Vaginal Clinical Isolates of Candida albicans Differentially Modulate Complosome Activation in Vaginal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Cell Culture Conditions
2.2. Vaginal Epithelial Cells (VECs)
2.3. Antibodies
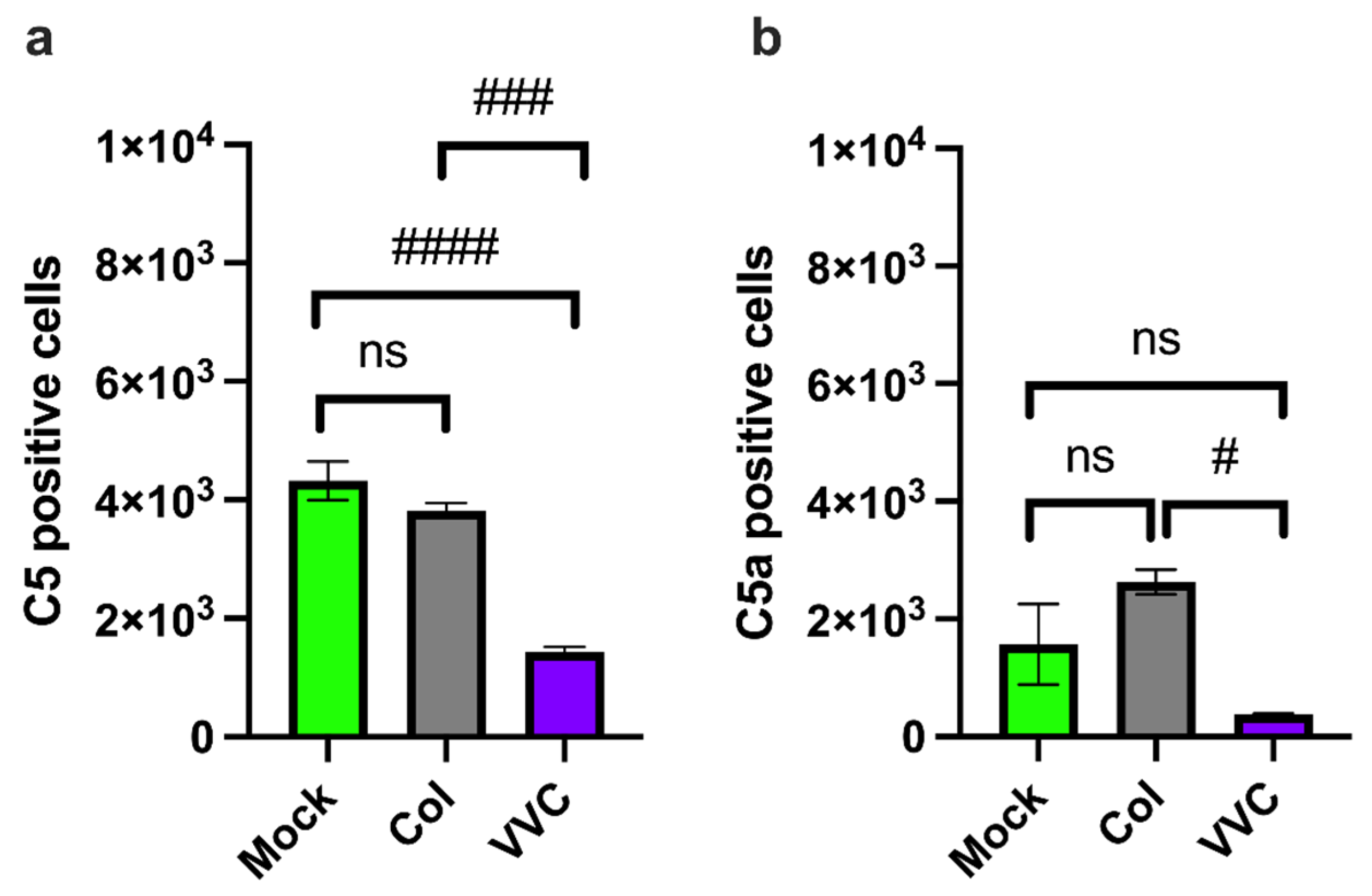
2.4. Flow Cytometry Analysis
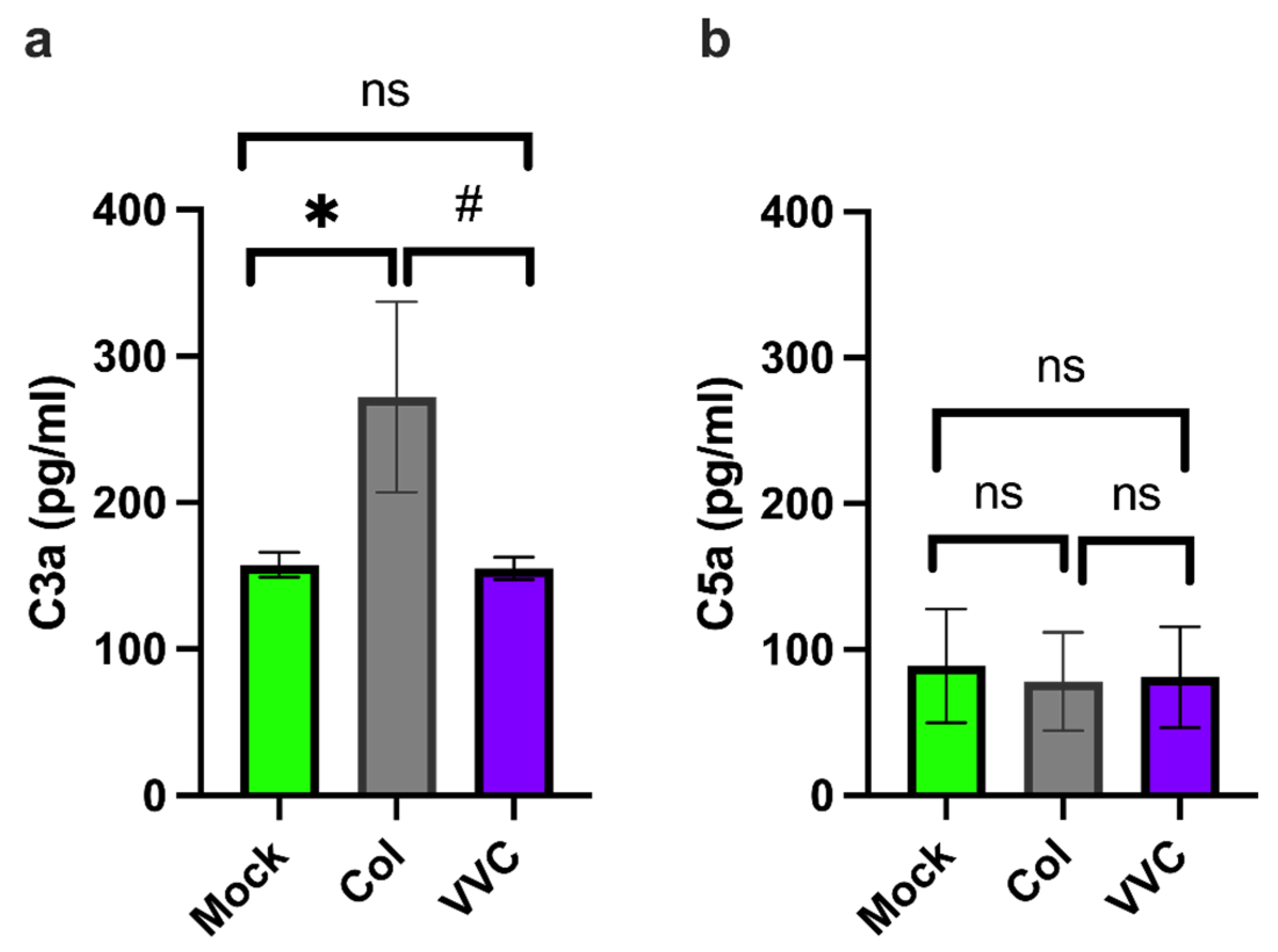
2.5. ELISA Test for the Detection of C3a and C5a in the Supernatant
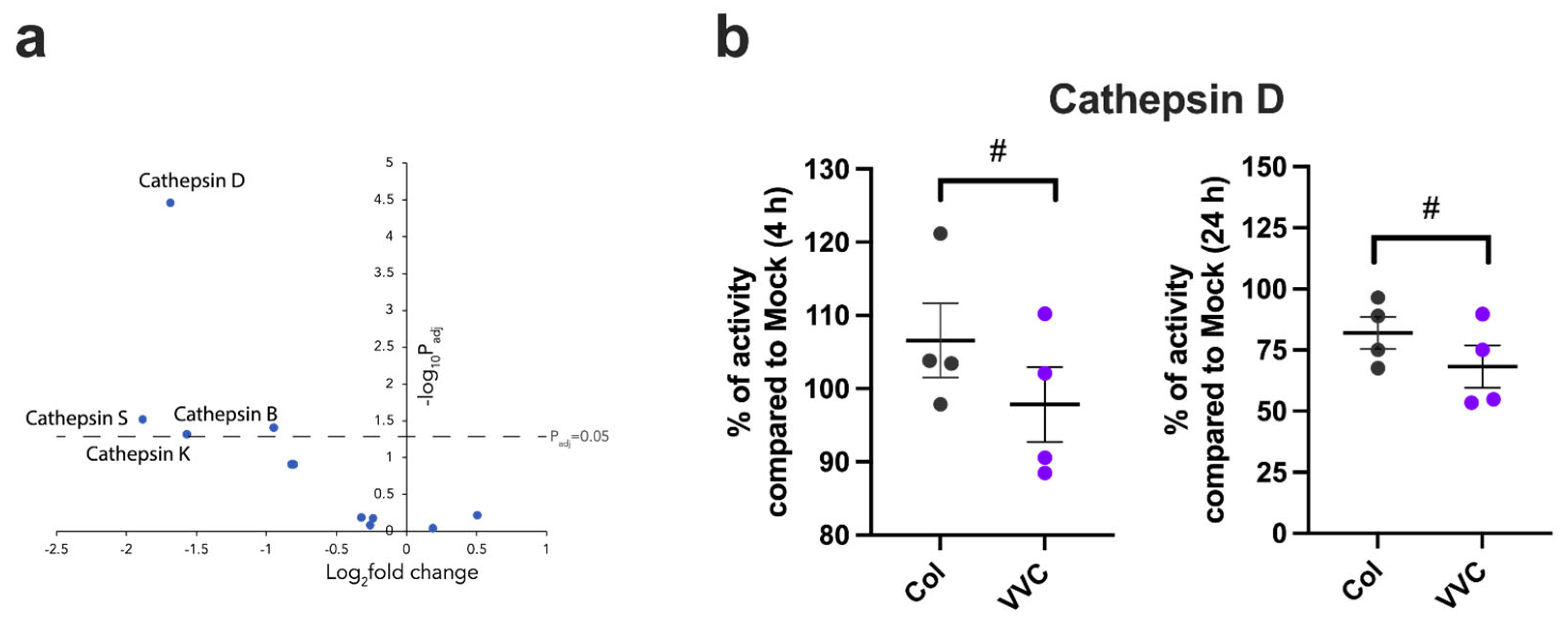
2.6. RNA Sequencing and Analysis
2.7. Cathepsin D Activity Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faria-Gonçalves, P.; Rolo, J.; Gaspar, C.; Oliveira, A.S.; Pestana, P.G.; Palmeira-de-Oliveira, R.; Gonçalves, T.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Recurrent Vulvovaginal Candida spp. Isolates Phenotypically Express Less Virulence Traits. Microb. Pathog. 2020, 148, 104471. [Google Scholar] [CrossRef] [PubMed]
- d’Enfert, C.; Kaune, A.-K.; Alaban, L.-R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The Impact of the Fungus-Host-Microbiota Interplay upon Candida Albicans Infections: Current Knowledge and New Perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Wheeler, R.T.; Pericolini, E. It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset. Front. Microbiol. 2021, 12, 692491. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; Vanthuyne, A. Vulvovaginal Candidiasis. Sex. Transm. Infect. 2006, 82, iv28–iv30. [Google Scholar] [CrossRef]
- Pericolini, E.; Perito, S.; Castagnoli, A.; Gabrielli, E.; Mencacci, A.; Blasi, E.; Vecchiarelli, A.; Wheeler, R.T. Epitope Unmasking in Vulvovaginal Candidiasis Is Associated with Hyphal Growth and Neutrophilic Infiltration. PLoS ONE 2018, 13, e0201436. [Google Scholar] [CrossRef]
- Sala, A.; Ardizzoni, A.; Spaggiari, L.; Vaidya, N.; Van Der Schaaf, J.; Rizzato, C.; Cermelli, C.; Mogavero, S.; Krüger, T.; Himmel, M.; et al. A New Phenotype in Candida-Epithelial Cell Interaction Distinguishes Colonization- versus Vulvovaginal Candidiasis-Associated Strains. MBio 2023, 14, e00107-23. [Google Scholar] [CrossRef]
- Kumwenda, P.; Cottier, F.; Hendry, A.C.; Kneafsey, D.; Keevan, B.; Gallagher, H.; Tsai, H.-J.; Hall, R.A. Estrogen Promotes Innate Immune Evasion of Candida Albicans through Inactivation of the Alternative Complement System. Cell Rep. 2022, 38, 110183. [Google Scholar] [CrossRef]
- Kenno, S.; Speth, C.; Rambach, G.; Binder, U.; Chatterjee, S.; Caramalho, R.; Haas, H.; Lass-Flörl, C.; Shaughnessy, J.; Ram, S.; et al. Candida Albicans Factor H Binding Molecule Hgt1p—A Low Glucose-Induced Transmembrane Protein Is Trafficked to the Cell Wall and Impairs Phagocytosis and Killing by Human Neutrophils. Front. Microbiol. 2019, 9, 3319. [Google Scholar] [CrossRef]
- Luo, S.; Dasari, P.; Reiher, N.; Hartmann, A.; Jacksch, S.; Wende, E.; Barz, D.; Niemiec, M.J.; Jacobsen, I.; Beyersdorf, N.; et al. The Secreted Candida Albicans Protein Pra1 Disrupts Host Defense by Broadly Targeting and Blocking Complement C3 and C3 Activation Fragments. Mol. Immunol. 2018, 93, 266–277. [Google Scholar] [CrossRef]
- Harpf, V.; Rambach, G.; Würzner, R.; Lass-Flörl, C.; Speth, C. Candida and Complement: New Aspects in an Old Battle. Front. Immunol. 2020, 11, 1471. [Google Scholar] [CrossRef]
- Heggi, M.T.; Nour El-Din, H.T.; Morsy, D.I.; Abdelaziz, N.I.; Attia, A.S. Microbial Evasion of the Complement System: A Continuous and Evolving Story. Front. Immunol. 2024, 14, 1281096. [Google Scholar] [CrossRef] [PubMed]
- Dühring, S.; Germerodt, S.; Skerka, C.; Zipfel, P.F.; Dandekar, T.; Schuster, S. Host-Pathogen Interactions between the Human Innate Immune System and Candida Albicans—Understanding and Modeling Defense and Evasion Strategies. Front. Microbiol. 2015, 6, 625. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.; Van Essen, M.F.; Van Kooten, C.; Trouw, L.A. Production of Complement Components by Cells of the Immune System. Clin. Exp. Immunol. 2017, 188, 183–194. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The Complement System. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Klos, A.; Tenner, A.J.; Johswich, K.-O.; Ager, R.R.; Reis, E.S.; Köhl, J. The Role of the Anaphylatoxins in Health and Disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef]
- Ghosh, M.; Rana, S. The Anaphylatoxin C5a: Structure, Function, Signaling, Physiology, Disease, and Therapeutics. Int. Immunopharmacol. 2023, 118, 110081. [Google Scholar] [CrossRef]
- Mastellos, D.C.; Hajishengallis, G.; Lambris, J.D. A Guide to Complement Biology, Pathology and Therapeutic Opportunity. Nat. Rev. Immunol. 2024, 24, 118–141. [Google Scholar] [CrossRef]
- Desai, J.V.; Kumar, D.; Freiwald, T.; Chauss, D.; Johnson, M.D.; Abers, M.S.; Steinbrink, J.M.; Perfect, J.R.; Alexander, B.; Matzaraki, V.; et al. C5a-Licensed Phagocytes Drive Sterilizing Immunity during Systemic Fungal Infection. Cell 2023, 186, 2802–2822.e22. [Google Scholar] [CrossRef]
- West, E.E.; Kemper, C. Complosome—The Intracellular Complement System. Nat. Rev. Nephrol. 2023, 19, 426–439. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, J.; Tomlinson, S.; Yuan, G.; He, S. The Role of the Complosome in Health and Disease. Front. Immunol. 2023, 14, 1146167. [Google Scholar] [CrossRef] [PubMed]
- Garred, P.; Hetland, G.; Mollnes, T.E.; Stoervold, G. Synthesis of C3, C5, C6, C7, C8, and C9 by Human Fibroblasts. Scand. J. Immunol. 1990, 32, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Posch, W.; Vosper, J.; Noureen, A.; Zaderer, V.; Witting, C.; Bertacchi, G.; Gstir, R.; Filipek, P.A.; Bonn, G.K.; Huber, L.A.; et al. C5aR Inhibition of Nonimmune Cells Suppresses Inflammation and Maintains Epithelial Integrity in SARS-CoV-2–Infected Primary Human Airway Epithelia. J. Allergy Clin. Immunol. 2021, 147, 2083–2097.e6. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kemper, C. Complement, Complosome, and Complotype: A Perspective. Eur. J. Immunol. 2023, 53, 2250042. [Google Scholar] [CrossRef]
- Li, X.X.; Fung, J.N.; Clark, R.J.; Lee, J.D.; Woodruff, T.M. Cell-Intrinsic C5a Synergizes with Dectin-1 in Macrophages to Mediate Fungal Killing. Proc. Natl. Acad. Sci. USA 2024, 121, e2314627121. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Konopko, A.; Jarczak, J.; Kazek, M.; Ratajczak, J.; Kucia, M. Complosome as a New Intracellular Regulatory Network in Both Normal and Malignant Hematopoiesis. Leukemia 2025, 39, 1571–1577. [Google Scholar] [CrossRef]
- Niyonzima, N.; Rahman, J.; Kunz, N.; West, E.E.; Freiwald, T.; Desai, J.V.; Merle, N.S.; Gidon, A.; Sporsheim, B.; Lionakis, M.S.; et al. Mitochondrial C5aR1 Activity in Macrophages Controls IL-1β Production Underlying Sterile Inflammation. Sci. Immunol. 2021, 6, eabf2489. [Google Scholar] [CrossRef]
- Desai, J.V.; Lionakis, M.S. C5-C5aR1-mediated Immune Responses during Fungal Infection: Clinical and Translational Implications. Clin. Transl. Med. 2023, 13, e1424. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Starick, M.; Aponte Alburquerque, R.; Kulkarni, H.S. Local Complement Activation and Modulation in Mucosal Immunity. Mucosal Immunol. 2024, 17, 739–751. [Google Scholar] [CrossRef]
- Kohli, A.; Islam, A.; Moyes, D.L.; Murciano, C.; Shen, C.; Challacombe, S.J.; Naglik, J.R. Oral and Vaginal Epithelial Cell Lines Bind and Transfer Cell-Free Infectious HIV-1 to Permissive Cells but Are Not Productively Infected. PLoS ONE 2014, 9, e98077. [Google Scholar] [CrossRef]
- Lafon, E.; Diem, G.; Witting, C.; Zaderer, V.; Bellmann-Weiler, R.M.; Reindl, M.; Bauer, A.; Griesmacher, A.; Fux, V.; Hoermann, G.; et al. Potent SARS-CoV-2-Specific T Cell Immunity and Low Anaphylatoxin Levels Correlate With Mild Disease Progression in COVID-19 Patients. Front. Immunol. 2021, 12, 684014. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for Large-Scale Genome and Gene Function Analysis with the PANTHER Classification System (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.; Mi, H. PANTHER: Making Genome-scale Phylogenetics Accessible to All. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; et al. Ensembl 2020. Nucleic Acids Res. 2019, 48, D682–D688. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- King, B.C.; Blom, A.M. Intracellular Complement and Immunometabolism: The Advantages of Compartmentalization. Eur. J. Immunol. 2024, 54, 2350813. [Google Scholar] [CrossRef]
- Wu, F.; Zou, Q.; Ding, X.; Shi, D.; Zhu, X.; Hu, W.; Liu, L.; Zhou, H. Complement Component C3a Plays a Critical Role in Endothelial Activation and Leukocyte Recruitment into the Brain. J. Neuroinflamm. 2016, 13, 23. [Google Scholar] [CrossRef]
- Dellière, S.; Sze Wah Wong, S.; Aimanianda, V. Soluble Mediators in Anti-Fungal Immunity. Curr. Opin. Microbiol. 2020, 58, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-J.; González, K.; Orasch, T.; Schmidt, F.; Brakhage, A.A. Manipulation of Host Phagocytosis by Fungal Pathogens and Therapeutic Opportunities. Nat. Microbiol. 2024, 9, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, F.; Yang, J.; Li, P.; Yan, D.; Yang, Y.; Zhang, W.; Ren, J.; Zhang, Z.; Wang, M. The Responses of the Gut Microbiota to MBL Deficiency. Mol. Immunol. 2020, 122, 99–108. [Google Scholar] [CrossRef]
- Choteau, L.; Parny, M.; François, N.; Bertin, B.; Fumery, M.; Dubuquoy, L.; Takahashi, K.; Colombel, J.-F.; Jouault, T.; Poulain, D.; et al. Role of Mannose-Binding Lectin in Intestinal Homeostasis and Fungal Elimination. Mucosal Immunol. 2016, 9, 767–776. [Google Scholar] [CrossRef]
- Lin, L.; Wang, M.; Zeng, J.; Mao, Y.; Qin, R.; Deng, J.; Ouyang, X.; Hou, X.; Sun, C.; Wang, Y.; et al. Sequence Variation of Candida Albicans Sap2 Enhances Fungal Pathogenicity via Complement Evasion and Macrophage M2-Like Phenotype Induction. Adv. Sci. 2023, 10, 2206713. [Google Scholar] [CrossRef]
- Ishii, M.; Beeson, G.; Beeson, C.; Rohrer, B. Mitochondrial C3a Receptor Activation in Oxidatively Stressed Epithelial Cells Reduces Mitochondrial Respiration and Metabolism. Front. Immunol. 2021, 12, 628062. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Hovhannisyan, H.; Gresnigt, M.S.; Iracane, E.; Oliveira-Pacheco, J.; Siscar-Lewin, S.; Seemann, E.; Qualmann, B.; Kalkreuter, T.; Müller, S.; et al. Candida Pathogens Induce Protective Mitochondria-Associated Type I Interferon Signalling and a Damage-Driven Response in Vaginal Epithelial Cells. Nat. Microbiol. 2021, 6, 643–657. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenno, S.; Pedretti, N.; Spaggiari, L.; Ardizzoni, A.; Comar, M.; Posch, W.; Wheeler, R.T.; Peppoloni, S.; Pericolini, E. Vaginal Clinical Isolates of Candida albicans Differentially Modulate Complosome Activation in Vaginal Epithelial Cells. J. Fungi 2025, 11, 501. https://doi.org/10.3390/jof11070501
Kenno S, Pedretti N, Spaggiari L, Ardizzoni A, Comar M, Posch W, Wheeler RT, Peppoloni S, Pericolini E. Vaginal Clinical Isolates of Candida albicans Differentially Modulate Complosome Activation in Vaginal Epithelial Cells. Journal of Fungi. 2025; 11(7):501. https://doi.org/10.3390/jof11070501
Chicago/Turabian StyleKenno, Samyr, Natalia Pedretti, Luca Spaggiari, Andrea Ardizzoni, Manola Comar, Wilfried Posch, Robert Treyde Wheeler, Samuele Peppoloni, and Eva Pericolini. 2025. "Vaginal Clinical Isolates of Candida albicans Differentially Modulate Complosome Activation in Vaginal Epithelial Cells" Journal of Fungi 11, no. 7: 501. https://doi.org/10.3390/jof11070501
APA StyleKenno, S., Pedretti, N., Spaggiari, L., Ardizzoni, A., Comar, M., Posch, W., Wheeler, R. T., Peppoloni, S., & Pericolini, E. (2025). Vaginal Clinical Isolates of Candida albicans Differentially Modulate Complosome Activation in Vaginal Epithelial Cells. Journal of Fungi, 11(7), 501. https://doi.org/10.3390/jof11070501











