Dermatophytoses Caused by Trichophyton indotineae: The First Case Reports in Malaysia and the Global Epidemiology (2018–2025)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Confirmation of T. indotineae Dermatophytoses
2.3. Data Collections and Analysis
3. Results
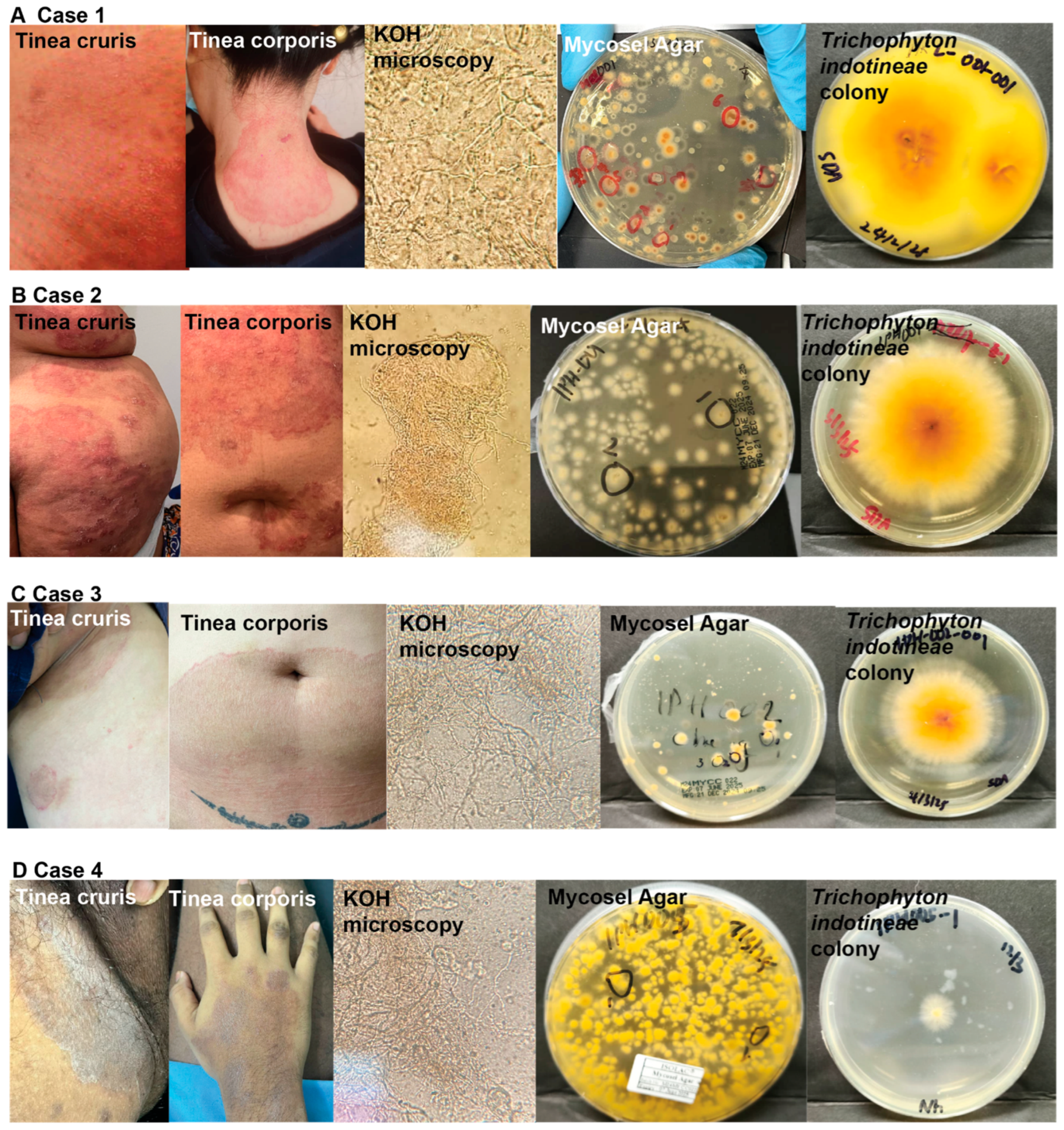
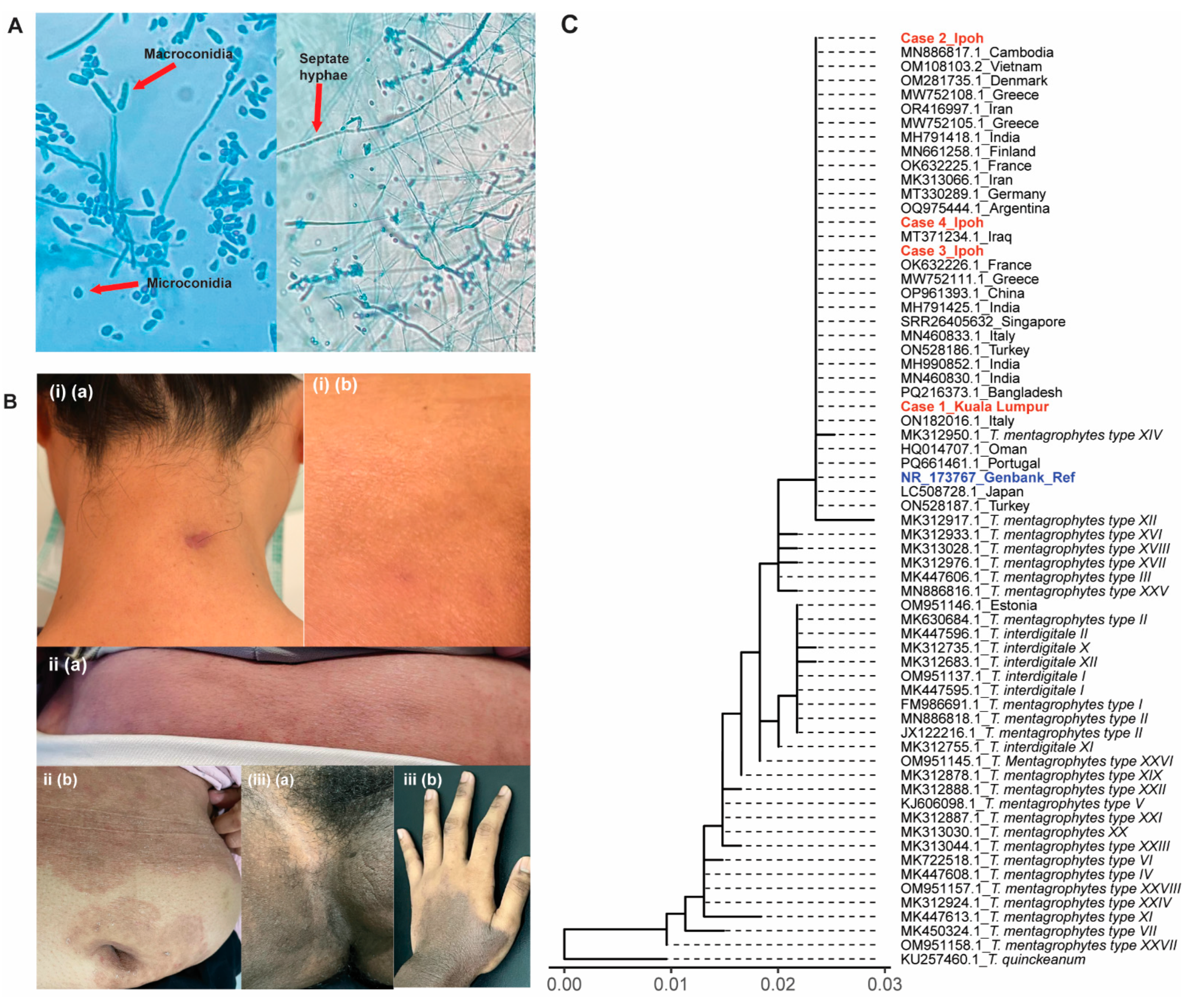
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Case 4
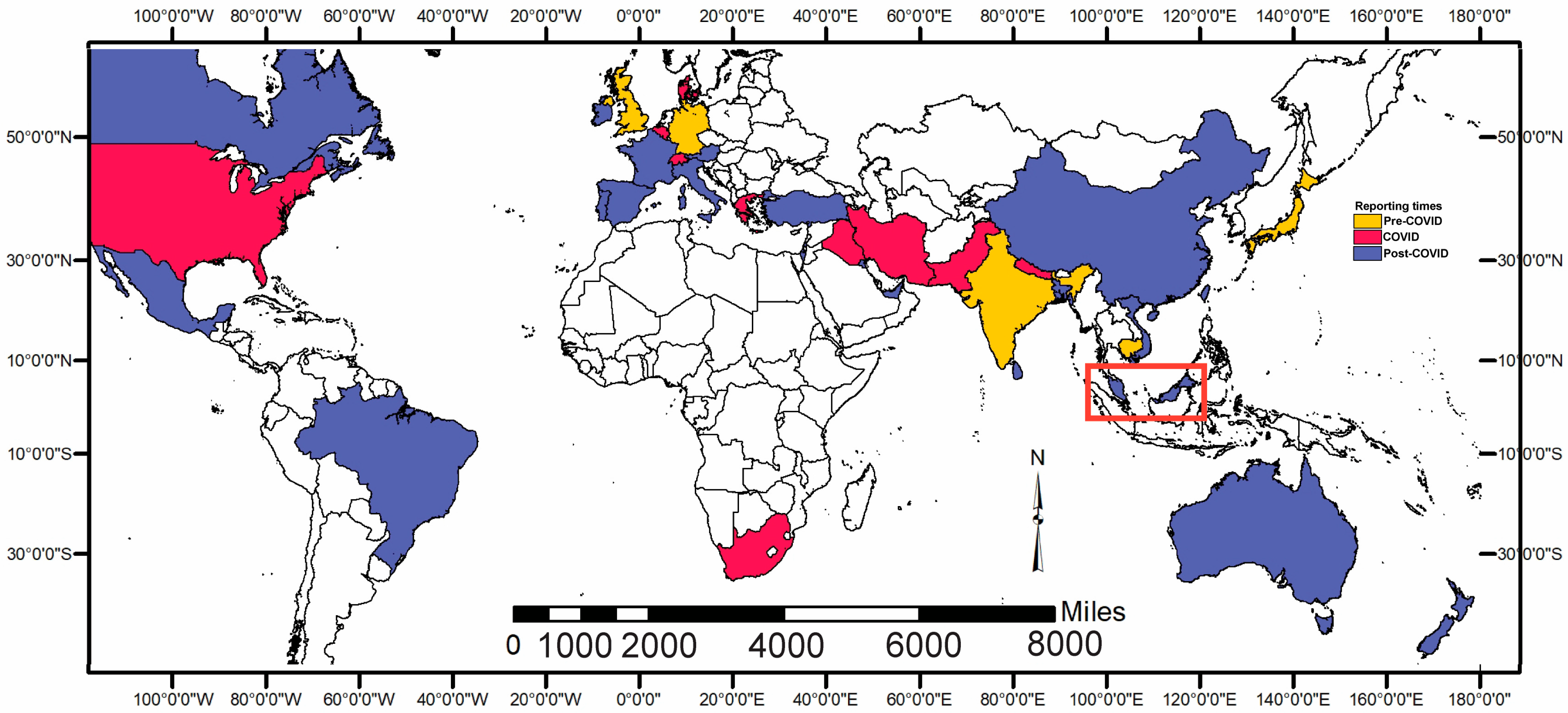
3.5. Cases Around the Globe
4. Discussion
4.1. Cases in Malaysia: Comparative Study with the World Records
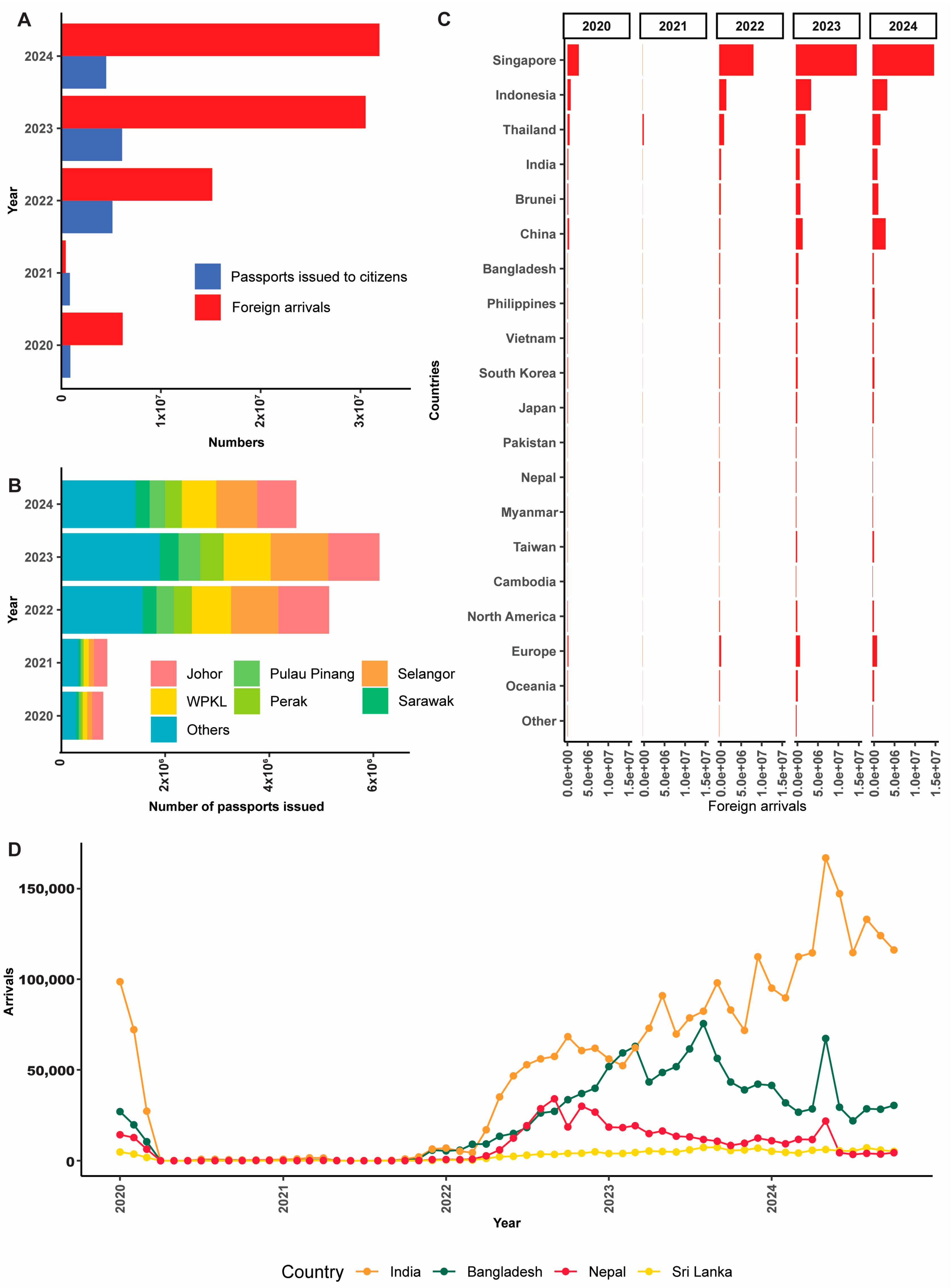
4.2. Delay Emergence of T. indotineae in Malaysia
4.3. Limitation of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase Chain Reaction |
| KOH | Potassium Hydroxide |
| ITS | Internal Transcribed Spacer |
| SNP | Single-Nucleotide Polymorphism |
| COVID-19 | Coronavirus Disease 2019 |
| FOMEMA | Foreign Workers Medical Examination Monitoring Agency |
| WHO | World Health Organization |
| CSV | Comma-Separated Values |
| NCBI | National Center of Biotechnology Information |
| BLAST | Basic Local Alignment Search Tool |
| LPCB | Lactophenol Cotton Blue |
| HIV | Human Immunodeficiency Virus |
References
- Zhan, P.; Liu, W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Frenkel, M. Dermatophyte Infections in Environmental Contexts. Res. Microbiol. 2015, 166, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, B.; Hay, R.; Morris-Jones, R. The Diagnosis and Management of Tinea. BMJ 2012, 344, e4380. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, C.; Bouchara, J.P.; Mignon, B. Updates on the Epidemiology of Dermatophyte Infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High Terbinafine Resistance in Trichophyton interdigitale Isolates in Delhi, India Harbouring Mutations in the Squalene Epoxidase Gene. Mycoses 2018, 61, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, J.; Kitagawa, H.; Noguchi, H.; Kano, R.; Hiruma, M.; Kamata, H.; Harada, K. Terbinafine-Resistant Strain of Trichophyton interdigitale Strain Isolated from a Tinea Pedis Patient. J. Dermatol. 2019, 46, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Süß, A.; Uhrlaß, S.; Ludes, A.; Verma, S.B.; Monod, M.; Krüger, C.; Nenoff, P. Extensive Tinea Corporis Due to a Terbinafine-Resistant Trichophyton mentagrophytes Isolate of the Indian Genotype in a Young Infant from Bahrain in Germany. Hautarzt 2019, 70, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, S.; Pchelin, I.M.; Zarei Mahmoudabadi, A.; Ansari, S.; Katiraee, F.; Rafiei, A.; Shokohi, T.; Abastabar, M.; Taraskina, A.E.; Kermani, F.; et al. Trichophyton mentagrophytes and T. interdigitale Genotypes Are Associated with Particular Geographic Areas and Clinical Manifestations. Mycoses 2019, 62, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M.; Hall, D.C.; Cooper, E.A.; Summerbell, R.C. The Emergence of Trichophyton indotineae: Implications for Clinical Practice. Int. J. Dermatol. 2023, 62, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Wong-O’Brien, B.; Lieberman, J.A.; Cookson, B.T.; Grinager, E.; Truong, T.T. The Brief Case: A Case of Tinea Corporis Caused by Drug-Resistant Trichophyton indotineae Identified by Broad-Range Fungal DNA Sequencing. J. Clin. Microbiol. 2024, 62, e00234-24. [Google Scholar] [CrossRef] [PubMed]
- Burmester, A.; Hipler, U.C.; Elsner, P.; Wiegand, C. Point Mutations in the Squalene Epoxidase Erg1 and Sterol 14-α Demethylase Erg11 Gene of T. indotineae Isolates Indicate that the Resistant Mutant Strains Evolved Independently. Mycoses 2022, 65, 97–102. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Liguori, G.; Cafarchia, C.; Triggiano, F.; Ciccarese, G.; Poli, M.A.; Ambrogio, F.; Bonamonte, D.; Cassano, N.; Vena, G.A.; et al. Cutaneous Infections Caused by Trichophyton indotineae: Case Series and Systematic Review. J. Clin. Med. 2025, 14, 1280. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Long, X.; Hu, W.; Zhu, J.; Jiang, Y.; Ahmed, S.; de Hoog, G.S.; Liu, W.; Jiang, Y. The Epidemic of the Multiresistant Dermatophyte Trichophyton indotineae Has Reached China. Front. Immunol. 2023, 13, 1113065. [Google Scholar] [CrossRef] [PubMed]
- Ponka, D.; Baddar, F. Microscopic Potassium Hydroxide Preparation. Can. Fam. Physician 2014, 60, 57. [Google Scholar] [PubMed]
- Wengenack, N.L.; Lainhart, W.; Wiederhold, N.P. CLSI M54-A: Principles and Procedures for Detection of Fungi in Clinical Specimens-Direct Examination and Culture; CLSI: Malvern, PA, USA, 2012; p. 164. [Google Scholar]
- ISO-1895; Mycosel Agar. ISOLAB: Shah Alam, Malaysia, 2024.
- Griffin, D.W.; Kellogg, C.A.; Peak, K.K.; Shinn, E.A. A Rapid and Efficient Assay for Extracting DNA from Fungi. Lett. Appl. Microbiol. 2002, 34, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Kobylak, N.; Bykowska, B.; Kurzyk, E.; Nowicki, R.; Brillowska-Dąbrowska, A. PCR and Real-Time PCR Approaches to the Identification of Arthroderma Otae Species Microsporum canis and Microsporum audouinii/Microsporum ferrugineum. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.; Colom, F.; Frasés, S.; Mulet, E.; Abad, J.L.; Alió, J.L. Detection and Identification of Fungal Pathogens by PCR and by ITS2 and 5.8S Ribosomal DNA Typing in Ocular Infections. J. Clin. Microbiol. 2001, 39, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Available online: https://iris.who.int/handle/10665/42330 (accessed on 17 May 2025).
- Harzing, A.-W. The Publish or Perish Book; Tarma Software Research Pty Limited: Melbourne, Australia, 2011. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. 2020. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 8 April 2025).
- Price, M.H. Mastering ArcGIS Pro; McGraw Hill: New York, NY, USA, 2023; p. 436. [Google Scholar]
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots (R Package Version 0.4.0). 2021. Available online: https://cloud.r-project.org/web/packages/ggpubr/index.html (accessed on 8 April 2025).
- Wilkinson, L. Ggplot2: Elegant Graphics for Data Analysis by WICKHAM, H. Biometrics 2011, 67, 678–679. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lillis, J.V.; Dawson, E.S.; Chang, R.; White, C.R. Disseminated Dermal Trichophyton rubrum Infection—An Expression of Dermatophyte Dimorphism? J. Cutan. Pathol. 2010, 37, 1168–1169. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasouli, A.; Barton, R.C.; Borman, A.M. Spread of Antifungal-Resistant Trichophyton indotineae, United Kingdom, 2017–2024. Emerg. Infect. Dis. 2025, 31, 192. [Google Scholar] [CrossRef] [PubMed]
- Astvad, K.M.T.; Hare, R.K.; Jørgensen, K.M.; Saunte, D.M.L.; Thomsen, P.K.; Arendrup, M.C. Increasing Terbinafine Resistance in Danish Trichophyton Isolates 2019–2020. J. Fungi 2022, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.M.C.; Ton Nu, P.A.; Le, C.C.; Ha, T.N.T.; Do, T.B.T.; Tran Thi, G. First Detection of Trichophyton indotineae Causing Tinea Corporis in Central Vietnam. Med. Mycol. Case Rep. 2022, 36, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Durdu, M.; Kandemir, H.; Karakoyun, A.S.; Ilkit, M.; Tang, C.; de Hoog, S. First Terbinafine-Resistant Trichophyton indotineae Isolates with Phe397Leu and/or Thr414His Mutations in Turkey. Mycopathologia 2023, 188, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Klonowski, E.; Verma, S.B.; Grigorjan, P.; Uhrlaβ, S. Trichophyton indotineae. Z. Gastroenterol. 2024, 62, 906–908. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.I.; Verma, S.B.; Illigner, G.M.; Uhrlaß, S.; Klonowski, E.; Burmester, A.; Noor, T.; Nenoff, P. Trichophyton mentagrophytes ITS Genotype VIII/Trichophyton indotineae Infection and Antifungal Resistance in Bangladesh. J. Fungi 2024, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, M.D.; Marzouk, S.; Bećiri, L. Widespread Dermatophytosis in a Healthy Adolescent: The First Report of Multidrug-Resistant Trichophyton indotineae Infection in the UAE. Acta Dermatovenerol. Alp. Pannonica Adriat. 2024, 33, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.V.; Gonçalves, R.D.J.; Valinoto, G.C.J.; Carvalho, G.d.S.M.; Celestrino, G.A.; Reis, A.P.C.; Lima, A.P.C.; da Costa, A.C.; Melhem, M.d.S.C.; Benard, G.; et al. First Case of Trichophyton indotineae in Brazil: Clinical and Mycological Criteria and Genetic Identification of Terbinafine Resistance. An. Bras. Dermatol. 2025, 100, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.C.; Wittig, F.; Krüger, C.; Nenoff, K.; Wiegand, C.; Saraswat, A.; et al. The Current Indian Epidemic of Superficial Dermatophytosis Due to Trichophyton mentagrophytes—A Molecular Study. Mycoses 2019, 62, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Verma, S.B.; Ebert, A.; Süß, A.; Fischer, E.; Auerswald, E.; Dessoi, S.; Hofmann, W.; Schmidt, S.; Neubert, K.; et al. Spread of Terbinafine-Resistant Trichophyton mentagrophytes Type VIII (India) in Germany–“The Tip of the Iceberg?”. J. Fungi 2020, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Efstathiou, I.; Theodoropoulos, K.; Pournaras, S.; Meletiadis, J. Molecular Epidemiology and Antifungal Susceptibility of Trichophyton Isolates in Greece: Emergence of Terbinafine-Resistant Trichophyton mentagrophytes Type VIII Locally and Globally. J. Fungi 2021, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Dellière, S.; Joannard, B.; Benderdouche, M.; Mingui, A.; Gits-Muselli, M.; Hamane, S.; Alanio, A.; Petit, A.; Gabison, G.; Bagot, M.; et al. Emergence of Diffi Cult-to-Treat Tinea Corporis Caused by Trichophyton mentagrophytes Complex Isolates, Paris, France. Emerg. Infect. Dis. 2022, 28, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Dellière, S.; Jabet, A.; Abdolrasouli, A. Current and Emerging Issues in Dermatophyte Infections. PLoS Pathog. 2024, 20, e1012258. [Google Scholar] [CrossRef] [PubMed]
- Koncan, R.; Benini, A.; Lo Cascio, G.; Di Meo, N.; Lepera, V.; Palladino, G.; Clemente, L.; Barbone, F.; Zalaudek, I. A Case of Double-Mutant Resistant Tinea Indotineae. JEADV Clin. Pract. 2024, 4, 248–252. [Google Scholar] [CrossRef]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.C.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-Wide Phenomenon of Antifungal Resistance in Dermatophytes: A Multicentre Study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Shaw, D.; Shankarnarayan, S.A.; Abhishek, S.A.; Dogra, S. Comprehensive Taxonomical Analysis of Trichophyton mentagrophytes/interdigitale Complex of Human and Animal Origin from India. J. Fungi 2023, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae—An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide—A Multidimensional Perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Uhrlaß, S.; Mey, S.; Koch, D.; Mütze, H.; Krüger, C.; Monod, M.; Nenoff, P. Dermatophytes and Skin Dermatophytoses in Southeast Asia—First Epidemiological Survey from Cambodia. Mycoses 2024, 67, e13718. [Google Scholar] [CrossRef] [PubMed]
- Sensitivity of Isolated Dermatophyte Strains to Antifungal Drugs in the Russian Federation. Available online: https://elibrary.ru/item.asp?id=41418751 (accessed on 28 April 2025).
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. Nov.: A New Highly Terbinafine-Resistant Anthropophilic Dermatophyte Species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, S.; Shamsizadeh, F.; Pchelin, I.M.; Rezaei-Matehhkolaei, A.; Mahmoudabadi, A.Z.; Valadan, R.; Ansari, S.; Katiraee, F.; Pakshir, K.; Zomorodian, K.; et al. Emergence of Terbinafine Resistant Trichophyton mentagrophytes in Iran, Harboring Mutations in the Squalene Epoxidase (SQLE) Gene. Infect. Drug Resist. 2020, 13, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Kakurai, M.; Harada, K.; Maeda, T.; Hiruma, J.; Kano, R.; Demitsu, T. Case of Tinea Corporis Due to Terbinafine-Resistant Trichophyton interdigitale. J. Dermatol. 2020, 47, e104–e105. [Google Scholar] [CrossRef] [PubMed]
- Kimura, U.; Hiruma, M.; Kano, R.; Matsumoto, T.; Noguchi, H.; Takamori, K.; Suga, Y. Caution and Warning: Arrival of Terbinafine-Resistant Trichophyton interdigitale of the Indian Genotype, Isolated from Extensive Dermatophytosis, in Japan. J. Dermatol. 2020, 47, e192–e193. [Google Scholar] [CrossRef] [PubMed]
- Mosam, A.; Shuping, L.; Naicker, S.; Maphanga, T.; Tsotetsi, E.; Mudau, R.; Maluleka, C.; Mpembe, R.; Ismail, H.; Singh, A.; et al. A Case of Antifungal-Resistant Ringworm Infection in KwaZulu-Natal Province, South Africa, Caused by Trichophyton indotineae; Public Health Bulletin South Africa: Johannesburg, South Africa, 2023. [Google Scholar]
- Usman, B.; Rehman, A.; Naz, I.; Anees, M. Prevalence and Antifungal Drug Resistance of Dermatophytes in the Clinical Samples from Pakistan. Acta Microbiol. Immunol. Hung. 2021, 68, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.; Theiler, M.; Bosshard, P.P. Epidemiological and Clinical Aspects of Trichophyton mentagrophytes/Trichophyton interdigitale Infections in the Zurich Area: A Retrospective Study Using Genotyping. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Sacheli, R.; Harag, S.; Dehavay, F.; Evrard, S.; Rousseaux, D.; Adjetey, A.; Seidel, L.; Laffineur, K.; Lagrou, K.; Hayette, M.P. Belgian National Survey on Tinea Capitis: Epidemiological Considerations and Highlight of Terbinafine-Resistant T. mentagrophytes with a Mutation on SQLE Gene. J. Fungi 2020, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Jabet, A.; Brun, S.; Normand, A.C.; Imbert, S.; Akhoundi, M.; Dannaoui, E.; Audiffred, L.; Chasset, F.; Izri, A.; Laroche, L.; et al. Extensive Dermatophytosis Caused by Terbinafine-Resistant Trichophyton indotineae, France. Emerg. Infect. Dis. 2022, 28, 229. [Google Scholar] [CrossRef] [PubMed]
- Posso-De Los Rios, C.J.; Tadros, E.; Summerbell, R.C.; Scott, J.A. Terbinafine Resistant Trichophyton indotineae Isolated in Patients With Superficial Dermatophyte Infection in Canadian Patients. J. Cutan. Med. Surg. 2022, 26, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; Cruciani, D.; Spina, S.; Piscioneri, V.; Natalini, Y.; Pezzotti, G.; Sabbatucci, M.; Papini, M. A Terbinafine Sensitive Trichophyton indotineae Strain in Italy: The First Clinical Case of Tinea Corporis and Onychomycosis. J. Fungi 2023, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Toutous Trellu, L.; Fontao, L.; Ninet, B. Towards an Early Clinical and Biological Resistance Detection in Dermatophytosis: About 2 Cases of Trichophyton indotineae. J. Fungi 2023, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.S.; Chaturvedi, S.; Zhu, Y.; Todd, G.C.; Yin, L.; Lopez, A.; Travis, L.; Smith, D.J.; Chiller, T.; Lockhart, S.R.; et al. Notes from the Field: First Reported U.S. Cases of Tinea Caused by Trichophyton indotineae—New York City. Morb. Mortal. Wkly. Rep. 2023, 72, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Santiso, G.; Romero, M.; Bonifaz, A.; Fernandez, M.; Marin, E. First Case Report of Tinea Corporis Caused by Trichophyton indotineae in Latin America. Med. Mycol. Case Rep. 2023, 41, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Indikova, I.; Teodorowicz, L.; Loydl, K.; Stary, A. Detection of Trichophyton indotineae in Austria: A Case Report. Available online: http://www.pilzambulatorium.at/web/index.php/forschung/publikationen/169-detection-of-trichophyton-indotineae-in-austria-a-case-report-2023 (accessed on 26 March 2025).
- Dashti, Y.; Alobaid, K.; Al-Rashidi, S.; Dashti, M.; AbdulMoneim, M.H.; Al-Enezi, M.; Abou-Chakra, N.; Jørgensen, K.M. Autochthonous Case of Trichophyton indotineae in Kuwait. J. Med. Mycol. 2023, 33, 101432. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasouli, A.; Borman, A.M.; Johnson, E.M.; Hay, R.J.; Arias, M. Terbinafine-Resistant Trichophyton indotineae Causing Extensive Dermatophytosis in a Returning Traveller, London, UK. Clin. Exp. Dermatol. 2024, 49, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Madarasingha, N.P.; Thabrew, H.; Uhrlass, S.; Eriyagama, S.; Reinal, D.; Jayasekera, P.I.; Nenoff, P. Dermatophytosis Caused by Trichophyton indotineae (Trichophyton mentagrophytes ITS Genotype VIII) in Sri Lanka. Am. J. Trop. Med. Hyg. 2024, 111, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.Y.; Halliday, C.L.; Chen, S.C.-A.; Koning, S.; Pawlikowski, J.; Du Cros, P.; Korman, T.M. Lessons from Practice Treatment-Resistant Tinea Caused by Trichophyton indotineae in Australia Tinea Cruris Infection in Patient 1. Med. Educ. 2024, 221, 192–194. [Google Scholar] [CrossRef]
- Quyền, N.M.; Quỳnh, N.T.N.; Hoàng, Đ.N.; Tuấn, L.Q.; Anh, L.T.; Bình, N.T.T.; Ánh, Đ.N. BÁO CÁO CA BỆNH: NẤM DA DO Trichophyton indotineae ĐẦU TIÊN TẠI MIỀN BẮC VIỆT NAM. Tạp Chí Y Dược Học Quân Sự 2024, 49, 128–136. [Google Scholar] [CrossRef]
- Tan, T.Y.; Wang, Y.S.; Wong, X.Y.A.; Rajandran, P.; Tan, M.G.; Tan, A.L.; Tan, Y.E. First Reported Case of Trichophyton indotineae Dermatophytosis in Singapore. Pathology 2024, 56, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Galili, E.; Lubitz, I.; Shemer, A.; Astman, N.; Pevzner, K.; Gazit, Z.; Segal, O.; Lyakhovitsky, A.; Halevi, S.; Baum, S.; et al. First Reported Cases of Terbinafine-Resistant Trichophyton indotineae Isolates in Israel: Epidemiology, Clinical Characteristics and Response to Treatment. Mycoses 2024, 67, e13812. [Google Scholar] [CrossRef] [PubMed]
- Villa-Gonzalez, J.M.; Pascual Ares, M.; López-Soria, L.M.; Gonzalez-Hermosa, M.R.; Gardeazabal García, J.; Lasa Elgezua, O. Extensive Tinea Corporis Caused by Trichophyton indotineae: Report of a Case in Spain. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e22. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, L.R.; Cronin, K.; Ruscica, S.; Patel, S.N.; Kus, J.V. Emergence of Terbinafine-Resistant Trichophyton indotineae in Ontario, Canada, 2014–2023. J. Clin. Microbiol. 2025, 63, e0153524. [Google Scholar] [CrossRef] [PubMed]
- Carroll, E.; Leahy, M.; Stanciu, M.; Laing, M. Trichophyton indotineae: First Case in Ireland and Response to Topical Griseofulvin. Clin. Exp. Dermatol. 2024, 49, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Anzawa, K.; Bernales-Mendoza, A.M.; Shimizu, A. Case of Tinea Corporis Caused by a Terbinafine-Sensitive Trichophyton indotineae Strain in a Vietnamese Worker in Japan. J. Dermatol. 2025, 52, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ferreira, J.; Vidal, B.; Veríssimo, C.; Sabino, R.; Marques, T.; Soares-de-Almeida, L.; Filipe, P. Extensive Recalcitrant Tinea Corporis Caused by Trichophyton indotineae. Mycopathologia 2025, 190, 20. [Google Scholar] [CrossRef] [PubMed]
- McKinney, W.P.; Blakiston, M.R.; Roberts, S.A.; Morris, A.J. Clinical Alert: Arrival of Terbinafine Resistant Trichophyton indotineae in New Zealand. N. Z. Med. J. 2025, 138, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, S.; Abou-Chakra, N.; Oldberg, K.; Chryssanthou, E.; Young, E. Terbinafine Resistant Trichophyton indotineae in Sweden. Acta. Derm. Venereol. 2025, 105, 42089. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wang, X.; Li, R. Dermatophyte Infection: From Fungal Pathogenicity to Host Immune Responses. Front. Immunol. 2023, 14, 1285887. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Kong, X.; Li, X.; Liu, W.; Liang, G. Prior Selection of Itraconazole in the Treatment of Recalcitrant Trichophyton indotineae Infection: Real-World Results from Retrospective Analysis. Mycoses 2024, 67, e13663. [Google Scholar] [CrossRef] [PubMed]
- Sonego, B.; Corio, A.; Mazzoletti, V.; Zerbato, V.; Benini, A.; di Meo, N.; Zalaudek, I.; Stinco, G.; Errichetti, E.; Zelin, E. Trichophyton indotineae, an Emerging Drug-Resistant Dermatophyte: A Review of the Treatment Options. J. Clin. Med. 2024, 13, 3558. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Polla Ravi, S.; Wang, T.; Bakotic, W.L.; Shemer, A. Mapping the Global Spread of T. indotineae: An Update on Antifungal Resistance, Mutations, and Strategies for Effective Management. Mycopathologia 2024, 189, 45. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, B.; Roskams, T.; Steger, P.; Van Steenbergen, W. Hepatotoxicity Related to Itraconazole: Report of Three Cases. Acta Clin. Belg. 2001, 56, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Rawal, H. Cardiotoxicity with Itraconazole. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Govt Extends Freeze on Foreign Worker Applications, No New Quota. Available online: https://theedgemalaysia.com/node/712299 (accessed on 26 March 2025).
- Assessment of Causes and Conributing Factors to Migrant Workers Becoming Undocumented in Malaysia. Available online: https://www.iom.int/resources/assessment-causes-and-contributing-factors-migrant-workers-becoming-undocumented-malaysia (accessed on 26 March 2025).
- Tighter Medical Requirements for Foreign Workers—Fomema. Available online: https://www.nst.com.my/news/nation/2023/12/994917/tighter-medical-requirements-foreign-workers-fomema (accessed on 26 March 2025).
- Rouhi, F.; Aboutalebian, S.; Rezaei-Matehkolaei, A.; Jahanshiri, Z.; Shidfar, M.R.; Chadeganipour, A.S.; Shadzi, S.; Kharazi, M.; Erami, M.; Mirhendi, H. Development and Evaluation of SYBR Green Real-Time PCR for Rapid and Specific Identification of Trichophyton indotineae. Mycoses 2025, 68, e70015. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.P.; Soo-Hoo, T.S.; Na, S.L.; Ang, L.S. Dermatophytes Isolated from Patients in University Hospital, Kuala Lumpur, Malaysia. Mycopathologia 2003, 155, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.P.; Saw, T.L.; Madasamy, M.; Soo Hoo, T.S. Onychomycosis in Malaysia. Mycopathologia 1999, 147, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Tzar, M.N.; Zetti, Z.R.; Ramliza, R.; Sharifah, A.S.; Leelavathi, M. Dermatomycoses in Kuala Lumpur, Malaysia. Sains Malays. 2014, 43, 1737–1742. [Google Scholar]
- Cañete-Gibas, C.F.; Mele, J.; Patterson, H.P.; Sanders, C.J.; Ferrer, D.; Garcia, V.; Fan, H.; David, M.; Wiederhold, N.P. Terbinafine-Resistant Dermatophytes and the Presence of Trichophyton indotineae in North America. J. Clin. Microbiol. 2023, 61, e00562-23. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.M.; Emeka, P.; Khan, A.H. Drug Information Activity and Nonprescription Requests Over the Malaysian Counter. Ther. Innov. Regul. Sci. 2013, 47, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Mijaljica, D.; Spada, F.; Harrison, I.P. Emerging Trends in the Use of Topical Antifungal-Corticosteroid Combinations. J. Fungi 2022, 8, 812. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Denning, D.W. The Impact of Corticosteroids on the Outcome of Fungal Disease: A Systematic Review and Meta-Analysis. Curr. Fungal Infect. Rep. 2023, 17, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Handog, E.B.; Enriquez-Macarayo, M.J. Topical Corticosteroid Abuse: Southeast Asia Perspective. In A Treatise on Topical Corticosteroids in Dermatology: Use, Misuse and Abuse; Springer: Singapore, 2018; pp. 209–222. [Google Scholar] [CrossRef]
- Iwen, P.C.; Hinrichs, S.H.; Rupp, M.E. Utilization of the Internal Transcribed Spacer Regions as Molecular Targets to Detect and Identify Human Fungal Pathogens. Med. Mycol. 2002, 40, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Kille, B.; Nute, M.G.; Huang, V.; Kim, E.; Phillippy, A.M.; Treangen, T.J. Parsnp 2.0: Scalable Core-Genome Alignment for Massive Microbial Datasets. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- GitHub—Tseemann/Snippy: Scissors: Rapid Haploid Variant Calling and Core Genome Alignment. Available online: https://github.com/tseemann/snippy (accessed on 28 April 2025).
- Croucher, N.J.; Page, A.J.; Connor, T.R.; Delaney, A.J.; Keane, J.A.; Bentley, S.D.; Parkhill, J.; Harris, S.R. Rapid Phylogenetic Analysis of Large Samples of Recombinant Bacterial Whole Genome Sequences Using Gubbins. Nucleic Acids Res. 2015, 43, e15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Er, Y.X.; Leong, K.F.; Foong, H.B.B.; Abdul Halim, A.A.; Kok, J.S.; Yap, N.J.; Tan, Y.C.; Tay, S.T.; Lim, Y.A.-L. Dermatophytoses Caused by Trichophyton indotineae: The First Case Reports in Malaysia and the Global Epidemiology (2018–2025). J. Fungi 2025, 11, 523. https://doi.org/10.3390/jof11070523
Er YX, Leong KF, Foong HBB, Abdul Halim AA, Kok JS, Yap NJ, Tan YC, Tay ST, Lim YA-L. Dermatophytoses Caused by Trichophyton indotineae: The First Case Reports in Malaysia and the Global Epidemiology (2018–2025). Journal of Fungi. 2025; 11(7):523. https://doi.org/10.3390/jof11070523
Chicago/Turabian StyleEr, Yi Xian, Kin Fon Leong, Henry Boon Bee Foong, Anis Amirah Abdul Halim, Jing Shun Kok, Nan Jiun Yap, Yuong Chin Tan, Sun Tee Tay, and Yvonne Ai-Lian Lim. 2025. "Dermatophytoses Caused by Trichophyton indotineae: The First Case Reports in Malaysia and the Global Epidemiology (2018–2025)" Journal of Fungi 11, no. 7: 523. https://doi.org/10.3390/jof11070523
APA StyleEr, Y. X., Leong, K. F., Foong, H. B. B., Abdul Halim, A. A., Kok, J. S., Yap, N. J., Tan, Y. C., Tay, S. T., & Lim, Y. A.-L. (2025). Dermatophytoses Caused by Trichophyton indotineae: The First Case Reports in Malaysia and the Global Epidemiology (2018–2025). Journal of Fungi, 11(7), 523. https://doi.org/10.3390/jof11070523





