification of the Fungi
2.3. Confrontation Assay
2.4. Isolation of Crude Extracts
2.5. Thin-Layer Chromatography (TLC) Analysis
2.6. Well Diffusion Method
2.7. Calculation of the Dry Substance Concentration of the Crude Extract That Causes a Minimum Observable Inhibition of Mycelial Growth (MIMGI) of the Target Fungi
2.8. Treatment of the Liquid Culture of P. blaeksleeanus with Crude Extract and Preparation of the Mycelial Lysate for the Measurement of Enzyme Activity
2.9. Quantification of the Protein Content and Enzyme Activities in the Mycelial Lysate
2.10. Statistical Comparisons and Curve Fitting
3. Results
3.1. Sequence Analysis
3.2. Susceptibility of the Tested Fungi to Trichoderma Isolates
3.3. Antifungal Potential of Trichoderma spp. Crude Extracts
3.4. Effects of T. harzianum-1S2-6 Extract on P. blakesleeanus Enzyme Activities
3.5. Confirmation of the Presence of Peptaibol-like Compounds in the Crude Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Sharma, S.; Kaur, I.; Nagpal, A.K. Chapter Three—Pesticides in Agriculture: Food Security vs. Food Safety. In Advances in Food Security and Sustainability; Elsevier: Amsterdam, The Netherlands, 2024; Volume 9, pp. 59–73. [Google Scholar]
- Manoharachary, C.; Singh, H.B.; Varma, A. Trichoderma: Agricultural Applications and Beyond; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Fischer, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 2022, 30, 384. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F. Indirect Effect of Pesticides on Insects and Other Arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 19, 2329. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Nawrocka, J.; Małolepsza, U. Diversity in plant systemic resistance induced by Trichoderma. Biol. Control 2013, 67, 149–156. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 3, 1160551. [Google Scholar] [CrossRef]
- Degenkolb, T.; von Dohren, H.; Nielsen, K.F.; Samuels, G.J.; Bruckner, H. Recent advances and future prospects in peptaibiotics, hydrophobin, and mycotoxin research, and their importance for chemotaxonomy of Trichoderma and Hypocrea. Chem. Biodivers. 2008, 5, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Chenthamara, K.; Zhang, J.; Atanasova, L.; Yang, D.; Miao, Y.; Rahimi, M.J.; Grujic, M.; Cai, F.; Pourmehdi, S.; et al. Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLoS Genet. 2018, 14, e1007322. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Dias, L.; Oliveira-Pinto, P.R.; Fernandes, J.O.; Regalado, L.; Mendes, R.; Teixeira, C.; Mariz-Ponte, N.; Gomes, P.; Santos, C. Peptaibiotics: Harnessing the potential of microbial secondary metabolites for mitigation of plant pathogens. Biotechnol. Adv. 2023, 68, 108223. [Google Scholar] [CrossRef]
- Vurro, M.; Gressel, J. Novel biotechnologies for biocontrol agent enhancement and management. In Exploiting the Interactions Between Fungal Antagonist, Pathogens and the Plant for Biocontrol; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Svensson, S.E.; Oliveira, A.O.; Adolfsson, K.H.; Heinmaa, I.; Root, A.; Kondori, N.; Ferreira, J.A.; Hakkarainen, M.; Zamani, A. Turning food waste to antibacterial and biocompatible fungal chitin/chitosan monofilaments. Int. J. Biol. Macromol. 2022, 209, 618–630. [Google Scholar] [CrossRef]
- Cheng, Q.; Dickwella Widanage, M.C.; Yarava, J.R.; Ankur, A.; Latgé, J.P.; Wang, P.; Wang, T. Molecular architecture of chitin and chitosan-dominated cell walls in zygomycetous fungal pathogens by solid-state NMR. Nat. Commun. 2024, 15, 8295. [Google Scholar] [CrossRef]
- Žerjav, M.; Schroers, H.J. First report of cucurbita fruit rot caused by Choanephora cucurbitarum in Slovenia. Plant Dis. 2019, 103, 760. [Google Scholar] [CrossRef]
- Yin, H.; Tian, M.; Peng, Y.; Qin, N.; Lü, H.; Ren, L.; Zhao, X. First Report on Choanephora cucurbitarum causing choanephora rot in Chenopodium plants and its sensitivity to fungicide. J. Fungi 2023, 9, 881. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I. Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef]
- Anees, M.; Tronsmo, A.; Edel-Hermann, V.; Hjeljord, L.G.; Héraud, C.; Steinberg, C. Characterization of field isolates of Trichoderma antagonistic against Rhizoctonia solani. Fungal Biol. 2010, 114, 691–701. [Google Scholar] [CrossRef]
- Asad, S.A.; Ali, N.; Hameed, A.; Khan, S.A.; Ahmad, R.; Bilal, M.; Shahzad, M.; Tabassum, A. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Pol. J. Microbiol. 2014, 63, 95–103. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of rhizoctonia seedling disease of soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Asad-Uz-Zaman, M.; Bhuiyan, M.R.; Khan, M.A.; Alam Bhuiyan, M.K.; Latif, M.A. Integrated options for the management of black root rot of strawberry caused by Rhizoctonia solani Kuhn. Comptes Rendus. Biol. 2015, 338, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.B.; Cerqueira, F.M.; Silva, R.d.N.; Ulhoa, C.J.; Lima, A.L. Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: Evaluation of coiling and hydrolytic enzyme production. Biotechnol. Lett. 2007, 29, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Halifu, S.; Deng, X.; Song, X.; Song, R.; Liang, X. Inhibitory mechanism of Trichoderma virens ZT05 on Rhizoctonia solani. Plants 2020, 19, 912. [Google Scholar] [CrossRef]
- Niu, X.; Thaochan, N.; Hu, Q. Diversity of linear non-ribosomal peptide in biocontrol fungi. J. Fungi 2020, 6, 61. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Vignolle, G.A.; Mach, R.L.; Mach-Aigner, A.R.; Derntl, C. Novel approach in whole genome mining and transcriptome analysis reveal conserved RiPPs in Trichoderma spp. BMC Genom. 2020, 21, 258. [Google Scholar] [CrossRef]
- Troian, R.F.; Steindorff, A.S.; Ramada, M.H.; Arruda, W.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: Evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol. Lett. 2014, 36, 2095–2101. [Google Scholar] [CrossRef]
- Risoli, S.; Cotrozzi, L.; Pisuttu, C.; Nali, C. Biocontrol agents of Fusarium head blight in wheat: A meta-analytic approach to elucidate their strengths and weaknesses. Phytopathology 2024, 114, 521–537. [Google Scholar] [CrossRef]
- Zaker, M. Natural plant products as eco-friendly fungicides for plant diseases control—A Review. Agriculturists 2016, 14, 134–141. [Google Scholar] [CrossRef]
- Food and Agriculture Organization for the United Nations. Achieving SDG2 Without Breaching the 1.5C Threshold: A Global Roadmap (2023). Available online: http://www.fao.org/interactive/sdg2-roadmap/assets/3d-models/inbrief-roadmap.pdf (accessed on 13 May 2025).
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 618–630. [Google Scholar] [CrossRef]
- Stevanović, K.S.; Čepkenović, B.; Križak, S.; Živić, M.Ž.; Todorović, N.V. ATP modulation of osmotically activated anionic current in the membrane of Phycomyces blakesleeanus sporangiophore. Sci. Rep. 2023, 13, 11897. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, K.S.; Čepkenović, B.; Križak, S.; Živić, M.Ž.; Todorović, N.V. Osmotically Activated Anion Current of Phycomyces Blakesleeanus—Filamentous Fungi Counterpart to Vertebrate Volume Regulated Anion Current. J. Fungi 2023, 9, 637. [Google Scholar] [CrossRef]
- Pajić, T.; Stevanović, K.; Todorović, N.V.; Krmpot, A.J.; Živić, M.; Savić-Šević, S.; Lević, S.M.; Stanić, M.; Pantelić, D.; Jelenković, B.; et al. In vivo femtosecond laser nanosurgery of the cell wall enabling patch-clamp measurements on filamentous fungi. Microsyst. Nanoeng. 2024, 10, 47. [Google Scholar] [CrossRef]
- Lukičić, J.; Cvetić Antić, T.; Živić, M.; Atlagić, K.; Mirčić, D.; Tanović, M.; Stanić, M. Activation of antioxidative metabolism in different growth stages of Phycomyces blakesleeanus mycelia exposed to vanadate. Compr. Plant Biol. 2025, 49, 195–210. [Google Scholar]
- Žižić, M.; Stanić, M.; Aquilanti, G.; Bajuk-Bogdanović, D.; Branković, G.; Rodić, I.; Živić, M.; Zakrzewska, J. Biotransformation of selenium in the mycelium of the fungus Phycomyces blakesleeanus. Anal. Bioanal. Chem. 2022, 414, 6213–6222. [Google Scholar] [CrossRef]
- Pajić, T.; Todorović, N.V.; Živić, M.; Nikolić, S.N.; Rabasović, M.D.; Clayton, A.H.A.; Krmpot, A.J. Label-free third harmonic generation imaging and quantification of lipid droplets in live filamentous fungi. Sci. Rep. 2022, 12, 18760. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Ivanović, Ž.; Blagojević, J.; Popović, S.; Vukojevč, J.; Ljaljević Grbić, M. Diversity and seasonal dynamics of culturable airborne fungi in a cultural heritage conservation facility. Int. Biodeterior. Biodegrad. 2021, 157, 105163. [Google Scholar] [CrossRef]
- Cerdá-Olmedo, E. Phycomyces and the biology of light and color. FEMS Microbiol. Rev. 2001, 25, 503–512. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA gene for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; p. 482. [Google Scholar]
- Tomah, A.A.; Abd Alamer, I.S.; Li, B.; Zhang, J.Z. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biol. Control 2020, 145, 104261. [Google Scholar] [CrossRef]
- Six, D.L.; De Beer, Z.W.; Duong, T.A.; Carroll, A.L.; Wingfield, M.J. Fungal associates of the lodgepole pine beetle, Dendroctonus murrayanae. Antonie Leeuwenhoek 2011, 100, 231–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez, M.A.; Venedikian, N.; Godeas, A. Fungal populations on sunflower (Helianthus annuus) anthosphere and their relation to susceptibility or olerance to Sclerotinia sclerotiorum attack. Mycopathologia 2001, 150, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, A.; Leitgeb, B.; Kredics, L.; Manczinger, L.; Vagvolgyi, C. A novel, image analysis-based method for the evaluation of in vitro antagonism. J. Microbiol. Methods 2006, 65, 619–622. [Google Scholar] [CrossRef]
- Tamandegani, P.R.; Marik, T.; Zafari, D.; Balázs, D.; Vágvölgyi, C.; Szekeres, A.; Kredics, L. Changes in peptaibol production of Trichoderma species during In Vitro antagonistic interactions with fungal plant pathogens. Biomolecules 2020, 10, 730. [Google Scholar] [CrossRef]
- Pandey, R.C.; Misra, R.; Rinehart, K.L., Jr. Visualization of N-protected peptides, amino acids and aminocyclitol antibiotics on a thin-layer chromatogram by ninhydrin. J. Chromatogr. A 1979, 170, 498–501. [Google Scholar] [CrossRef]
- Cerda-Olmedo, E. Standard growth conditions and variations. In Phycomyces; Cerda-Olmedo, E., Lipson, E.D.E., Eds.; Cold Spring Harbor Laboratory: New York, NY, USA, 1987; pp. 337–339. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Del Rio, L.A.; Ortega, M.G.; Lopez, A.L.; Gorge, J.L. A more sensitive modification of the catalase assay with the Clark oxygen electrode: Application to the kinetic study of the pea leaf enzyme. Anal. Biochem. 1977, 80, 409–415. [Google Scholar] [CrossRef]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Tančić, S.; Skrobonja, J.; Lalošević, M.; Jevtić, R.; Vidić, M. Impact of Trichoderma spp. on soybean seed germination and potential antagonistic effect on Sclerotinia sclerotiorum. Pestic. Phytomed. 2013, 28, 181–185. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, H.; Zhang, F.; Guo, N.; Wang, Y.; Chen, L.; Ji, X.; Li, C. Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Biochem. 2016, 100, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Bhale, U.N.; Wagh, P.M.; Rajkonda, J.N. Antagonistic confrontation of Trichoderma spp. against fruit rot pathogens on Sapodilla (Manilkara zapota L.). J. Yeast Fungal Res. 2013, 4, 5–11. [Google Scholar]
- Kotasthane, A.; Agrawal, T.; Kushwah, R.; Rahatkar, O.V. In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. Eur. J. Plant Pathol. 2014, 141, 523–543. [Google Scholar] [CrossRef]
- Budha, C.B.; Shrestha, S.M.; Manandhar, H.K.; Baidya, S. Evaluation of Trichoderma spp. against wirestem disease of cauliflower caused by Rhizoctonia solani. J. Agric. Environ. 2023, 24, 127–136. [Google Scholar] [CrossRef]
- Singh, S.; Balodi, R.; Meena, P.N.; Singhal, S. Biocontrol activity of Trichoderma harzianum, Bacillus subtilis and Pseudomonas fluorescens against Meloidogyne incognita, Fusarium oxysporum and Rhizoctonia solani. Indian. Phytopathol. 2021, 74, 703–714. [Google Scholar] [CrossRef]
- Vitti, A.; Bevilacqua, V.; Logozzo, G.; Bochicchio, R.; Amato, M.; Nuzzaci, M. Seed coating with Trichoderma harzianum T-22 of Italian durum wheat increases protection against Fusarium culmorum-induced crown rot. Agriculture 2022, 12, 714. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Atanasova, L.; Crom, S.L.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef]
- Kredics, L.; Antal, Z.; Szekeres, A.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C.; Nagy, E. Extracellular proteases of Trichoderma species. A review. Acta Microbiol. Immunol. Hung. 2005, 52, 169–184. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Singh, B.; Raja, M.; Ismaiel, A.; Lakshman, D.K.; Tomar, M.; Sharma, P. Genetic diversity and antagonistic properties of Trichoderma strains from the crop rhizospheres in southern Rajasthan, India. Sci. Rep. 2024, 14, 8610. [Google Scholar] [CrossRef] [PubMed]
- Andrés, P.A.; Alejandra, P.M.; Benedicto, M.C.; Nahuel, R.I.; Clara, B.M. A Comparative study of different strains of Trichoderma under different conditions of temperature and pH for the control of Rhizoctonia solani. Agric. Sci. 2022, 13, 702–714. [Google Scholar]
- Purwantisari, S.; Nurbayani, F.A.; Safina, M.F.I.; Choiriyah, M. Antagonistic activity of Trichoderma harzianum against Aspergillus parasiticus and Mucor circinelloides in corn plant (Zea mays L.). Planta Trop. 2025, 13, 38–51. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Koeppe, R.E.; Andersen, O.S. Intrinsic lipid curvature and bilayer elasticity as regulators of channel function: A comparative single-molecule study. Int. J. Mol. Sci. 2024, 25, 2758. [Google Scholar] [CrossRef]
- Fox, R.; Richards, F. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-Å resolution. Nature 1982, 300, 325–330. [Google Scholar] [CrossRef]
- Nagao, T.; Mishima, D.; Javkhlantugs, N.; Wang, J.; Ishioka, D.; Yokota, K.; Norisada, K.; Kawamura, I.; Ueda, K.; Naito, A. Structure and orientation of antibiotic peptide alamethicin in phospholipid bilayers as revealed by chemical shift oscillation analysis of solid state nuclear magnetic resonance and molecular dynamics. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2789–2798. [Google Scholar] [CrossRef]
- Meyer, C.E.; Reusser, F. A polypeptide antibacterial agent isolated from Trichoderma viride. Experientia 1967, 23, 85–86. [Google Scholar] [CrossRef]
- Béven, L.; Duval, D.; Rebuffat, S.; Riddell, F.G.; Bodo, B.; Wróblewski, H. Membrane permeabilisation and antimycoplasmic activity of the 18-residue peptaibols, trichorzins PA. Biochim. Biophys. Acta. 1998, 1372, 78–90. [Google Scholar] [CrossRef]
- Alfaro-Vargas, P.; Bastos-Salas, A.; Muñoz-Arrieta, R.; Pereira-Reyes, R.; Redondo-Solano, M.; Fernández, J.; Mora-Villalobos, A.; López-Gómez, J.P. Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide. J. Fungi 2022, 8, 1037. [Google Scholar] [CrossRef]
- Mueller, D.S.; Dorrance, A.E.; Derksen, R.C.; Ozkan, E.; Kurle, J.E.; Grau, C.R.; Gaska, J.M.; Hartman, G.L.; Bradley, C.A.; Pedersen, W.L. Efficacy of fungicides on Sclerotinia sclerotiorum and their potential for control of Sclerotinia stem rot on soybean. Plant Dis. 2002, 86, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Marik, T.; Tyagi, C.; Racić, G.; Rakk, D.; Szekeres, A.; Vágvölgyi, C.; Kredics, L. New 19-Residue peptaibols from Trichoderma clade viride. Microorganisms 2018, 12, 85. [Google Scholar] [CrossRef]
- Xiao-Yan, S.; Qing-Tao, S.; Shu-Tao, X.; Xiu-Lan, C.; Cai-Yun, S.; Yu-Zhong, Z. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiol. Lett. 2006, 260, 119–125. [Google Scholar] [CrossRef]
- Lian, H.; Li, R.; Ma, G.; Zhao, Z.; Zhang, T.; Li, M. The effect of Trichoderma harzianum agents on physiological-biochemical characteristics of cucumber and the control effect against Fusarium wilt. Sci. Rep. 2023, 13, 17606. [Google Scholar] [CrossRef]
- Wonglom, P.; Ruangwong, O.-U.; Poncheewin, W.; Arikit, S.; Riangwong, K.; Sunpapao, A. Trichoderma-bioenriched vermicompost induces defense response and promotes plant growth in thai rice variety “Chor Khing”. J. Fungi 2024, 10, 582. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Tohge, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Villalobos-Escobedo, J.M.; Esparza-Reynoso, S.; Pelagio-Flores, R.; López-Ramírez, F.; Ruiz-Herrera, L.F.; López-Bucio, J.; Herrera-Estrella, A. The fungal NADPH oxidase is an essential element for the molecular dialog between Trichoderma and Arabidopsis. Plant J. 2020, 103, 2178–2192. [Google Scholar] [CrossRef]
- Lu, M.; Wen, T.; Guo, M.; Li, Q.; Peng, X.; Zhang, Y.; Lu, Z.; Wang, J.; Xu, Y.; Zhang, C. Regulation of intracellular reactive oxygen species levels after the development of Phallus rubrovolvatus rot disease due to Trichoderma koningii mycoparasitism. J. Fungi 2023, 9, 525. [Google Scholar] [CrossRef]
- Ojaghian, S.; Wang, L.; Xie, G.L.; Zhang, J.Z. Effect of volatiles produced by Trichoderma spp. on expression of glutathione transferase genes in Sclerotinia sclerotiorum. Biol. Control 2019, 136, 103999. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, Y.; Rahimi, M.J.; Zhu, H.; Steindorff, A.; Schiessler, S.; Cai, F.; Pang, G.; Chenthamara, K.; Xu, Y.; et al. Guttation capsules containing hydrogen peroxide: An evolutionarily conserved NADPH oxidase gains a role in wars between related fungi. Environ. Microbiol. 2019, 21, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Mironenka, J.; Różalska, S.; Soboń, A.; Bernat, P. Trichoderma harzianum metabolites disturb Fusarium culmorum metabolism: Metabolomic and proteomic studies. Microbiol. Res. 2021, 249, 126770. [Google Scholar] [CrossRef] [PubMed]
- Rodić, I.; Žižić, M.; Lukičić, J.; Stanić, M.; Gianoncelli, A.; Bonanni, V.; Zakrzewska, J.; Živić, M.; Cvetić Antić, T. Metabolic changes in Phycomyces blakesleeanus mycelia during selenite reduction and cellular localization of synthesized SeNPs. World J. Microb. Biot. 2025, 41, 254. [Google Scholar] [CrossRef]
- Nartey, L.K.; Pu, Q.; Zhu, W.; Zhang, S.; Li, J.; Yao, Y.; Hu, X. Antagonistic and plant growth promotion effects of Mucor moelleri, a potential biocontrol agent. Microbiol. Res. 2022, 255, 126922. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Morel, M.; Ngadin, A.A.; Droux, M.; Jacquot, J.P.; Gelhaye, E. The fungal glutathione S-transferase system. Evidence of new classes in the wood-degrading basidiomycete Phanerochaete chrysosporium. Cell. Mol. Life Sci. 2009, 66, 3711–3725. [Google Scholar] [CrossRef]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-related enzymes and proteins: A review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- de Castro, C.; del Valle, P.; Rúa, J.; García-Armesto, M.R.; Gutiérrez-Larraínzar, M.; Busto, F.; de Arriaga, D. Antioxidant defence system during exponential and stationary growth phases of Phycomyces blakesleeanus: Response to oxidative stress by hydrogen peroxide. Fungal Biol. 2013, 117, 275–287. [Google Scholar] [CrossRef]
- Ye, W.; Chen, Y.; Zhang, W.; Liu, T.; Liu, Y.; Li, M.; Li, S.; Xu, L.; Liu, H. Potential anti-Candida albicans mechanism of Trichoderma acid from Trichoderma spirale. Int. J. Mol. Sci. 2023, 24, 5445. [Google Scholar] [CrossRef]
- Setargie, A.; Wang, C.; Zhang, L.; Xu, Y. Chromatographic and mass spectroscopic guided discovery of Trichoderma peptaibiotics and their bioactivity. Eng. Microbiol. 2024, 4, 100135. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, C.R.; Vilcinskas, A.; Brückner, H.; Degenkolb, T. The sequences of the eleven-residue peptaibiotics: Suzukacillins-B. Chem. Biodivers. 2013, 10, 827–837. [Google Scholar] [CrossRef] [PubMed]

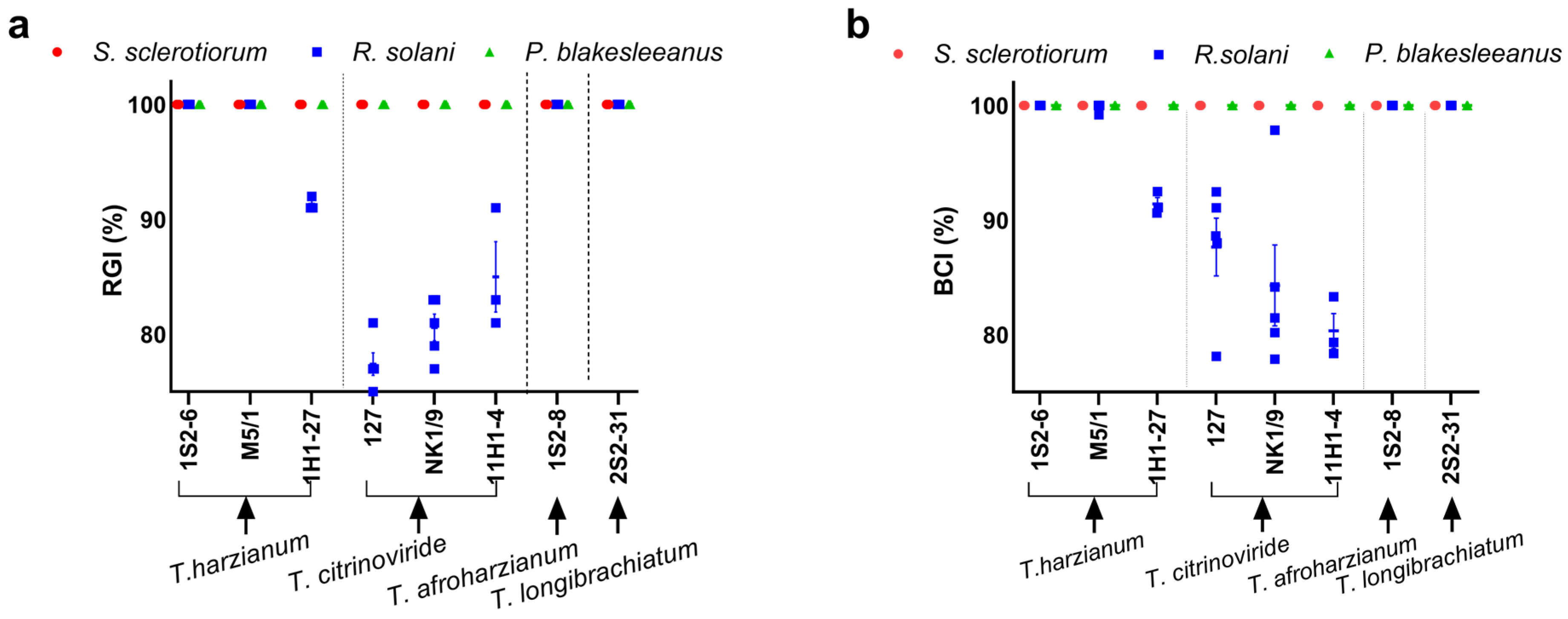
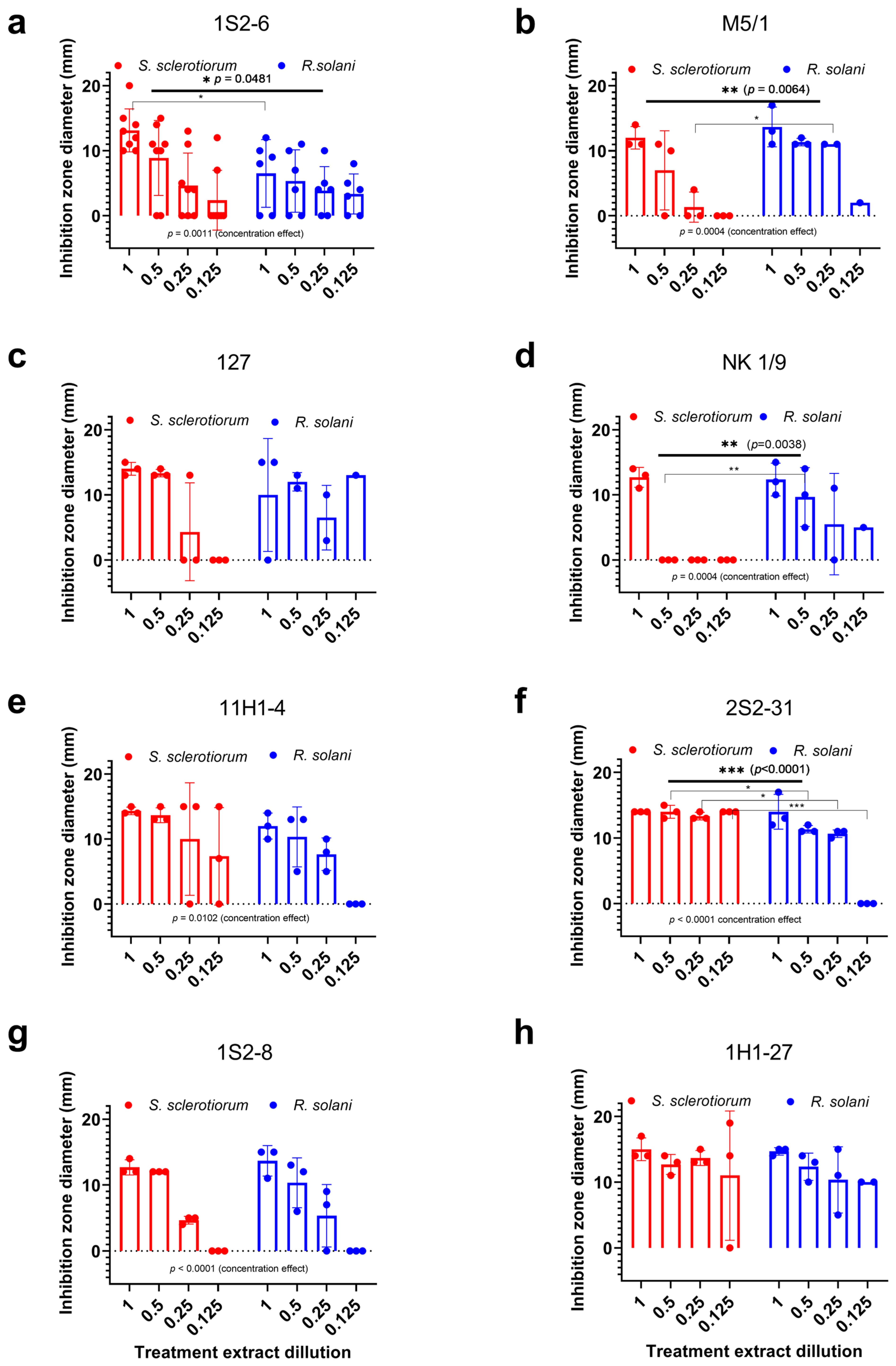
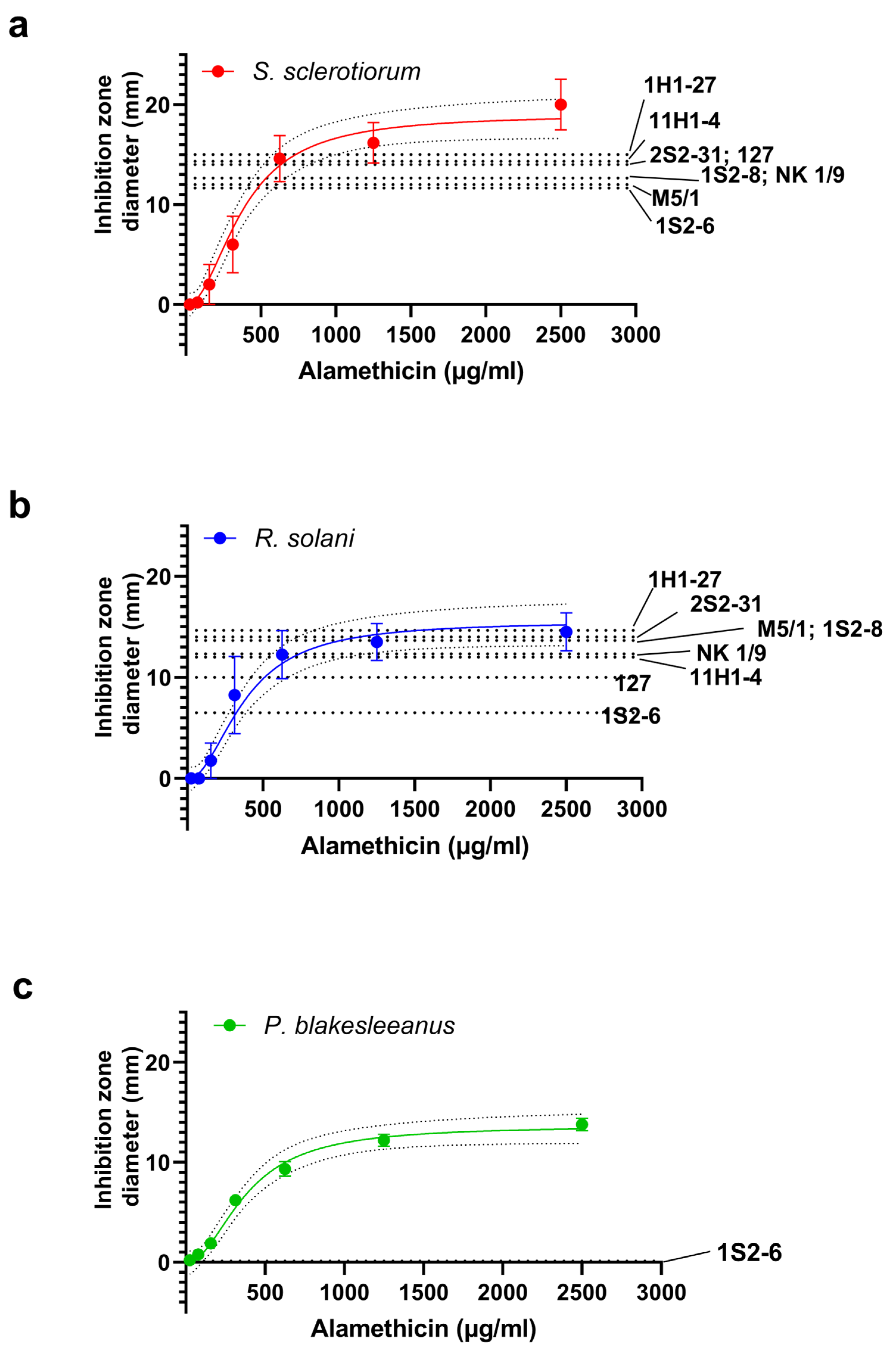


| Strain Code | Species | Accession Number |
|---|---|---|
| 1S2-8 | Trichoderma afroharzianum | PQ496494 |
| 11H1-4 | Trichoderma citrinoviride | PQ496497 |
| 1H1-27 | Trichoderma harzianum | PQ496510 |
| 1S2-6 | Trichoderma harzianum | PQ496529 |
| 2S2-31 | Trichoderma longibrachiatum | PQ496530 |
| 127 | Trichoderma citrinoviride | PQ496531 |
| NK 1/9 | Trichoderma citrinoviride | PQ601324 |
| M5/1 | Trichoderma harzianum | PQ651500 |
| K-500 | Sclerotinia sclerotiorum | PQ496532 |
| K-499 | Rhizoctonia solani | PQ496533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atlagić, K.; Cvetić Antić, T.; Lukičić, J.; Kruščić, K.; Živić, M.; Unković, N.; Pajić, T.; Stevanović, K.; Todorović, N.V. Biocontrol Potential of Native Trichoderma Strains Toward Soil-Borne Phytopathogenic and Saprotrophic Fungi. J. Fungi 2025, 11, 535. https://doi.org/10.3390/jof11070535
Atlagić K, Cvetić Antić T, Lukičić J, Kruščić K, Živić M, Unković N, Pajić T, Stevanović K, Todorović NV. Biocontrol Potential of Native Trichoderma Strains Toward Soil-Borne Phytopathogenic and Saprotrophic Fungi. Journal of Fungi. 2025; 11(7):535. https://doi.org/10.3390/jof11070535
Chicago/Turabian StyleAtlagić, Kristina, Tijana Cvetić Antić, Jovana Lukičić, Katarina Kruščić, Miroslav Živić, Nikola Unković, Tanja Pajić, Katarina Stevanović, and Nataša V. Todorović. 2025. "Biocontrol Potential of Native Trichoderma Strains Toward Soil-Borne Phytopathogenic and Saprotrophic Fungi" Journal of Fungi 11, no. 7: 535. https://doi.org/10.3390/jof11070535
APA StyleAtlagić, K., Cvetić Antić, T., Lukičić, J., Kruščić, K., Živić, M., Unković, N., Pajić, T., Stevanović, K., & Todorović, N. V. (2025). Biocontrol Potential of Native Trichoderma Strains Toward Soil-Borne Phytopathogenic and Saprotrophic Fungi. Journal of Fungi, 11(7), 535. https://doi.org/10.3390/jof11070535







