Pathogen Enzyme-Mediated Alkoxyamine Homolysis as a Killing Mechanism of Aspergillus fumigatus
Abstract
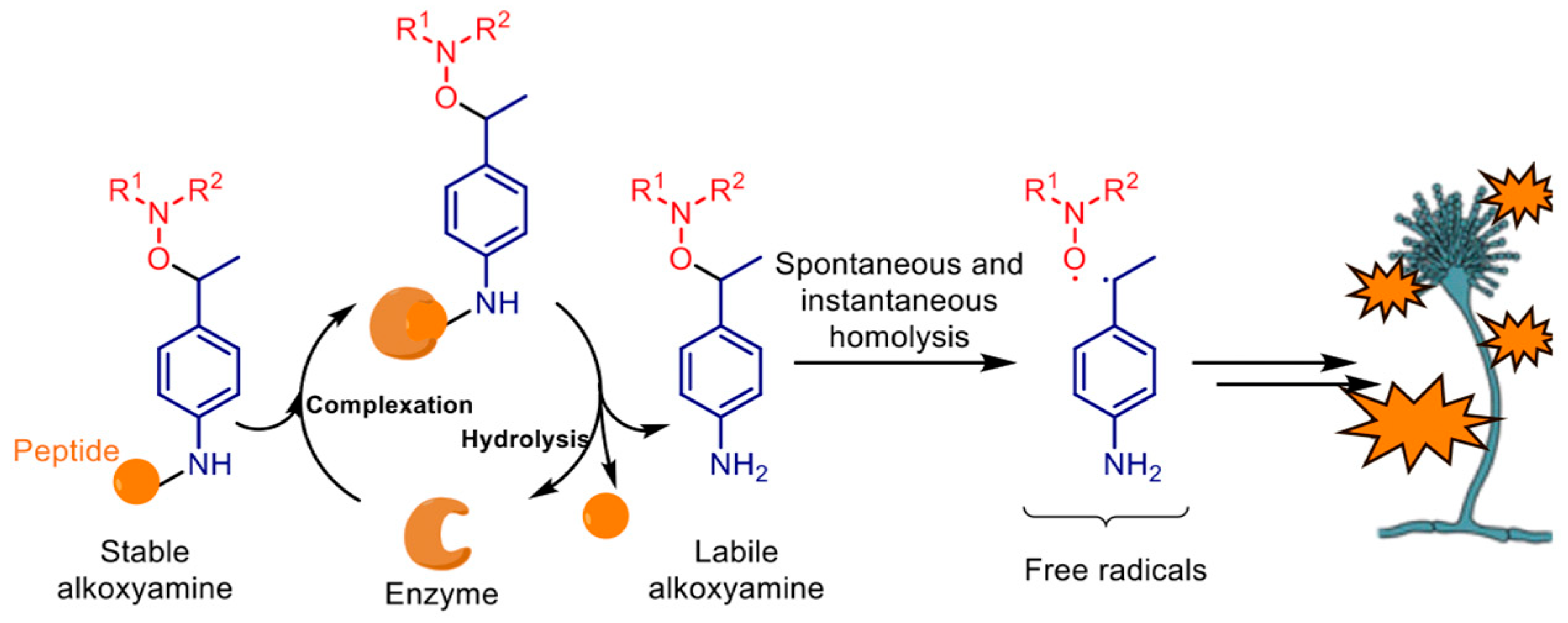
1. Introduction
2. Materials and Methods
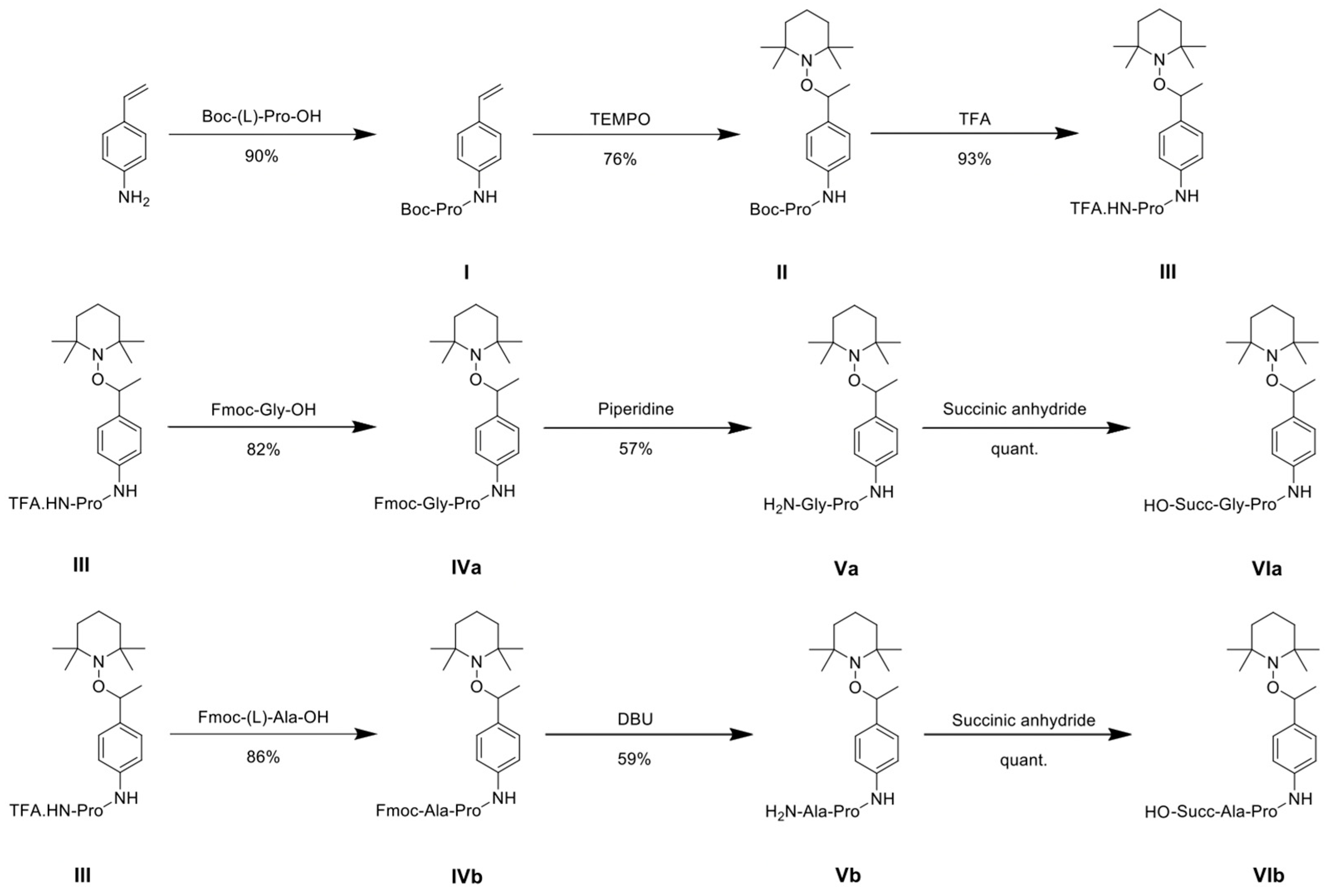
2.1. Alkoxyamine Synthesis
2.2. Stability of Prodrugs
2.3. Kinetics of Prodrug Enzymatic Activation
2.4. Fungal Cultures
2.5. Exposure of A. fumigatus Spores to Alkoxyamines
2.6. Exposure of Epithelial Cells to Alkoxyamine Va
2.7. Statistics
3. Results
3.1. Prodrug Synthesis
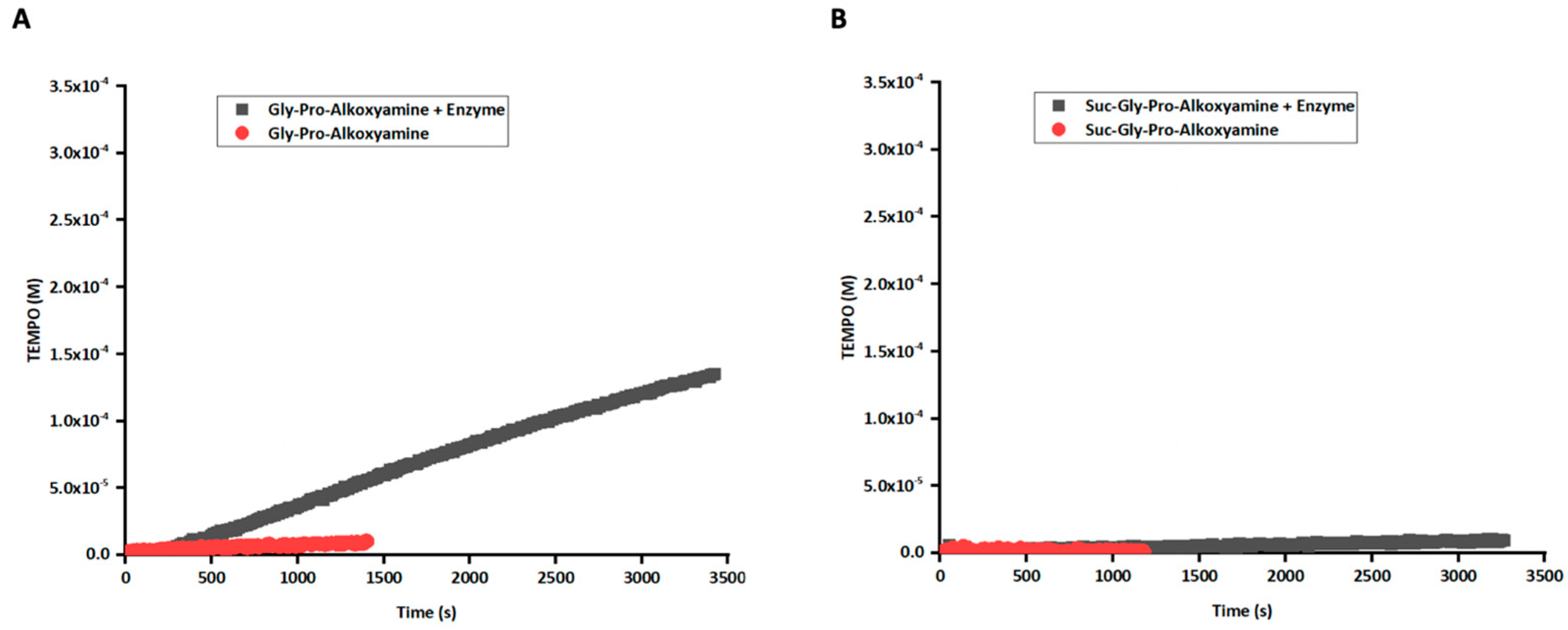
3.2. Stability of Prodrugs
3.3. Enzymatic Activation of Prodrug
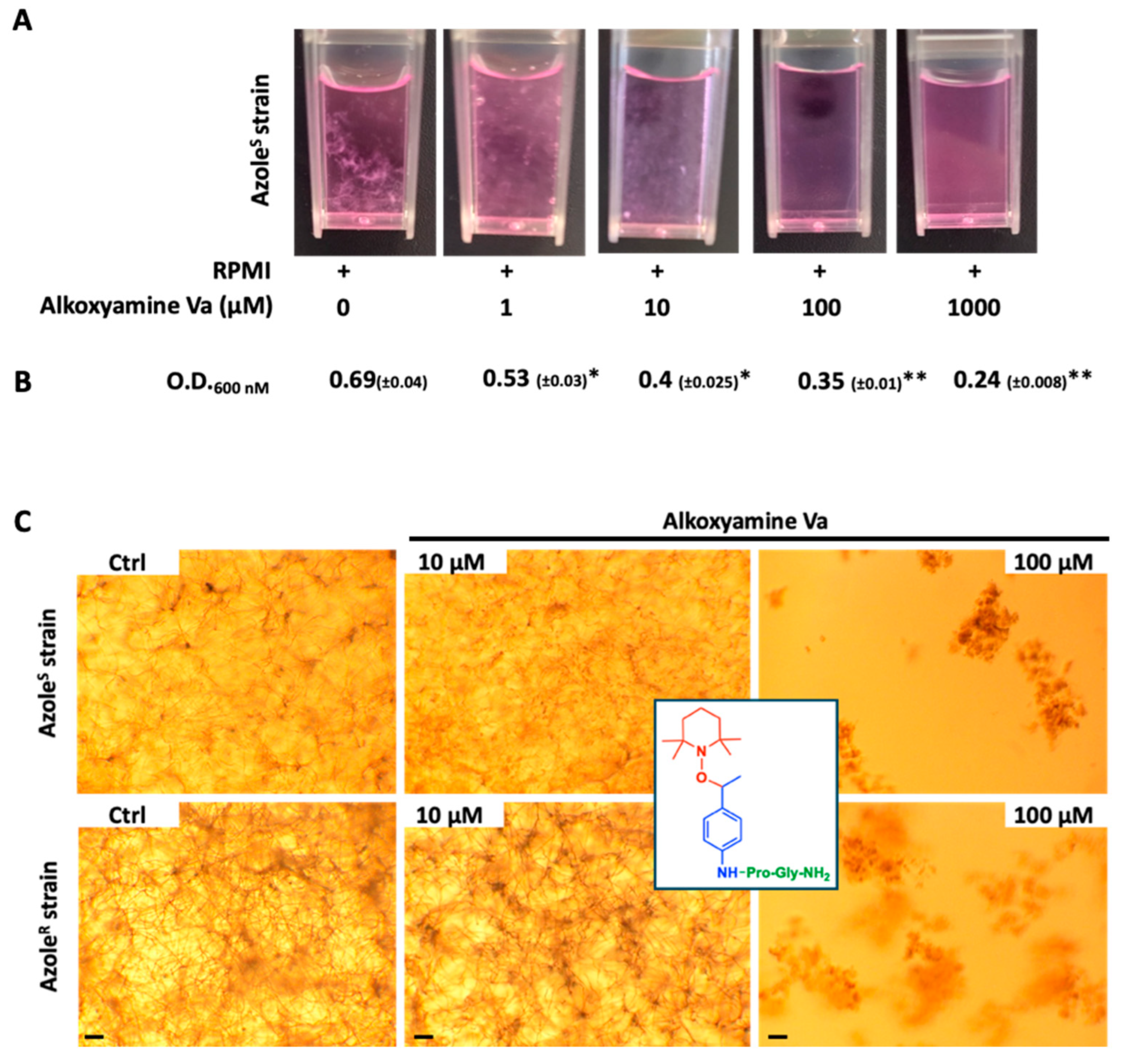
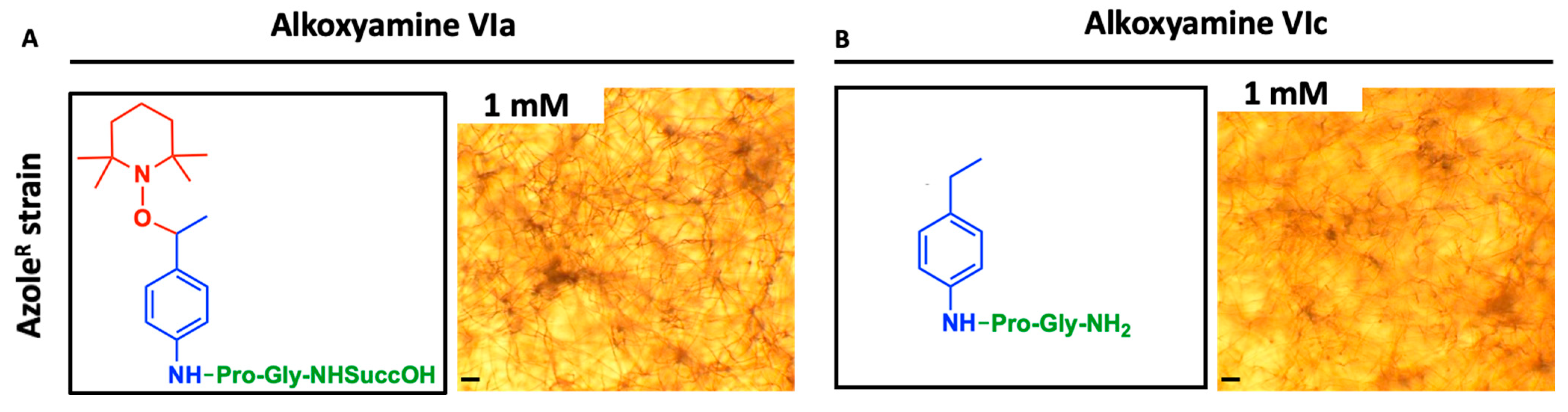
3.4. Killing of A. fumigatus by Alkoxyamines
3.5. Antifungal Efficiency of Alkoxyamines Requires Both Defined Peptide Substrate and Radical Structure
3.6. Alteration of Epithelial Cell Monolayer Following Exposure to Alkoxyamine Va
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. Semin. Immunopathol. 2015, 37, 141–152. [Google Scholar] [CrossRef]
- Dupont, D.; Menotti, J.; Turc, J.; Miossec, C.; Wallet, F.; Richard, J.C.; Argaud, L.; Paulus, S.; Wallon, M.; Ader, F.; et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID-19). Med. Mycol. 2021, 59, 110–114. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Burgel, P.R.; Paugam, A.; Hubert, D.; Martin, C. Aspergillus fumigatus in the cystic fibrosis lung: Pros and cons of azole thera-py. Infect. Drug Resist. 2016, 20, 229–238. [Google Scholar] [CrossRef]
- Logan, A.; Wolfe, A.; Williamson, J.C. Antifungal Resistance and the Role of New Therapeutic Agents. Curr. Infect. Dis. Rep. 2022, 24, 105–116. [Google Scholar] [CrossRef]
- Yang, Y.L.; Xiang, Z.J.; Yang, J.H.; Wang, W.J.; Xu, Z.C.; Xiang, R.L. Adverse Effects Associated With Currently Commonly Used Antifungal Agents: A Network Meta-Analysis and Systematic Review. Front. Pharmacol. 2021, 12, 697330. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Moncelet, D.; Voisin, P.; Koonjoo, N.; Bouchaud, V.; Massot, P.; Parzy, E.; Audran, G.; Franconi, J.M.; Thiaudière, E.; Marque, S.R.; et al. Alkoxyamines: Toward a New Family of Theranostic Agents against Cancer. Mol. Pharmaceutics 2014, 11, 2412–2419. [Google Scholar] [CrossRef]
- Piris, P.; Buric, D.; Yamasaki, T.; Huchedé, P.; Rossi, M.; Matteudi, M.; Montero, M.P.; Rodallec, A.; Appay, R.; Roux, C.; et al. Conditional generation of free radicals by selective activation of alkoxyamines: Towards more effective and less toxic targeting of brain tumors. Chem. Sci. 2023, 14, 7988–7998. [Google Scholar] [CrossRef]
- Reyser, T.; To, T.H.; Egwu, C.; Paloque, L.; Nguyen, M.; Hamouy, A.; Stigliani, J.L.; Bijani, C.; Augereau, J.M.; Joly, J.P.; et al. Alkoxyamines Designed as Potential Drugs against Plasmodium and Schistosoma Parasites. Molecules 2020, 25, 3838. [Google Scholar] [CrossRef]
- Embo-Ibouanga, A.W.; Nguyen, M.; Paloque, L.; Joly, J.P.; Bikanga, R.; Augereau, J.M.; Robert, A.; Audran, G.; Mellet, P.; Boissier, J.; et al. Dynamic covalent bonding (DCB): The bond lability of alkoxyamines as drugs against Schisto-soma mansoni and Plasmodium falciparum. Org. Biomol. Chem. 2025, 23, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Filliâtre, M.; Seren, S.; Embo-Ibouanga, A.W.; Joly, J.P.; Bouchaud, V.; Kelkoul, I.; Marque, S.R.A.; Audran, G.; Voisin, P.; Mellet, P. Intrinsic Proteolytic Activities from Cancer Cells Are Sufficient to Activate Alkoxyamine Prodrugs and Induce Cell Death. ACS Omega 2024, 9, 39004–39012. [Google Scholar] [CrossRef]
- Embo-Ibouanga, A.W.; Nguyen, M.; Joly, J.P.; Coustets, M.; Augereau, J.M.; Paloque, L.; Vanthuyne, N.; Bikanga, R.; Robert, A.; Benoit-Vical, F.; et al. Peptide-Alkoxyamine Drugs: An Innovative Approach to Fight Schistosomiasis: “Digging Their Graves with Their Forks”. Pathogens 2024, 13, 482. [Google Scholar] [CrossRef]
- Marque, S.R.A.; Le Mercier, C.; Tordo, P.; Fischer, H. Factors Influencing the C-O-Bond Homolysis of Trialkylhydroxylamines. Macromolecules 2000, 33, 4403–4410. [Google Scholar] [CrossRef]
- Persoz, C.; Leleu, C.; Achard, S.; Fasseu, M.; Menotti, J.; Meneceur, P.; Momas, I.; Derouin, F.; Seta, N. Sequential air-liquid ex-posure of human respiratory cells to chemical and biological pollutants. Toxicol. Lett. 2011, 207, 53–59. [Google Scholar] [CrossRef]
- Bouyssi, A.; Trecourt, A.; Déméautis, T.; Persat, F.; Glehen, O.; Wallon, M.; Devouassoux, G.; Bentaher, A.; Menotti, J. Aspergillus fumigatus is responsible for inflammation in a murine model of chronic obstructive pulmo-nary disease exacerbation. Respir Res. 2025, 26, 25. [Google Scholar] [CrossRef] [PubMed]
- Boxio, R.; Wartelle, J.; Nawrocki-Raby, B.; Lagrange, B.; Malleret, L.; Hirche, T.; Taggart, C.; Pacheco, Y.; Devouassoux, G.; Bentaher, A. Neutrophil elastase cleaves epithelial cadherin in acutely injured lung epithelium. Respir. Res. 2016, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Bouyssi, A.; Déméautis, T.; Trecourt, A.; Delles, M.; Agostini, F.; Monneret, G.; Glehen, O.; Wallon, M.; Persat, F.; Devouassoux, G.; et al. Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores. J. Fungi. 2023, 9, 682. [Google Scholar] [CrossRef]
- Beauvais, A.; Monod, M.; Wyniger, J.; Debeaupuis, J.P.; Grouzmann, E.; Brakch, N.; Svab, J.; Hovanessian, A.G.; Latgé, J.P. Dipeptidyl-peptidase IV secreted by Aspergillus fumigatus, a fungus pathogenic to humans. Infect. Immun. 1997, 65, 3042–3047. [Google Scholar] [CrossRef]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef]
- Embo-Ibouanga, A.W.; Nguyen, M.; Paloque, L.; Coustets, M.; Joly, J.P.; Augereau, J.M.; Vanthuyne, N.; Bikanga, R.; Coquin, N.; Robert, A.; et al. Hybrid Peptide-Alkoxyamine Drugs: A Strategy for the Development of a New Family of Antiplasmodial Drugs. Molecules 2024, 29, 1397. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.A.; Sysoeva, G.M.; Nikolin, V.P.; Kaledin, V.I.; Tretyakov, E.V.; Edeleva, M.V.; Balakhnin, S.M.; Lushnikova, E.L.; Audran, G.; Mark, S. Comparative Study of Toxicity of Alkoxyamines In Vitro and In Vivo. Bull. Exp. Biol. Med. 2017, 164, 49–53, Erratum in: Bull. Exp. Biol. Med. 2018, 165, 176. https://doi.org/10.1007/s10517-018-4123-9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filliâtre, M.; Voisin, P.; Seren, S.; Kelkoul, I.; Glehen, O.; Mellet, P.; Thétiot-Laurent, S.; Menotti, J.; Marque, S.R.A.; Audran, G.; et al. Pathogen Enzyme-Mediated Alkoxyamine Homolysis as a Killing Mechanism of Aspergillus fumigatus. J. Fungi 2025, 11, 503. https://doi.org/10.3390/jof11070503
Filliâtre M, Voisin P, Seren S, Kelkoul I, Glehen O, Mellet P, Thétiot-Laurent S, Menotti J, Marque SRA, Audran G, et al. Pathogen Enzyme-Mediated Alkoxyamine Homolysis as a Killing Mechanism of Aspergillus fumigatus. Journal of Fungi. 2025; 11(7):503. https://doi.org/10.3390/jof11070503
Chicago/Turabian StyleFilliâtre, Marion, Pierre Voisin, Seda Seren, Ines Kelkoul, Olivier Glehen, Philippe Mellet, Sophie Thétiot-Laurent, Jean Menotti, Sylvain R. A. Marque, Gérard Audran, and et al. 2025. "Pathogen Enzyme-Mediated Alkoxyamine Homolysis as a Killing Mechanism of Aspergillus fumigatus" Journal of Fungi 11, no. 7: 503. https://doi.org/10.3390/jof11070503
APA StyleFilliâtre, M., Voisin, P., Seren, S., Kelkoul, I., Glehen, O., Mellet, P., Thétiot-Laurent, S., Menotti, J., Marque, S. R. A., Audran, G., & Bentaher, A. (2025). Pathogen Enzyme-Mediated Alkoxyamine Homolysis as a Killing Mechanism of Aspergillus fumigatus. Journal of Fungi, 11(7), 503. https://doi.org/10.3390/jof11070503








