Biocontrol Strategies Against Plant-Parasitic Nematodes Using Trichoderma spp.: Mechanisms, Applications, and Management Perspectives
Abstract
1. Introduction
2. Taxonomy, Morphological Characteristics, and Environmental Adaptation of Trichoderma spp.
3. Interactions of Trichoderma with Plants and Other Microorganisms
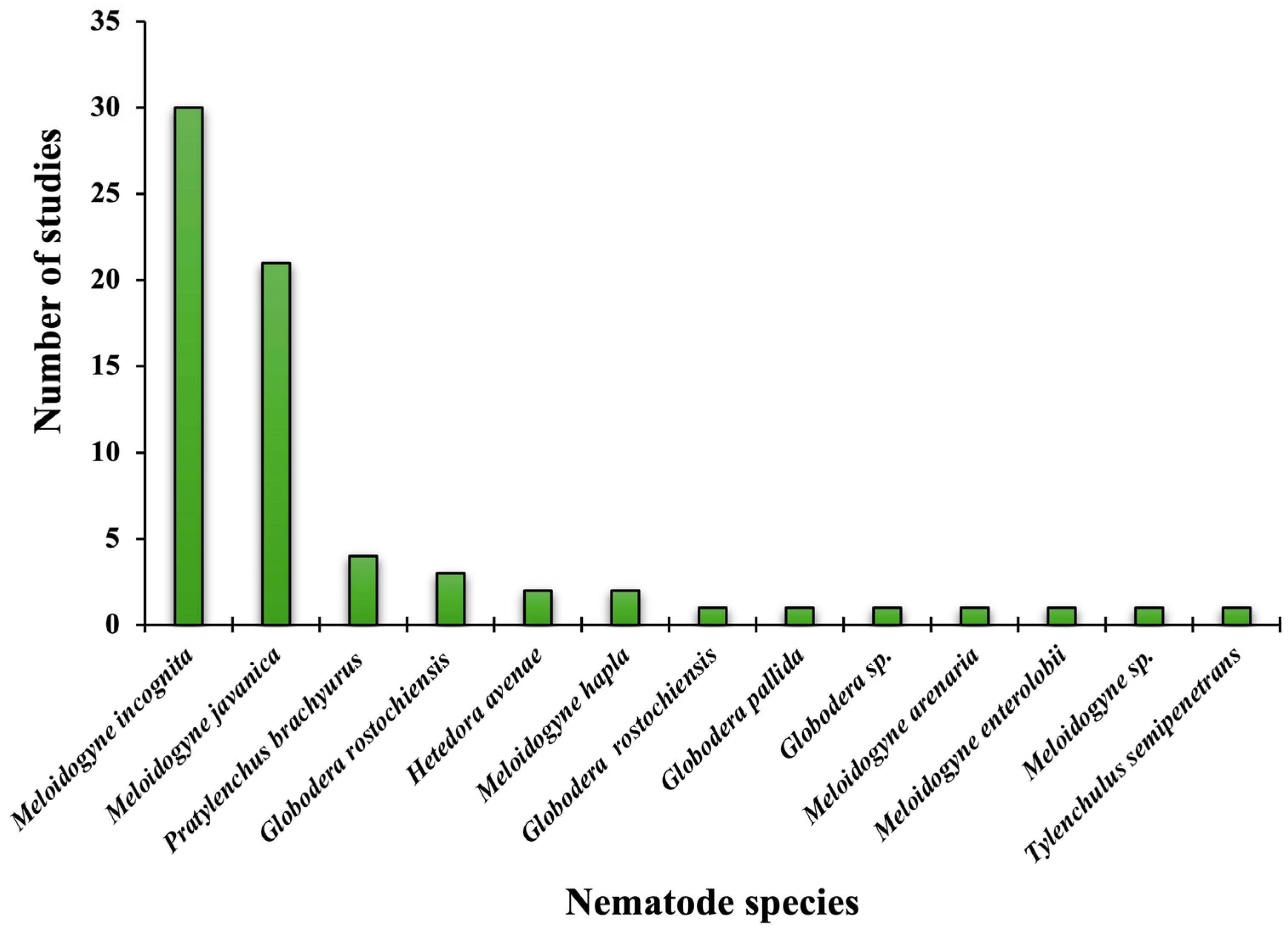
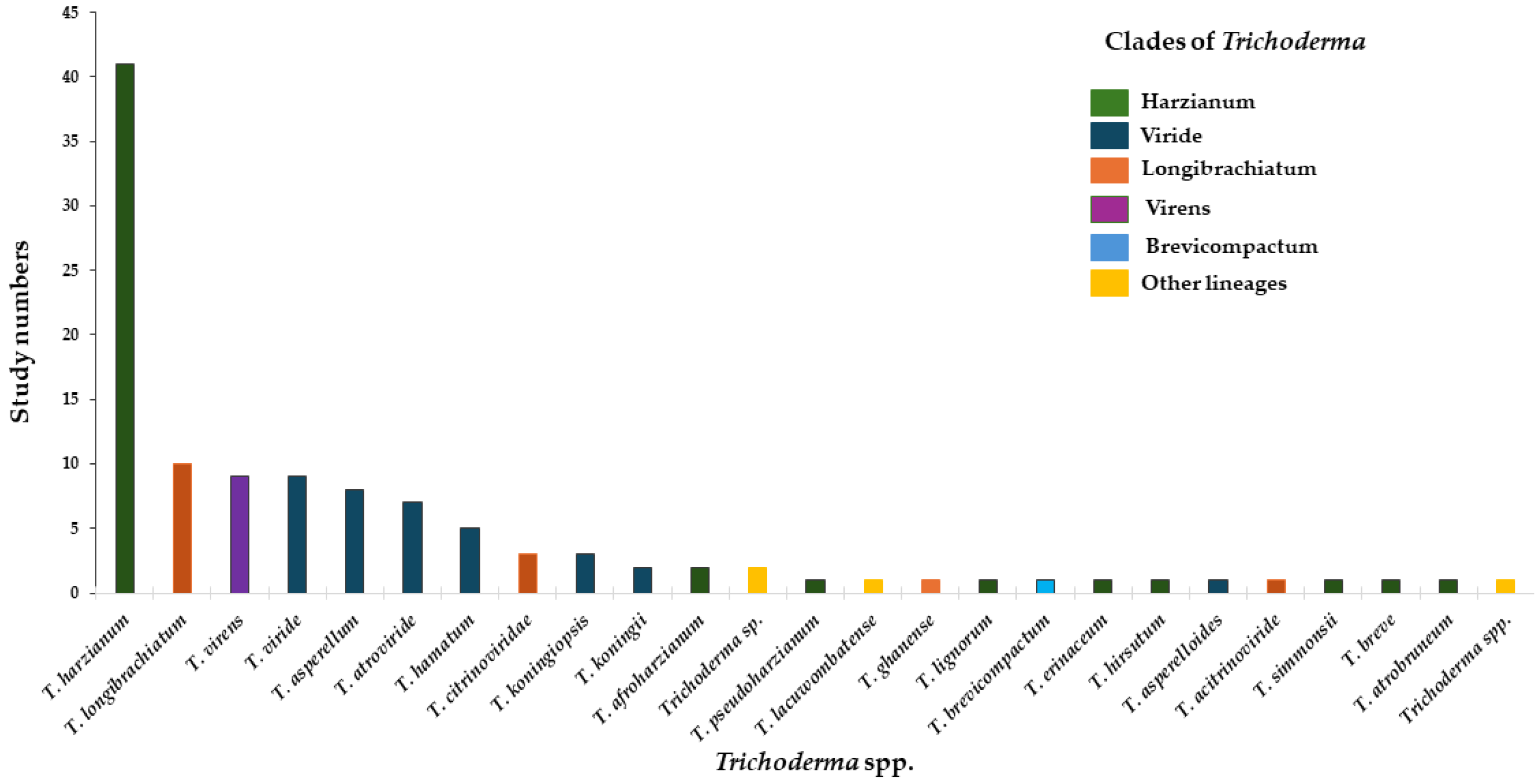
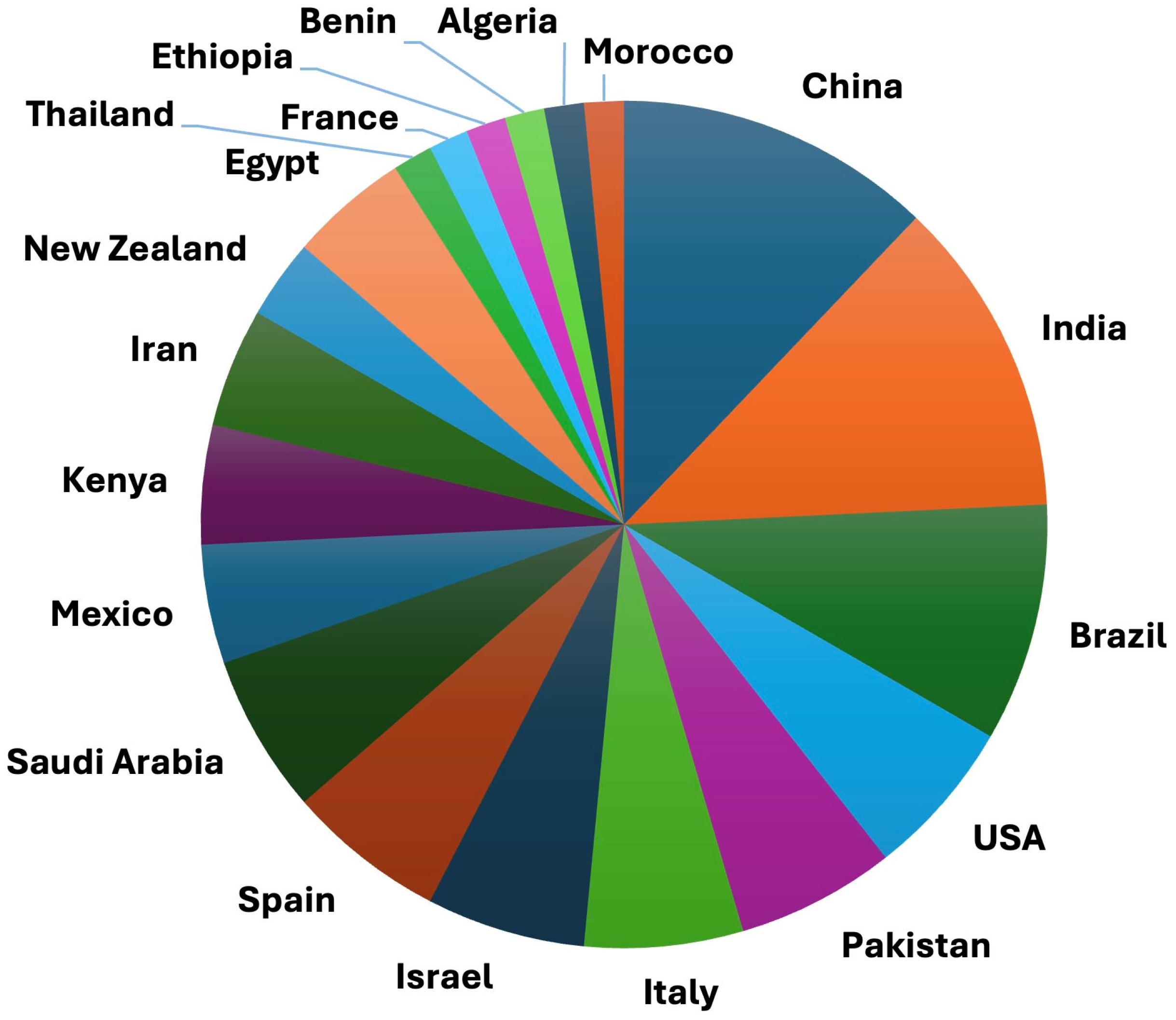
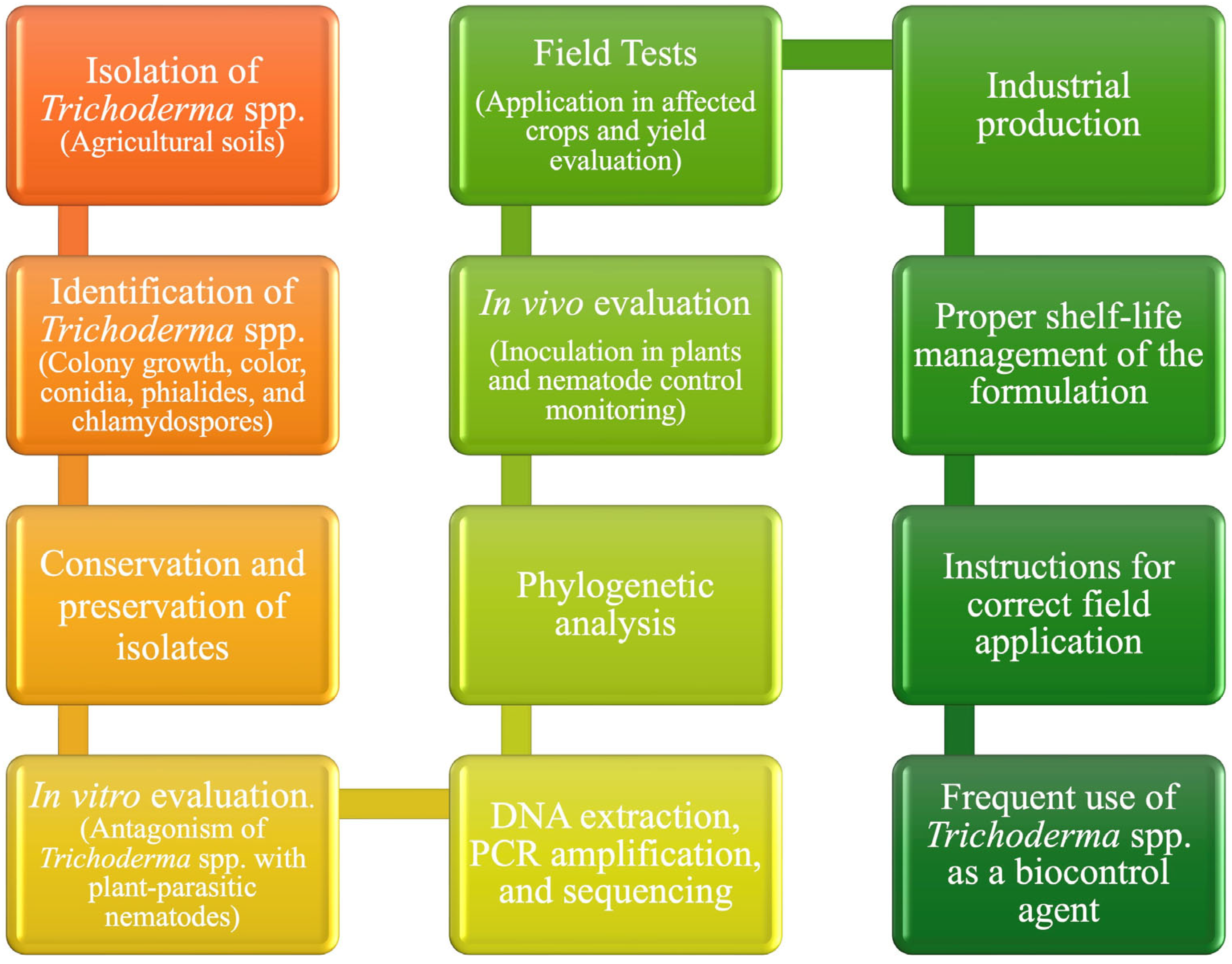
4. Application of Trichoderma in Nematode Control
| Trichoderma Species and Application Type | Mechanism of Action and Study Type | Nematode | Country and Crop | Results and References |
|---|---|---|---|---|
| T. harzianum and T. koningi/C.S | Antagonism/In vivo | M. arenaria | USA/Corn | Reduction in egg production [43] |
| T. longibrachiatum/C.F | Antagonism/In vitro | Meloidogyne spp., Heterodera sachari, G. rostochiensis | France | Inhibited movement of infective juveniles [51] |
| T. harzianum rifai/- | Parasitism/In vitro | G. rostochiensis | Pakistan/Potato | Trichoderma penetrated cysts and eggs, causing larval death [52] |
| T. harzianum and T. lignorum/C.F | Parasitism/In vivo | M. javanica | Israel/Tomato | Enhanced plant growth and reduced gall formation [44] |
| T. virens/C.F | Antagonism/In vitro and in vivo | M. incognita | USA/Tomato | 42% fewer eggs and J2 per gram of roots [53] |
| T. harzianum/C.S | Parasitism/In vitro | M. javanica | Israel/Tomato | Ability to colonize eggs and second-stage juveniles (J2) [45] |
| T. harzianum/C.F | Antagonism/In vitro | M. incognita | Spain | Significant reduction in eggs [54] |
| T. atrovirens and T. harzianum/C.S | Parasitism/In vitro | M. javanica | Israel/Tomato | Nematode biocontrol activity [55] |
| T. harzianum/C.S | Parasitism/In vitro and in vivo | M. javanica | Iran/Tomato | Reduces egg hatching and activates defense enzymes [56] |
| T. asperellum, T. harzianum, T. brevicompactum, T. hamatum and T. erinaceum/C.S | Antagonism/In vivo | M. incognita | Benin/Tomato and carrot | Lowers J2 density, cuts egg production by 86%, and increases tomato yield by 30% [57] |
| T. harzianum/C.F | Parasitism/In vitro and in vivo | M. javanica | Iran/Tomato | Inhibited egg hatching, 84% reduction in egg parasitism, and decreased nematode damage [58] |
| T. harzianum/C.S | Parasitism/In vitro and in vivo | M. incognita | Brazil/Cucumber | Inhibited movement of 60% of eggs and J2 [59] |
| T. harzianum/C.S | Antibiosis and induction of resistance of the plant/In vivo | M. enterolobii | Thailand/Guava | Reduced nematode numbers and stopped J2 development [60] |
| T. harzianum/C.S | Parasitism/In vivo | M. javanica | Saudi Arabia/Tomato | 89% of eggs infected; reduced egg hatching by 8.8% and caused 64.5% J2 mortality [61] |
| T. harzianum/C.S | Parasitism/In vivo | G. rostochiensis | Pakistan/Tomato | Cyst wall or egg surface penetration was chemical and mechanical [52] |
| T. longibrachiatum/C.S | Parasitism/In vitro and in vivo | H. avenae | China/Wheat | The parasitic effects of T. longibrachiatum were >91% after 18 days [62] |
| T. longibrachiatum/C.S | Parasitism/In vitro | M. incognita | China/Cucumber | Strong lethal effect (>88%) and improved plant growth [63] |
| T. harzianum/C.S | Antagonism/In vitro | M. incognita | Mexico/Tomato | Reproduction was reduced by 87–90%, nematode damage and gall formation decreased and plant height and dry biomass increased [64] |
| T. harzianum and T. viride/C.S | Antagonism/In vivo | M. javanica | Saudi Arabia/Tomato | Suppression of nematode reproduction and gall formation, increased tomato plant growth [65] |
| T. harzianum/C.S | Antagonism/In vitro and in vivo | M. incognita | Ethiopia/Tomato | 80% of J2 mortality at 72 h [66] |
| T. asperellum, T. harzianum, T. virens, T. atroviride, T. lacuwombatense, T. viride/C.S | Antagonism/In vivo | M. hapla | New Zealand/Tomato | Trichoderma strains reduced 1.1 eggs mL soil−1 and suppressed galling by 42–88% [67] |
| T. harzianum/C.S | Antagonism/In vivo | G. pallida | USA/Potato | 60% reduction in nematode reproduction [68] |
| T. atroviride/C.S | Induce resistance/In vivo | M. javanica | Spain/Tomato | Reductions of 42% in galls, 60% in egg masses, and 90% in adult nematodes [69] |
| T. harzianum, T. atroviride, T. virens/C.S | Antagonism/In vivo | M. incognita | Mexico/Bell pepper | Egg production reduced by 63% and female production by 14.3%; plant growth enhanced [70] |
| T. harzianum/C.S | Induced resistance/In vivo | M. incognita | Spain/Tomato | Host defenses enhanced during infection, varying by parasitism stage [71] |
| T. longibrachiatum/C.S | Parasitism and induced resistance/In vivo | P. brachyurus and M. javanica | Brazil/Soybean | All treatments effectively controlled P. brachyurus and M. javanica [72] |
| T. harzianum/C.S | Parasitism/In vivo | M. incognita | Italy/Tomato | Root colonization primed Systemic Acquired Resistance against root-knot nematodes [73] |
| T. longibrachiatum/C.S | Parasitism/In vivo | H. avenae | China/Wheat | 89.8% reduction in cysts and juveniles in soil, and 88.3% reduction in J2 and females in roots [74] |
| T. harzianum, T. asperellum and T. longibrachiatum/- | In vitro | M. javanica | Morocco/Olive | Trichoderma strains killed 50% of the J2s [75] |
| T. harzianum, T. hamatum, T. viride, T. virens and T. koningii/C.S and C.F | In vivo | M. incognita | India/Tomato | Culture suspensions caused the greatest reduction in hatching and juvenile mortality [76] |
| T. viride/C.S | Antagonism/In vivo | M. incognita | India/Tomato | Increased shoot weight and decreased root weight of tomato, with dose-dependent reductions in galls, egg masses and eggs per egg mass [77] |
| T. harzianum and T. viride/C.S | Antagonism/In vivo | M. incognita | Pakistan/Tomato | Significant reductions in number of galls, egg masses, eggs per egg mass and reproductive factors of M. incognita in a dose-dependent manner [78] |
| T. harzianum, T. viride, and T. virens/C.S | Parasitism/In vivo | M. incognita | Egypt/Pea | 78 to 89% reduction in nematode numbers and gall numbers [79] |
| T. koningiopsis/C.S and C.F | Enzymatic hydrolysis/In vitro | M. javanica and M. incognita | Brazil | High nematode mortality when applied as an enzymatic filtrate or conidial suspension [80] |
| T. citrinoviride/C.S | Antagonism/In vivo | M. incognita | China/Tomato | Egg hatching inhibition 90% and promoted the growth of tomato plants [48] |
| T. harzianum, T. afroharzianum, T. hirsutum/C.S | Parasitism/In vitro | G. rostochiensis and Meloidogyne spp. | Algeria/Tomato | Mortality above 70% [81] |
| T. pseudoharzianum, T. koingiopsis, T. asperelloides, T. afroharzianum, T. acitrinoviride, T. hamatum, T. viride/C.F | Antagonism/In vitro | M. incognita | China/Chili | Only the secondary metabolites of T. virens showed strong nematicidal activity, causing the highest egg hatch inhibition and J2 mortality [82] |
| T. longibrachiatum/C.F | Induce resistance/In vitro | M. incognita | China/Marine algae | The metabolite cyclodepsipeptides 7–9 showed moderate nematicidal activities [83] |
| T. asperellum and T. harzianum (commercial formulates)/C.S | Antagonism/In vitro | M. incognita | Spain/Tomato and cucumber | The number of egg masses and eggs per plant were reduced. Induced resistance to M. incognita in tomato but not in cucumber [10] |
| T. harzianum/C.S | Parasitism/In vivo | M. incognita | China/Tomato | Nematode reduction percentage of 62%. The gall number per plant decreased by 75% [14] |
| T. hamatum/C.F | Induce resistance/In vitro | M. incognita | Saudi Arabia/Tomato | Egg hatch inhibition was 78% and juvenile stage mortality rate was 89% [49] |
| T. harzianum, T. viride and T. virens/C.S | Induce resistance/In vivo | M. javanica | Egypt/Peanut | The highest percentages reduction in J2 in soil (being 81%) was recorded with T. viride, followed by T. harzianum (77%) and T. virens (73%) [84] |
| T. asperellum and T. harzianum/C.F | Antibiosis/In vivo | P. brachyurus | Brazil/Soybean | Both isolates have nematicide effects that improve J2 mortality by 41–65% [85] |
| T. citrinoviride, T. ghanense, T. harzianum, T. koningiopsis, T. simmonsii, and T. virens/C.F | Antibiosis/In vitro | M. javanica and M. incognita | Mexico/Tomato | The most lethal strains were T. harzianum, T. koningiopsis, T. ghanense and T. virens, which caused 51–100% mortality of J2 of both nematodes [47] |
| T. virens/C.S | Antagonism/In vitro and in vivo | M. incognita | India/Chickpea | Reduction in J2 hatching [50] |
| T. harzianum/C.S | Antibiosis/In vivo | M. javanica | Egypt/Tomato | The penetration rates of nematodes, as well as the number of J2, females, egg mass, and galls were significantly reduced [86] |
| T. asperellum/C.S | In vivo | M. incognita | India/Okra | Hatching suppression 96% and J2 mortality 90% [9] |
| Trichoderma Species with Different Products | Mechanism of Action and Study Type | Application Type | Nematode | Country and Crop | Results and References |
|---|---|---|---|---|---|
| T. harzianum + neem, karanj, and castor oil cakes | Parasitism/In vivo | C.S | Tylenchulus semipenetrans | India/lime | Trichoderma in combination with vegetable oils showed good control of the nematode [87] |
| T. virens + Burkholderia cepacia | Antagonism/In vivo | C.F. | M. incognita | USA/Bell pepper | T. virens suppresses M. incognita; when combined, it decreases effectiveness [53] |
| T. harzianum + Pseudomonas fluorescens | Antagonism/In vitro and in vivo | C.F | M. javanica | Pakistan/Tomato | Mixtures of P. fluorescens and T. harzianum improve nematode biocontrol [90] |
| T. asperellum and T. atroviride with Monoclonal and polyclonal antibodies | Parasitism/In vitro | C.S | M. javanica | Israel/Tomato | Trichoderma parasitism increased with antibodies in bioassays [93] |
| T. longibrachiatum and cadusafos | Parasitism/In vivo | C.S | M. javanica | Iran/Zuchini | The optimal concentrations for best plant growth and lowest nematode reproduction were 1.7 mg a.i. kg−1 soil and 108 conidia mL−1 [94] |
| Bacillus licheniformis, B. subtilis, T. longibrachiatum | Parasitism and induced resistance/In vivo | C.S | P. brachyurus and M. javanica | Brazil/Soybean | Nematode reduction percentage of 34–40% for P. brachyurus and 88–92% for M. javanica [72] |
| T. asperellum, T. atroviride, Trichoderma sp. and Purpureocillium lilacinum | Antagonism/In vivo | C.S | M. javanica | Kenya/Pineapple | Reduced nematode egg and egg mass production, lowering root galling damage by 60.8–81.8% and increasing root mass growth [95] |
| T. viride, T. harzianum, Trichoderma sp. | Antibiosis/In vitro | C.F | M. incognita race 2 | India/Tomato | Culture filtrates of Trichoderma significantly induced inhibition of egg hatching and mortality of M. incognita race 2 [96] |
| T. asperellum, B. subtilis, Purpureocillium lilacinum, and abamectin | Antagonism/In vivo | C.S | Pratylenchus brachyurus | Brazil/Soybean | Reduction in the reproduction factor: T. asperellum 56%, B. subtilis 78%, and the combination of T. asperellum with B. subtilis and/or P. lilacinum 72.2% [91] |
| T. harzianum and Pochonia chlamydosporia | Antagonism/In vivo | C.S | M. incognita | Italy/Tomato | Tomato plants pre-treated with a mixture of beneficial bio-control agents (BCAs), as soil-drenches, were less sensitive to infection of the root-knot nematode [92] |
| T. harzianum, T. atroviride, T. longibrachiatum and carob galactomannan biopolymer | Antagonism/In vivo | C.S | M. incognita | Italy/Tomato | Coating tomato roots with the carob galactomannan biopolymer followed by soil application of selected Trichoderma strains reduced the root galling index [88] |
| Bacillus megatarium, B. subtilis, T. harzianum | Antibiosis/In vivo | C.S and C.F | M. incognita | India/Sweet basil | Reducing M. incognita infestation by 46 to 72%. A consortium of BM and TH was the most potent treatment [97] |
| T. harzianum and arbuscular mycorrhizae | Antibiosis/In vivo | C.S | M. javanica | Egypt/Tomato | The lowest number of juveniles was observed in the case of either single mycorrhizal inoculation (45%) or in combination with T. harzianum (55%) [86] |
| 1,3- dichloropropene with T. harzianum and an organic fertilizer | Antagonism/In vivo | C.S | M. incognita | Italy/Tomato | The greatest nematicidal effect was caused by a combination of the three products [89] |
| T. asperellum, T. hamatum, T. atrobruneum, and Clonostachys rosea | In vitro | C.S | Globodera spp. | Kenya/Potato | T. asperellum and T. breve suppressed nematode egg hatching by 50%, while T. breve specifically reduced egg viability by 41% |
| T. harzianum. and Bacillus velezensis | Antibiosis/In vitro | C.S | M. javanica | Iran/Tomato | Significant nematicidal activity, inhibiting egg hatching (16–45%) and inducing J2 mortality (30–46%) [98] |
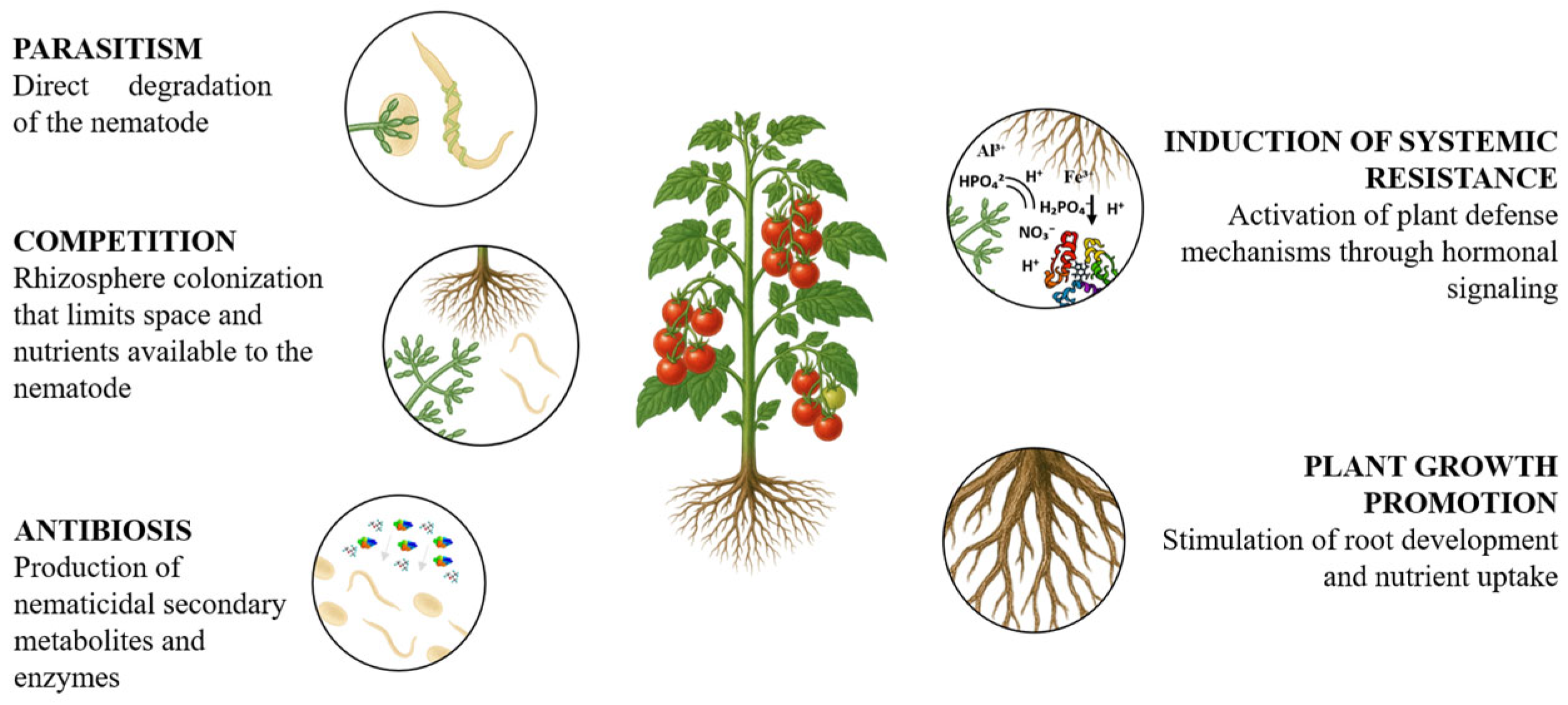
5. Mechanisms of Action of Trichoderma Against Nematodes
5.1. Parasitism
5.2. Secondary Metabolite Production (Antibiosis)
5.3. Competition for Resources and Rhizosphere Colonization
5.4. Induction of Systemic Resistance in Plants
6. Commercial Applications, Limitations, and Future Perspectives of Trichoderma spp. in Nematode Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mesa-Valle, C.M.; Garrido-Cardenas, J.A.; Cebrian-Carmona, J.; Talavera, M.; Manzano-Agugliaro, F. Global research on plant nematodes. Agronomy 2020, 10, 1148. [Google Scholar] [CrossRef]
- Ning, J.; Zhou, J.; Wang, H.; Liu, Y.; Ahmad, F.; Feng, X.; Fu, Y.; Gu, X.; Zhao, L. Parallel evolution of C-type lectin domain gene family sizes in insect-vectored nematodes. Front. Plant Sci. 2022, 13, 856826. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Chitwood, D.J. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture–Agricultural Research Service. Pest Manag. Sci. Former. Pestic. Sci. 2003, 59, 748–753. [Google Scholar] [CrossRef]
- Nicol, J.; Turner, S.; Coyne, D.L.; Nijs, L.d.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, Switzerland, 2011; pp. 21–43. [Google Scholar]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- TariqJaveed, M.; Farooq, T.; Al-Hazmi, A.S.; Hussain, M.D.; Rehman, A.U. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021, 183, 107626. [Google Scholar] [CrossRef]
- Saharan, R.; Patil, J.; Yadav, S.; Kumar, A.; Goyal, V. The nematicidal potential of novel fungus, Trichoderma asperellum FbMi6 against Meloidogyne incognita. Sci. Rep. 2023, 13, 6603. [Google Scholar] [CrossRef]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial formulates of Trichoderma induce systemic plant resistance to Meloidogyne incognita in tomato and the effect is additive to that of the Mi-1.2 resistance gene. Front. Microbiol. 2020, 10, 3042. [Google Scholar] [CrossRef]
- Agbessenou, A.; Akutse, K.S.; Yusuf, A.A.; Khamis, F.M. The endophyte Trichoderma asperellum M2RT4 induces the systemic release of methyl salicylate and (Z)-jasmone in tomato plant affecting host location and herbivory of Tuta absoluta. Front. Plant Sci. 2022, 13, 860309. [Google Scholar] [CrossRef]
- Natural Resources Defense Council. ¿Cuáles Son los Efectos del Cambio Climático? Available online: https://www.nrdc.org/es/stories/cuales-son-efectos-cambio-climatico (accessed on 14 January 2025).
- Tayyrov, A.; Schmieder, S.S.; Bleuler-Martinez, S.; Plaza, D.F.; Künzler, M. Toxicity of potential fungal defense proteins towards the fungivorous nematodes Aphelenchus avenae and Bursaphelenchus okinawaensis. Appl. Environ. Microbiol. 2018, 84, e02051-18. [Google Scholar] [CrossRef]
- Yan, Y.; Mao, Q.; Wang, Y.; Zhao, J.; Fu, Y.; Yang, Z.; Peng, X.; Zhang, M.; Bai, B.; Liu, A. Trichoderma harzianum induces resistance to root-knot nematodes by increasing secondary metabolite synthesis and defense-related enzyme activity in Solanum lycopersicum L. Biol. Control 2021, 158, 104609. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Yang, H.-T.; Zhang, X.-J.; Zhou, F.-Y.; Wu, X.-Q.; Xie, X.-Y.; Zhao, X.-Y.; Zhou, H.-Z. Five new species of Trichoderma from moist soils in China. MycoKeys 2022, 87, 133. [Google Scholar] [CrossRef]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Sousa, T.F.; Reça, B.N.P.V.; Castro, G.S.; da Silva, I.J.S.; Caniato, F.F.; de Araújo Júnior, M.B.; Yamagishi, M.E.B.; Koolen, H.H.F.; Bataglion, G.A.; Hanada, R.E. Trichoderma agriamazonicum sp. nov.(Hypocreaceae), a new ally in the control of phytopathogens. Microbiol. Res. 2023, 275, 127469. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhuang, W.-Y. Trichoderma shennongjianum and Trichoderma tibetense, two new soil-inhabiting species in the Strictipile clade. Mycoscience 2016, 57, 311–319. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; Alarcón, A. Trichoderma: Importancia agrícola, biotecnológica, y sistemas de fermentación para producir biomasa y enzimas de interés industrial. Chil. J. Agric. Anim. Sci. 2019, 35, 98–112. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.d.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Boddy, L.; Hiscox, J. Fungal ecology: Principles and mechanisms of colonization and competition by saprotrophic fungi. Microbiol. Spectr. 2016, 4, 1–16. [Google Scholar] [CrossRef]
- Jouhten, P.; Pitkänen, E.; Pakula, T.; Saloheimo, M.; Penttilä, M.; Maaheimo, H. 13C-metabolic flux ratio and novel carbon path analyses confirmed that Trichoderma reesei uses primarily the respirative pathway also on the preferred carbon source glucose. BMC Syst. Biol. 2009, 3, 1–16. [Google Scholar] [CrossRef]
- Bailey, B.A.; Melnick, R.L. The endophytic Trichoderma. In Trichoderma: Biology and Applications; CABI: Wallingford, UK, 2013; pp. 152–172. [Google Scholar]
- Cortés-Hernández, F.d.C.; Alvarado-Castillo, G.; Sánchez-Viveros, G. Trichoderma spp., an alternative for sustainable agriculture: A review. Rev. Colomb. De Biotecnol. 2023, 25, 73–87. [Google Scholar] [CrossRef]
- Natsiopoulos, D.; Topalidou, E.; Mantzoukas, S.; Eliopoulos, P.A. Endophytic Trichoderma: Potential and prospects for plant health management. Pathogens 2024, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A. Evolution and comparative genomics of the most common Trichoderma species. BMC Genom. 2019, 20, 485. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- El Enshasy, H.A.; Ambehabati, K.K.; El Baz, A.F.; Ramchuran, S.; Sayyed, R.; Amalin, D.; Dailin, D.J.; Hanapi, S.Z. Trichoderma: Biocontrol agents for promoting plant growth and soil health. In Agriculturally Important Fungi for Sustainable Agriculture: Volume 2: Functional Annotation for Crop Protection; Springer: Cham, Switzerland, 2020; pp. 239–259. [Google Scholar]
- Jemo, M.; Nkenmegne, S.; Buernor, A.B.; Raklami, A.; Ambang, Z.; Souleyamanou, A.; Ouhdouch, Y.; Hafidi, M. Mycorrhizas and Trichoderma fungi increase the accumulation of secondary metabolites in grain legume leaves and suppress foliar diseases in field-grown conditions of the humid forest of Cameroon. BMC Plant Biol. 2023, 23, 582. [Google Scholar] [CrossRef]
- Martínez, B.; Infante, D.; Reyes, Y. Trichoderma spp. y su función en el control de plagas en los cultivos. Rev. Protección Veg. 2013, 28, 1–11. [Google Scholar]
- Yang, R.; Qin, Z.; Wang, J.; Zhang, X.; Xu, S.; Zhao, W.; Huang, Z. The interactions between arbuscular mycorrhizal fungi and Trichoderma longibrachiatum enhance maize growth and modulate root metabolome under increasing soil salinity. Microorganisms 2022, 10, 1042. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Mehetre, S.T.; Mukherjee, P.K. Trichoderma Improves Nutrient Use Efficiency in Crop Plants. In Nutrient Use Efficiency: From Basics to Advances; Rakshit, A., Singh, H.B., Sen, A., Eds.; Springer: New Delhi, India, 2015; pp. 173–180. [Google Scholar]
- Sharma, S.; Kour, D.; Rana, K.L.; Dhiman, A.; Thakur, S.; Thakur, P.; Thakur, S.; Thakur, N.; Sudheer, S.; Yadav, N. Trichoderma: Biodiversity, ecological significances, and industrial applications. In Recent Advancement in White Biotechnology Through Fungi: Volume 1: Diversity and Enzymes Perspectives; Springer: Cham, Switzerland, 2019; pp. 85–120. [Google Scholar]
- Medina-García, L.R. Los hongos micorrízicos arbusculares y su rol en los agroecosistemas. Cultiv. Trop. 2022, 43, e14. [Google Scholar]
- Betancourt Tituaña, H.F. Sinergismo Entre Hongos Micorrícicos y Trichoderma harzianum en el Control del Nematodo Nacobbus aberrans en Plantas de Tomate (Solanum lycopersicum L.). Master’s Thesis, Universidad Nacional de La Plata, Buenos Aires, Argentina, 2020. [Google Scholar]
- Asghar, W.; Craven, K.D.; Kataoka, R.; Mahmood, A.; Asghar, N.; Raza, T.; Iftikhar, F. The application of Trichoderma spp., an old but new useful fungus, in sustainable soil health intensification: A comprehensive strategy for addressing challenges. Plant Stress 2024, 12, 100455. [Google Scholar] [CrossRef]
- Hu, J.; Chen, K.; Li, J.; Wei, Y.; Wang, Y.; Wu, Y.; Yang, H.; Zhou, Y.; Ryder, M.; Denton, M.D. Large-scale Trichoderma diversity was associated with ecosystem, climate and geographic location. Environ. Microbiol. 2020, 22, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Firdu, Z.; Alemu, T.; Assefa, F. Field performance of Trichoderma harzianum AAUT14 and Bacillus subtilis AAUB95 on faba bean (Vicia faba L.) growth promotion and management of chocolate spot (Botrytis fabae Sard.). IJPSS 2020, 32, 35–45. [Google Scholar] [CrossRef]
- Duc, N.; Mayer, Z.; Pék, Z.; Helyes, L.; Posta, K. Combined inoculation of arbuscular mycorrhizal fungi, Pseudomonas fluorescens and Trichoderma spp. for enhancing defense enzymes and yield of three pepper cultivars. Appl. Ecol. Environ. Res. 2017, 15, 1815–1829. [Google Scholar] [CrossRef]
- Velmourougane, K.; Prasanna, R.; Singh, S.; Chawla, G.; Kumar, A.; Saxena, A.K. Modulating rhizosphere colonisation, plant growth, soil nutrient availability and plant defense enzyme activity through Trichoderma viride-Azotobacter chroococcum biofilm inoculation in chickpea. Plant Soil 2017, 421, 157–174. [Google Scholar] [CrossRef]
- Windham, G.; Windham, M.; Williams, W. Effects of Trichoderma spp. on maize growth and Meloidogyne arenaria reproduction. Plant Dis. 1989, 73, 493–495. [Google Scholar] [CrossRef]
- Spiegel, Y.; Chet, I. Evaluation of Trichoderma spp. as a biocontrol agent against soilborne fungi and plant-parasitic nematodes in Israel. Integr. Pest Manag. Rev. 1998, 3, 169–175. [Google Scholar] [CrossRef]
- Sharon, E.; Bar-Eyal, M.; Chet, I.; Herrera-Estrella, A.; Kleifeld, O.; Spiegel, Y. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Phytopathology 2001, 91, 687–693. [Google Scholar] [CrossRef]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species-a diverse group of novel and important plant parasites. In Root-Knot Nematodes; CABI: Wallingford, UK, 2009; pp. 1–17. [Google Scholar]
- Moo-Koh, F.A.; Cristóbal-Alejo, J.; Andrés, M.F.; Martín, J.; Reyes, F.; Tun-Suárez, J.M.; Gamboa-Angulo, M. In vitro assessment of organic and residual fractions of nematicidal culture filtrates from thirteen tropical Trichoderma strains and metabolic profiles of most-active. J. Fungi 2022, 8, 82. [Google Scholar] [CrossRef]
- Fan, H.; Yao, M.; Wang, H.; Zhao, D.; Zhu, X.; Wang, Y.; Liu, X.; Duan, Y.; Chen, L. Isolation and effect of Trichoderma citrinoviride Snef1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC Microbiol. 2020, 20, 299. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bani Mfarrej, M.F.; Nadeem, H.; Ahamad, L.; Hashem, M.; Alamri, S.; Gupta, R.; Ahmad, F. Trichoderma virens mitigates the root-knot disease progression in the chickpea plant. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2022, 72, 775–787. [Google Scholar] [CrossRef]
- Djian, C.; Pijarowski, L.; Ponchet, M.; Arpin, N.; Favre-Bonvin, J. Acetic acid: A selective nematicidal metabolite from culture filtrates of Paecilomyces lilacinus (Thom) Samson and Trichoderma longibrachiatum Rifai. Nematologica 1991, 37, 101–112. [Google Scholar]
- Saifullah, S.; Khan, N. Low temperature scanning electron microscopic studies on the interaction of Globodera rostochiensis Woll. and Trichoderma harzianum Rifai. Pak. J. Bot. 2014, 43, 357–361. [Google Scholar]
- Meyer, S.L.; Massoud, S.I.; Chitwood, D.J.; Roberts, D.P. Evaluation of Trichoderma virens and Burkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematology 2000, 2, 871–879. [Google Scholar]
- Suarez, B.; Rey, M.; Castillo, P.; Monte, E.; Llobell, A. Isolation and characterization of PRA1, a trypsin-like protease from the biocontrol agent Trichoderma harzianum CECT 2413 displaying nematicidal activity. Appl. Microbiol. Biotechnol. 2004, 65, 46–55. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Viterbo, A.; Bar-Eyal, M.; Nagan, H.; Samuels, G.J.; Spiegel, Y. Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur. J. Plant Pathol. 2007, 118, 247–258. [Google Scholar] [CrossRef]
- Sahebani, N.; Hadavi, N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 2008, 40, 2016–2020. [Google Scholar] [CrossRef]
- Affokpon, A.; Coyne, D.L.; Htay, C.C.; Agbèdè, R.D.; Lawouin, L.; Coosemans, J. Biocontrol potential of native Trichoderma isolates against root-knot nematodes in West African vegetable production systems. Soil Biol. Biochem. 2011, 43, 600–608. [Google Scholar] [CrossRef]
- Naserinasab, F.; Sahebani, N.; Etebarian, H.R. Biological control of Meloidogyne javanica by Trichoderma harzianum BI and salicylic acid on tomato. Afr. J. Food Sci. 2011, 5, 276–280. [Google Scholar]
- Mascarin, G.M.; Bonfim Junior, M.; de Araújo Filho, J. Trichoderma harzianum reduces population of Meloidogyne incognita in cucumber plants under greenhouse conditions. J. Entomol. Nematol. 2012, 4, 54–57. [Google Scholar]
- Jindapunnapat, K.; Chinnasri, B.; Kwankuae, S. Biological control of root-knot nematodes (Meloidogyne enterolobii) in guava by the fungus Trichoderma harzianum. J. Dev. Sustain. Agric. 2013, 8, 110–118. [Google Scholar]
- Elgorban, A.M.; Abdel-Wahab, M.A.; Bahkali, A.H. Biocontrol of Meloidogyne javanica on Tomato Plants by Hypocrea lixii (the Teleomorph of Trichoderma harzianum). CLEAN–Soil Air Water 2014, 42, 1464–1469. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B.; Xue, Y. The parasitic and lethal effects of Trichoderma longibrachiatum against Heterodera avenae. Biol. Control 2014, 72, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Biocontrol potential of a native species of Trichoderma longibrachiatum against Meloidogyne incognita. Appl. Soil Ecol. 2015, 94, 21–29. [Google Scholar] [CrossRef]
- Pinzón Espinoza, L.; Candelero de la Cruz, J.; Tun Suárez, J.; Reyes Oregel, V.; Cristóbal Alejo, J. Meloidogyne incognita control in tomato (Solanum lycopersicum L.) to the implementation of Trichoderma harzianum. Fitosanidad 2015, 19, 5–11. [Google Scholar]
- Al-Hazmi, A.S.; TariqJaveed, M. Effects of different inoculum densities of Trichoderma harzianum and Trichoderma viride against Meloidogyne javanica on tomato. Saudi J. Biol. Sci. 2016, 23, 288–292. [Google Scholar] [CrossRef]
- Feyisa, B.; Lencho, A.; Selvaraj, T.; Amb, P.; Getaneh, G. Evaluation of some botanicals and Trichoderma harzianum for the management of tomato root-knot nematode (Meloidogyne incognita (Kofoid and White) Chit Wood). Adv. Crop Sci. Technol. 2016, 4, 201. [Google Scholar] [CrossRef]
- Braithwaite, M.; Clouston, A.; Minchin, R.; Yardley, J.; Nieto-Jacobo, M.F.; Mendoza-Mendoza, A.; Steyaert, J.; Hill, R.; Marshall, J.; Stewart, A. The density-dependent effect of initial nematode population levels on the efficacy of Trichoderma as a bio-nematicide against Meloidogyne hapla on tomato. Australas. Plant Pathol. 2016, 45, 473–479. [Google Scholar] [CrossRef]
- Contina, J.; Dandurand, L.; Knudsen, G. Use of GFP-tagged Trichoderma harzianum as a tool to study the biological control of the potato cyst nematode Globodera pallida. Appl. Soil Ecol. 2017, 115, 31–37. [Google Scholar] [CrossRef]
- Medeiros, H.A.d.; Araújo Filho, J.V.d.; Freitas, L.G.d.; Castillo, P.; Rubio, M.B.; Hermosa, R.; Monte, E. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep. 2017, 7, 40216. [Google Scholar] [CrossRef]
- Herrera-Parra, E.; Cristóbal-Alejo, J.; Ramos-Zapata, J.A. Trichoderma strains as growth promoters in Capsicum annuum and as biocontrol agents in Meloidogyne incognita. Chil. J. Agric. Res. 2017, 77, 318–324. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; Van Wees, S.C. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Miamoto, A.; Silva, M.T.R.e.; Dias-Arieira, C.R.; Puerari, H.H. Alternative products for Pratylenchus brachyurus and Meloidogyne javanica management in soya bean plants. J. Phytopathol. 2017, 165, 635–640. [Google Scholar] [CrossRef]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. Plant Cell Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Ji, W.; Xu, B.; Hou, B.; Liu, J. Mechanisms and characterization of Trichoderma longibrachiatum T6 in suppressing nematodes (Heterodera avenae) in wheat. Front. Plant Sci. 2017, 8, 1491. [Google Scholar] [CrossRef]
- Aït Hamza, M.; Lakhtar, H.; Tazi, H.; Moukhli, A.; Fossati-Gaschignard, O.; Miché, L.; Roussos, S.; Ferji, Z.; El Mousadik, A.; Mateille, T. Diversity of nematophagous fungi in Moroccan olive nurseries: Highlighting prey-predator interactions and efficient strains against root-knot nematodes. Biol. Control 2017, 114, 14–23. [Google Scholar] [CrossRef]
- Khan, M.R.; Ahmad, I.; Ahamad, F. Effect of pure culture and culture filtrates of Trichoderma species on root-knot nematode, Meloidogyne incognita infesting tomato. Indian Phytopathol. 2018, 71, 265–274. [Google Scholar] [CrossRef]
- Sonkar, S.; Bhatt, J.; Meher, J.; Kashyap, P. Bio-efficacy of Trichoderma viride against the root-knot nematode (Meloidogyne incognita) in tomato plant. J. Pharmacogn. Phytochem 2018, 7, 2010–2014. [Google Scholar]
- Mukhtar, T.; Jabbar, A.; Raja, M.; Javed, H. Management of root-knot nematode, Meloidogyne incognita, in tomato with two Trichoderma species. Pak. J. Zool. 2018, 50, 1589–1592. [Google Scholar] [CrossRef]
- El-Nagdi, W.M.; Youssef, M.M.; El-Khair, H.A.; Abd-Elgawad, M.M. Effect of certain organic amendments and Trichoderma species on the root-knot nematode, Meloidogyne incognita, infecting pea (Pisum sativum L.) plants. Egypt. J. Biol. Pest Control 2019, 29, 75. [Google Scholar] [CrossRef]
- Baldoni, D.B.; Antoniolli, Z.I.; Mazutti, M.A.; Jacques, R.J.S.; Dotto, A.C.; de Oliveira Silveira, A.; Ferraz, R.C.; Soares, V.B.; de Souza, A.R.C. Chitinase production by Trichoderma koningiopsis UFSMQ40 using solid state fermentation. Braz. J. Microbiol. 2020, 51, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Benttoumi, N.; Colagiero, M.; Sellami, S.; Boureghda, H.; Keddad, A.; Ciancio, A. Diversity of nematode microbial antagonists from Algeria shows occurrence of nematotoxic Trichoderma spp. Plants 2020, 9, 941. Plants 2020, 9, 941. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef]
- Du, F.-Y.; Ju, G.-L.; Xiao, L.; Zhou, Y.-M.; Wu, X. Sesquiterpenes and cyclodepsipeptides from marine-derived fungus Trichoderma longibrachiatum and their antagonistic activities against soil-borne pathogens. Mar. Drugs 2020, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Elkelany, U.; Hammam, M.; El-Nagdi, W.; Abd-El-Khair, H. Field application of Trichoderma spp. for controlling the root-knot nematode, Meloidogyne javanica in peanut plants. Egypt. J. Agronematology 2021, 20, 85–100. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Almeida, N.O.; Côrtes, M.V.d.C.B.; Júnior, M.L.; da Rocha, M.R.; Ulhoa, C.J. Biological control of Pratylenchus brachyurus with isolates of Trichoderma spp. on soybean. Biol. Control 2021, 152, 104425. [Google Scholar] [CrossRef]
- Nafady, N.A.; Sultan, R.; El-Zawahry, A.M.; Mostafa, Y.S.; Alamri, S.; Mostafa, R.G.; Hashem, M.; Hassan, E.A. Effective and promising strategy in management of tomato root-knot nematodes by Trichoderma harzianum and arbuscular mycorrhizae. Agronomy 2022, 12, 315. [Google Scholar] [CrossRef]
- Parvatha Reddy, P.; Rao, M.; Nagesh, M. Management of the citrus nematode Tylenchulus semipenetrans by integration of Trichoderma harzianum with oil cakes [India]. Nematol. Mediterr. 1996, 24, 265–267. [Google Scholar]
- D’Errico, G.; Mormile, P.; Malinconico, M.; Bolletti Censi, S.; Lanzuise, S.; Crasto, A.; Woo, S.L.; Marra, R.; Lorito, M.; Vinale, F. Trichoderma spp. and a carob (Ceratonia siliqua) galactomannan to control the root-knot nematode Meloidogyne incognita on tomato plants. Can. J. Plant Pathol. 2021, 43, 267–274. [Google Scholar] [CrossRef]
- d’Errico, G.; Greco, N.; Vinale, F.; Marra, R.; Stillittano, V.; Davino, S.W.; Woo, S.L.; D’Addabbo, T. Synergistic effects of Trichoderma harzianum, 1, 3 dichloropropene and organic matter in controlling the root-knot nematode Meloidogyne incognita on tomato. Plants 2022, 11, 2890. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.; Shaukat, S. Trichoderma harzianum enhances the production of nematicidal compounds in vitro and improves biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Lett. Appl. Microbiol. 2004, 38, 169–175. [Google Scholar] [CrossRef]
- Oliveira, K.C.L.d.; Araújo, D.v.d.; Meneses, A.C.d.; Silva, J.M.e.; Tavares, R.L.C. Biological management of Pratylenchus brachyurus in soybean crops. Rev. Caatinga 2019, 32, 041–051. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Spiegel, Y. Improved attachment and parasitism of Trichoderma on Meloidogyne javanica in vitro. Eur. J. Plant Pathol. 2009, 123, 291–299. [Google Scholar] [CrossRef]
- Sokhandani, Z.; Moosavi, M.R.; Basirnia, T. Optimum concentrations of Trichoderma longibrachiatum and cadusafos for controlling Meloidogyne javanica on zucchini plants. J. Nematol. 2016, 48, 54. [Google Scholar] [CrossRef]
- Kiriga, A.W.; Haukeland, S.; Kariuki, G.M.; Coyne, D.L.; Beek, N.V. Effect of Trichoderma spp. and Purpureocillium lilacinum on Meloidogyne javanica in commercial pineapple production in Kenya. Biol. Control 2018, 119, 27–32. [Google Scholar] [CrossRef]
- Devi, G.; Bora, L. Effect of some biocontrol agents against root-knot nematode (Meloidogyne incognita race2). Int. J. Environ. Agric. Biotechnol. 2018, 3, 265260. [Google Scholar] [CrossRef]
- Tiwari, S.; Pandey, R.; Gross, A. Identification of rhizospheric microorganisms that manages root knot nematode and improve oil yield in sweet basil (Ocimum basilicum L.). Agronomy 2021, 11, 570. [Google Scholar] [CrossRef]
- Rostami, M.; Shahbazi, S.; Soleimani, R.; Ghorbani, A. Optimizing sustainable control of Meloidogyne javanica in tomato plants through gamma radiation-induced mutants of Trichoderma harzianum and Bacillus velezensis. Sci. Rep. 2024, 14, 17774. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Inbar, J.; Chet, I. The role of lectins in recognition and adhesion of the mycoparasitic fungus Trichoderma spp. to its host. Towar. Anti-Adhes. Ther. Microb. Dis. 1996, 408, 229–231. [Google Scholar]
- Zeilinger, S.; Atanasova, L. Sensing and regulation of mycoparasitism-relevant processes in Trichoderma. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–55. [Google Scholar]
- Sivasithamparam, K.; Ghisalberti, E. Secondary metabolism in Trichoderma and Gliocladium. In Trichoderma and Gliocladium; Kubicek, C.P., Harman, G.E., Eds.; Francis & Taylor Ltd.: London, UK, 1998; Volume 1, pp. 139–191. [Google Scholar]
- Yang, Z.; Yu, Z.; Lei, L.; Xia, Z.; Shao, L.; Zhang, K.; Li, G. Nematicidal effect of volatiles produced by Trichoderma sp. J. Asia-Pac. Entomol. 2012, 15, 647–650. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Li, M.F.; Li, G.H.; Zhang, K.Q. Non-Volatile Metabolites from Trichoderma spp. Metabolites 2019, 9, 58. Metabolites 2019, 9, 58. [Google Scholar] [CrossRef]
- Anitha, R.; Murugesan, K. Production of gliotoxin on natural substrates by Trichoderma virens. J. Basic Microbiol. Int. J. Biochem. Physiol. Genet. Morphol. Ecol. Microorg. 2005, 45, 12–19. [Google Scholar]
- Li, G.; Zhang, K.; Xu, J.; Dong, J.; Liu, Y. Nematicidal substances from fungi. Recent Pat. Biotechnol. 2007, 1, 212–233. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Bordallo, J.; Lopez-Llorca, L.; Jansson, H.B.; Salinas, J.; Persmark, L.; Asensio, L. Colonization of plant roots by egg-parasitic and nematode-trapping fungi. New Phytol. 2002, 154, 491–499. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, Y.; Yin, N.; Li, Y.; Ling, J.; Mao, Z.; Yang, Y.; Xie, B. Analysis of the activity and biological control efficacy of the Bacillus subtilis strain Bs-1 against Meloidogyne incognita. Crop Prot. 2019, 122, 125–135. [Google Scholar] [CrossRef]
- Dube, B.; Smart, G.C., Jr. Biological control of Meloidogyne incognita by Paecilomyces lilacinus and Pasteuria penetrans. J. Nematol. 1987, 19, 222. [Google Scholar]
- Jansson, H.-B.; Lopez-Llorca, L.V. Control of nematodes by fungi. Mycol. Ser. 2004, 21, 205–216. [Google Scholar]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Fernández, I.; Sánchez-Guzmán, M.J.; Jung, S.C.; Pascual, J.A.; Pozo, M.J. Deciphering the hormonal signalling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front. Plant Sci. 2013, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Philp, J.; Li, J.; Wei, Y.; Li, H.; Yang, K.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.D. Trichoderma harzianum inoculation reduces the incidence of clubroot disease in Chinese cabbage by regulating the rhizosphere microbial community. Microorganisms 2020, 8, 1325. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Forghani, F.; Hajihassani, A. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. 2020, 11, 1125. [Google Scholar] [CrossRef]
- Hinterdobler, W.; Li, G.; Spiegel, K.; Basyouni-Khamis, S.; Gorfer, M.; Schmoll, M. Trichoderma reesei isolated from Austrian soil with high potential for biotechnological application. Front. Microbiol. 2021, 12, 552301. [Google Scholar] [CrossRef]
- Panchalingam, H.; Powell, D.; Adra, C.; Foster, K.; Tomlin, R.; Quigley, B.L.; Nyari, S.; Hayes, R.A.; Shapcott, A.; Kurtböke, D.İ. Assessing the various antagonistic mechanisms of Trichoderma strains against the brown root rot pathogen Pyrrhoderma noxium infecting heritage fig trees. J. Fungi 2022, 8, 1105. [Google Scholar] [CrossRef]
- Mohiddin, F.A.; Padder, S.A.; Bhat, A.H.; Ahanger, M.A.; Shikari, A.B.; Wani, S.H.; Bhat, F.A.; Nabi, S.U.; Hamid, A.; Bhat, N.A. Phylogeny and optimization of Trichoderma harzianum for chitinase production: Evaluation of their antifungal behaviour against the prominent soil borne phyto-pathogens of temperate India. Microorganisms 2021, 9, 1962. [Google Scholar] [CrossRef] [PubMed]
- Izuogu, N. Nematicidal Effect of Trichoderma harzianum T22 on Meloidogyne incognita (Kofoid & White) Chitwood, Infecting Celosia argentea TLV8. J. Agric. Res. Dev. 2013, 12, 35–43. [Google Scholar]
- Cumagun, C.J.R. Chapter 39—Advances in Formulation of Trichoderma for Biocontrol. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 527–531. [Google Scholar]
- Wang, Y.; Chen, H.; Ma, L.; Gong, M.; Wu, Y.; Bao, D.; Zou, G. Use of CRISPR-Cas tools to engineer Trichoderma species. Microb. Biotechnol. 2022, 15, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.B.P.R.; Oliveira-Santos, N.; Novais, D.P.S.d.; Cruz-Magalhães, V.; Loguercio, L.L. Beneficial plants-Trichoderma interactions on host tolerance to abiotic stresses: A meta-analysis. Front. Plant Physiol. 2025, 3, 1569221. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From’omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef]
- Comite, E.; El-Nakhel, C.; Rouphael, Y.; Ventorino, V.; Pepe, O.; Borzacchiello, A.; Vinale, F.; Rigano, D.; Staropoli, A.; Lorito, M. Bioformulations with beneficial microbial consortia, a bioactive compound and plant biopolymers modulate sweet basil productivity, photosynthetic activity and metabolites. Pathogens 2021, 10, 870. [Google Scholar] [CrossRef]
| Mechanism of Action | Against Nematodes | Against Fungal Pathogens |
|---|---|---|
| Production of secondary metabolites | Gliotoxin, viridin, cyclosporin A, acetic acid—inhibit egg hatching and juvenile development [48,51,107,116] | Peptaibols, gliotoxin, 6-pentyl-α-pyrone—inhibit fungal growth and spore germination [117] |
| Production of lytic enzymes | Proteases, chitinases—degrade cuticle or eggshell [49,78,118] | Chitinases, glucanases—degrade fungal cell walls [82] |
| Induced systemic resistance (ISR) | Activation of jasmonic acid/ethylene pathways—increased plant-defense compounds [10] | Similar activation to ISR—enhanced plant resistance to fungal infection [10,119] |
| Direct physical interaction | Limited or absent [45] | Mycoparasitism: coiling, penetration, and degradation of fungal hyphae [28] |
| Competition for space and nutrients | Present in the rhizosphere [19] | Strong competition on root and rhizoplane surfaces [120,121] |
| Plant growth promotion | Enhances plant tolerance to nematode stress [10,65] | Improves plant vigor, indirectly reducing fungal susceptibility [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Soto, M.B.; Tovar-Pedraza, J.M.; Solano-Báez, A.R.; Bayardo-Rosales, H.; Márquez-Licona, G. Biocontrol Strategies Against Plant-Parasitic Nematodes Using Trichoderma spp.: Mechanisms, Applications, and Management Perspectives. J. Fungi 2025, 11, 517. https://doi.org/10.3390/jof11070517
Contreras-Soto MB, Tovar-Pedraza JM, Solano-Báez AR, Bayardo-Rosales H, Márquez-Licona G. Biocontrol Strategies Against Plant-Parasitic Nematodes Using Trichoderma spp.: Mechanisms, Applications, and Management Perspectives. Journal of Fungi. 2025; 11(7):517. https://doi.org/10.3390/jof11070517
Chicago/Turabian StyleContreras-Soto, María Belia, Juan Manuel Tovar-Pedraza, Alma Rosa Solano-Báez, Heriberto Bayardo-Rosales, and Guillermo Márquez-Licona. 2025. "Biocontrol Strategies Against Plant-Parasitic Nematodes Using Trichoderma spp.: Mechanisms, Applications, and Management Perspectives" Journal of Fungi 11, no. 7: 517. https://doi.org/10.3390/jof11070517
APA StyleContreras-Soto, M. B., Tovar-Pedraza, J. M., Solano-Báez, A. R., Bayardo-Rosales, H., & Márquez-Licona, G. (2025). Biocontrol Strategies Against Plant-Parasitic Nematodes Using Trichoderma spp.: Mechanisms, Applications, and Management Perspectives. Journal of Fungi, 11(7), 517. https://doi.org/10.3390/jof11070517







