Immunoregulation in Fungal Infections: A Review and Update on the Critical Role of Myeloid-Derived Suppressor Cells
Abstract
1. Introduction
2. MDSCs May Play Contradictory Roles When Stimulated by Fungi or Fungal Extracts
3. MDSCs Worsen the Disease in Paracoccidioides brasiliensis Infection
4. MDSC Recruitment by Cryptococcus neoformans Worsens Host Defense
5. Fungi-Induced MDSCs Can Play a Role in the Fight Against Cancer Tumors
6. MDSCs Act as Drivers of the “Trained Tolerogenic Immunity” Induced by Low-Virulence Fungi Infection
7. Conclusion Remarks and Therapeutic Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clark, T.A.; Hajjeh, R.A. Recent trends in the epidemiology of invasive mycoses. Curr. Opin. Infect. Dis. 2002, 15, 569–574. [Google Scholar] [CrossRef]
- Fleming, R.V.; Walsh, T.J.; Anaissie, E.J. Emerging and less common fungal pathogens. Infect. Dis. Clin. N. Am. 2002, 16, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Goldman, M.J.; Wheat, L.J. Mucosal and invasive fungal infections in HIV/AIDS. Eur. J. Med. Res. 2022, 7, 236–241. [Google Scholar]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Mei-Sheng Riley, M. Invasive Fungal Infections Among Immunocompromised Patients in Critical Care Settings: Infection Prevention Risk Mitigation. Crit. Care Nurs. Clin. N. Am. 2021, 33, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Puccetti, P.; Bistoni, F. Fungi, dendritic cells and receptors: A host perspective for fungal virulence. Trends Microbiol. 2002, 10, 508–514. [Google Scholar] [CrossRef]
- Loures, F.V.; Röhm, M.; Lee, C.K.; Santos, E.; Wang, J.P.; Specht, C.A.; Calich, V.L.G.; Urban, C.F.; Levitz, S.M. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS Pathog. 2015, 11, e1004643. [Google Scholar] [CrossRef]
- Araújo, E.F.; Medeiros, D.H.; Galdino, N.A.L.; Condino-Neto, A.; Calich, V.L.G.; Loures, F.V. Tolerogenic Plasmacytoid Dendritic Cells Control Paracoccidioides brasiliensis Infection by Inducting Regulatory T Cells in an IDO-Dependent Manner. PLoS Pathog. 2016, 12, e1006115. [Google Scholar] [CrossRef]
- Medina, E.; Hartl, D. Myeloid-Derived Suppressor Cells in Infection: A General Overview. J. Innate Immun. 2018, 10, 407–413. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Youn, J.I.; Gabrilovich, D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010, 40, 2969–2975. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Rieber, N.; Singh, A.; Öz, H.; Carevic, M.; Bouzani, M.; Amich, J.; Ost, M.; Ye, Z.; Ballbach, M.; Schäfer, I.; et al. Pathogenic fungi regulate immunity by inducing neutrophilic myeloid-derived suppressor cells. Cell Host Microbe 2015, 17, 507–514. [Google Scholar] [CrossRef]
- Ost, M.; Singh, A.; Peschel, A.; Mehling, R.; Rieber, N.; Hartl, D. Myeloid-Derived Suppressor Cells in Bacterial Infections. Front. Cell. Infect. Microbiol. 2016, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Ademe, M. Paradoxes in the Phenotype, Frequency and Roles of Myeloid-Derived Suppressor Cells During HIV Infection. HIV AIDS 2020, 12, 151–156. [Google Scholar] [CrossRef]
- Cheng, P.; Corzo, C.A.; Luetteke, N.; Yu, B.; Nagaraj, S.; Bui, M.M.; Ortiz, M.; Nacken, W.; Sorg, C.; Vogl, T.; et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008, 29, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.G.; Liebertz, D.J.; Epstein, A.L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 2010, 15, 2273–2284. [Google Scholar] [CrossRef]
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011, 118, 5498–5505. [Google Scholar] [CrossRef]
- Preite, N.W.; Kaminski, V.L.; Borges, B.M.; Calich, V.L.G.; Loures, F.V. Myeloid-derived suppressor cells are associated with impaired Th1 and Th17 responses and severe pulmonary paracoccidioidomycosis which is reversed by anti-Gr1 therapy. Front. Immunol. 2023, 14, 1039244. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Kullberg, B.J.; van der Meer, J.W.; Gow, N.A.; Netea, M.G. Host-microbe interactions: Innate pattern recognition of fungal pathogens. Curr. Opin. Microbiol. 2008, 11, 305–312. [Google Scholar] [CrossRef]
- Jouault, T.; Sarazin, A.; Martinez-Esparza, M.; Fradin, C.; Sendid, B.; Poulain, D. Host responses to a versatile commensal: PAMPs and PRRs interplay leading to tolerance or infection by Candida albicans. Cell. Microbiol. 2009, 11, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P. Tasting the fungal cell wall. Cell. Microbiol. 2010, 12, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Innate antifungal immunity: The key role of phagocytes. Annu. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Joly, S.; Ma, N.; Sadler, J.J.; Soll, D.R.; Cassel, S.L.; Sutterwala, F.S. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J. Immunol. 2009, 183, 3578–3581. [Google Scholar] [CrossRef]
- Saïd-Sadier, N.; Padilla, E.; Langsley, G.; Ojcius, D.M. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE. 2010, 5, e10008. [Google Scholar] [CrossRef]
- Gross, O.; Gewies, A.; Finger, K.; Schäfer, M.; Sparwasser, T.; Peschel, C.; Förster, I.; Ruland, J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006, 44, 651–656. [Google Scholar] [CrossRef]
- Gross, O.; Poeck, H.; Bscheider, M.; Dostert, C.; Hannesschläger, N.; Endres, S.; Hartmann, G.; Tardivel, A.; Schweighoffer, E.; Tybulewicz, V.; et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009, 459, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, V.D.; Preite, N.W.; Borges, B.M.; Dias-Melicio, L.A.; Soares, Â.M.; Calich, V.L.; Loures, F.V. The immunosuppressive activity of myeloid-derived suppressor cells in murine Paracoccidioidomycosis relies on Indoleamine 2,3-dioxygenase activity and Dectin-1 and TLRs signaling. Sci. Rep. 2023, 13, 12391. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Wang, Z.W.; Li, F.; Zhou, L.H.; Jiang, Y.S.; Yu, Y.; Ma, H.H.; Zhu, L.P.; Qu, J.M.; Jia, X.M. Inhibition of myeloid-derived suppressor cell arginase-1 production enhances T-cell-based immunotherapy against Cryptococcus neoformans infection. Nat. Commun. 2022, 13, 4074. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tao, N.; Liu, X.; Li, X.; Tang, J.; Ma, C.; Xu, X.; Shao, H.; Hou, B.; Wang, H.; et al. Polysaccharide from Lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLoS ONE 2012, 7, e51751. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhu, X.; Wang, Y.; Liu, W.; Gong, W. Polysaccharide Agaricus blazei Murill stimulates myeloid-derived suppressor cell differentiation from M2 to M1 type, which mediates inhibition of tumor immune evasion via the Toll-like receptor 2 pathway. Immunology 2015, 146, 379–391. [Google Scholar] [CrossRef]
- Mueller-Leisse, J.; Brueggemann, S.; Bouzani, M.; Schmitt, A.L.; Einsele, H.; Loeffler, J. Polymorphonuclear neutrophils and granulocytic myeloid-derived suppressor cells inhibit natural killer cell activity toward Aspergillus fumigatus. Med. Mycol. 2015, 53, 622–629. [Google Scholar] [CrossRef]
- Singh, A.; Lelis, F.; Braig, S.; Schäfer, I.; Hartl, D.; Rieber, N. Differential regulation of myeloid-derived suppressor cells by Candida species. Front. Microbiol. 2016, 7, 1624. [Google Scholar] [CrossRef]
- Lilly, E.A.; Yano, J.; Esher, S.K.; Hardie, E.; Fidel, P.L., Jr.; Noverr, M.C. Spectrum of trained innate immunity induced by low-virulence Candida species against lethal polymicrobial intra-abdominal infection. Infect. Immun. 2019, 87, e00348-e19. [Google Scholar] [CrossRef]
- Wang, T.; Fan, C.; Yao, A.; Xu, X.; Zheng, G.; You, Y.; Jiang, C.; Zhao, X.; Hou, Y.; Hung, M.C.; et al. The Adaptor Protein CARD9 Protects against Colon Cancer by Restricting Mycobiota-Mediated Expansion of Myeloid-Derived Suppressor Cells. Immunity 2018, 49, 504–514.e4. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Wu, X. Ganoderma lucidum polysaccharide (GLP) enhances antitumor immune response by regulating differentiation and inhibition of MDSCs via a CARD9-NF-κB-IDO pathway. Biosci. Rep. 2020, 40, BSR20201170. [Google Scholar] [CrossRef]
- Lilly, E.A.; Bender, B.E.; Esher, R.S.; Fidel, P.L., Jr.; Noverr, M.C. Trained innate immunity induced by vaccination with low-virulence Candida species mediates protection against several forms of fungal sepsis via Ly6G+ Gr-1+ leukocytes. mBio 2021, 12, e0254821. [Google Scholar] [CrossRef]
- Peng, K.T.; Chen, J.L.; Kuo, L.T.; Yu, P.A.; Hsu, W.H.; Lee, C.W.; Chang, P.J.; Huang, T.Y. GMI, an immunomodulatory peptide from Ganoderma microsporum, restrains periprosthetic joint infections via modulating the functions of myeloid-derived suppressor cells and effector T cells. Int. J. Mol. Sci. 2021, 22, 6854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Y.; Chen, Y.; Yin, Y.; Chen, Y.; Chen, Q.; Hou, Y.; Shen, S.; Lv, M.; Wang, T. Gut fungi enhances immunosuppressive function of myeloid-derived suppressor cells by activating PKM2-dependent glycolysis to promote colorectal tumorigenesis. Exp. Hematol. Oncol. 2022, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Harriett, A.J.; Righi, S.E.; Lilly, E.A.; Fidel, P.L., Jr.; Noverr, M.C. Efficacy of Candida dubliniensis and fungal β-glucans in inducing trained innate immune protection against inducers of sepsis. Front. Cell. Infect. Microbiol. 2022, 12, 898030. [Google Scholar] [CrossRef]
- Edgerton, M.; Rojas, I.; Kumar, R.; Li, R.; Salvatori, O.; Abrams, S.; Irimia, D. Neutrophil swarms containing myeloid-derived suppressor cells are crucial for limiting oral mucosal infection by C. albicans. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Liu, N.N.; Yi, C.X.; Wei, L.Q.; Zhou, J.A.; Jiang, T.; Hu, C.C.; Wang, L.; Wang, Y.Y.; Zou, Y.; Zhao, Y.K.; et al. The intratumor mycobiome promotes lung cancer progression via myeloid-derived suppressor cells. Cancer Cell 2023, 41, 1927–1944.e9. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhu, L.; Yang, Q.; Zhou, J. β-Glucan subverts the function of myeloid cells in neonates. J. Immunol. Res. 2024, 2024, 2765001. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Twardowski, P.; Lau, C.; Chan, Y.S.; Wong, K.; Xiao, S.; Wang, J.; Wu, X.; Frankel, P.; et al. Reduction of myeloid-derived suppressor cells in prostate cancer murine models and patients following white button mushroom treatment. Clin. Transl. Med. 2024, 14, e70048. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Pan, X.; Li, P.; Ren, Z.; Wang, X.; Chen, Y.; Shen, S.; Wang, T.; Lin, A. IL-1β mediates Candida tropicalis-induced immunosuppressive function of MDSCs to foster colorectal cancer. Cell Commun. Signal. 2024, 22, 408. [Google Scholar] [CrossRef]
- Li, X.; Ruan, Q.; Yang, W.; Tian, H.; Wu, N.; Qadir, J.; Wang, J.; Hu, H.; Liu, Y.; Cai, M.; et al. Polysaccharide isolated from Grifola frondosa eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T cells responses. Int. J. Biol. Sci. 2024, 20, 664–679. [Google Scholar] [CrossRef]
- Kaminski, V.L.; Borges, B.M.; Santos, B.V.; Preite, N.W.; Calich, V.L.G.; Loures, F.V. MDSCs use a complex molecular network to suppress T-cell immunity in a pulmonary model of fungal infection. Front. Cell. Infect. Microbiol. 2024, 14, 1392744. [Google Scholar] [CrossRef]
- Preite, N.W.; Kaminski, V.L.; Borges, B.M.; Dos Santos, B.V.; Calich, V.L.G.; Loures, F.V. Specific depletion of myeloid-derived suppressor cells by the chemotherapy agent 5-fluorouracil enhances protective immune response in paracoccidioidomycosis. J. Infect. Dis. 2024, 230, 1279–1290. [Google Scholar] [CrossRef]
- Guimarães-DE-Oliveira, J.C.; Diniz-Lima, I.; Ferreira-Dos-Santos, I.M.; Silva-Junior, E.B.D.; Covre, L.P.; Freire-DE-Lima, M.; Fonseca, L.M.D.; Morrot, A.; Freire-DE-Lima, L.; Mendonça-Previato, L.; et al. Recruitment of Polymorphonuclear Myeloid-Derived Suppressor Cells During Cryptococcus neoformans Infection. An. Acad. Bras. Ciencias 2025, 97, e20240985. [Google Scholar] [CrossRef] [PubMed]
- Lasser, S.A.; Ozbay Kurt, F.G.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef]
- Twardowski, P.; Kanaya, N.; Frankel, P.; Synold, T.; Ruel, C.; Pal, S.K.; Junqueira, M.; Prajapati, M.; Moore, T.; Tryon, T.; et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus-induced prostate-specific antigen responses. Cancer 2015, 121, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Hagen, F.; Puccia, R.; Hahn, R.C.; de Camargo, Z.P. Paracoccidioides and Paracoccidioidomycosis in the 21st Century. Mycopathologia 2023, 188, 129–133. [Google Scholar] [CrossRef]
- Peçanha, P.M.; Peçanha-Pietrobom, P.M.; Grão-Velloso, T.R.; Rosa Júnior, M.; Falqueto, A.; Gonçalves, S.S. Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment. J. Fungi 2022, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Bazan, S.B.; Costa, T.A.; de Araújo, E.F.; Feriotti, C.; Loures, F.V.; Pretel, F.D.; Calich, V.L.G. Loss- and gain-of-function approaches indicate a dual role exerted by regulatory T cells in pulmonary paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2015, 9, e0004189. [Google Scholar] [CrossRef]
- McEwen, J.G.; Bedoya, V.; Patiño, M.M.; Salazar, M.E.; Restrepo, A.M. Experimental murine paracoccidiodomycosis induced by the inhalation of conidia. J Med. Vet. Mycol. 1987, 25, 165–175. [Google Scholar] [CrossRef]
- Kashino, S.S.; Fazioli, R.A.; Cafalli-Favati, C.; Meloni-Bruneri, L.H.; Vaz, C.A.; Burger, E.; Singer, L.M.; Calich, V.L. Resistance to Paracoccidioides brasiliensis infection is linked to a preferential Th1 immune response, whereas susceptibility is associated with absence of IFN-gamma production. J. Interferon Cytokine Res. 2000, 20, 89–97. [Google Scholar] [CrossRef]
- Pagliari, C.; Fernandes, E.R.; Stegun, F.W.; da Silva, W.L.; Seixas Duarte, M.I.; Sotto, M.N. Paracoccidioidomycosis: Cells expressing IL17 and Foxp3 in cutaneous and mucosal lesions. Microb. Pathog. 2011, 50, 263–267. [Google Scholar] [CrossRef]
- de Castro, L.F.; Ferreira, M.C.; da Silva, R.M.; Blotta, M.H.; Longhi, L.N.; Mamoni, R.L. Characterization of the immune response in human paracoccidioidomycosis. J. Infect. 2013, 67, 470–485. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, E.F.; Feriotti, C.; Galdino, N.A.L.; Preite, N.W.; Calich, V.L.G.; Loures, F.V. The IDO-AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front. Immunol. 2017, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Galdino, N.A.L.; Loures, F.V.; de Araújo, E.F.; da Costa, T.A.; Preite, N.W.; Calich, V.L.G. Depletion of regulatory T cells in ongoing paracoccidioidomycosis rescues protective Th1/Th17 immunity and prevents fatal disease outcome. Sci. Rep. 2018, 8, 16544. [Google Scholar] [CrossRef]
- Cavassani, K.A.; Campanelli, A.P.; Moreira, A.P.; Vancim, J.O.; Vitali, L.H.; Mamede, R.C.; Martinez, R.; Silva, J.S. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J. Immunol. 2006, 177, 5811–5818. [Google Scholar] [CrossRef]
- Ferreira, M.C.; de Oliveira, R.T.; da Silva, R.M.; Blotta, M.H.; Mamoni, R.L. Involvement of regulatory T cells in the immunosuppression characteristic of patients with paracoccidioidomycosis. Infect. Immun. 2010, 78, 4392–4401. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Green, K.A.; Ma, C.; Hoffmann, F.W.; Hoffmann, P.R.; Green, W.R. Depletion of monocytic myeloid-derived suppressor cells in LP-BM5 murine retroviral infection has a positive impact on virus-induced host immunodeficiency. Virology 2024, 600, 110247. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Firacative, C.; Lizarazo, J.; Illnait-Zaragozí, M.T.; Castañeda, E. Latin American Cryptococcal Study Group. The status of cryptococcosis in Latin America. Memórias Inst. Oswaldo Cruz 2018, 113, e170554. [Google Scholar] [CrossRef]
- Stenzel, W.; Müller, U.; Köhler, G.; Heppner, F.L.; Blessing, M.; McKenzie, A.N.; Brombacher, F.; Alber, G. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am. J Pathol. 2009, 174, 486–496. [Google Scholar] [CrossRef]
- Voelz, K.; May, R.C. Cryptococcal interactions with the host immune system. Eukaryot. Cell 2010, 9, 835–846. [Google Scholar] [CrossRef]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef]
- Man, S.M.; Zhu, Q.; Zhu, L.; Liu, Z.; Karki, R.; Malik, A.; Sharma, D.; Li, L.; Malireddi, R.K.; Gurung, P.; et al. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell 2015, 162, 45–58. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Tang, C.; Sun, H.; Kadoki, M.; Han, W.; Ye, X.; Makusheva, Y.; Deng, J.; Feng, B.; Qiu, D.; Tan, Y.; et al. Blocking Dectin-1 prevents colorectal tumorigenesis by suppressing prostaglandin E2 production in myeloid-derived suppressor cells and enhancing IL-22 binding protein expression. Nat. Commun. 2023, 14, 1493. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Yamabayashi, H. A zymosanlike substance extracted from Candida albicans. Med. J. Osaka Univ. 1958, 9, 11–21. [Google Scholar]
- Mankiewicz, E.; Livak, M. Effect of Candida albicans on the evolution of experimental tuberculosis. Nature 1960, 187, 250–251. [Google Scholar] [CrossRef]
- Carlson, E. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infect. Immun. 1982, 38, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E. Enhancement by Candida albicans of Staphylococcus aureus, Serratia marcescens, and Streptococcus faecalis in the establishment of infection in mice. Infect. Immun. 1983, 39, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Klaerner, H.G.; Uknis, M.E.; Acton, R.D.; Dahlberg, P.S.; Carlone-Jambor, C.; Dunn, D.L. Candida albicans and Escherichia coli are synergistic pathogens during experimental microbial peritonitis. J. Surg. Res. 1997, 70, 161–165. [Google Scholar] [CrossRef]
- Sawyer, R.G.; Adams, R.B.; May, A.K.; Rosenlof, L.K.; Pruett, T.L. Development of Candida albicans and C. albicans/Escherichia coli/Bacteroides fragilis intraperitoneal abscess models with demonstration of fungus-induced bacterial translocation. J. Med. Vet. Mycol. 1995, 33, 49–52. [Google Scholar] [CrossRef]
- Esher, S.K.; Fidel, P.L., Jr.; Noverr, M.C. Candida/Staphylococcal polymicrobial intra-abdominal infection: Pathogenesis and perspectives for a novel form of trained innate immunity. J. Fungi. 2019, 5, 37. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Cheng, F.; Yu, B.; Nefedova, Y.; Sotomayor, E.; Lush, R.; Gabrilovich, D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003, 63, 4441–4449. [Google Scholar]
- Delano, M.J.; Scumpia, P.O.; Weinstein, J.S.; Coco, D.; Nagaraj, S.; Kelly-Scumpia, K.M.; O’Malley, K.A.; Wynn, J.L.; Antonenko, S.; Al-Quran, S.Z.; et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007, 204, 1463–1474. [Google Scholar] [CrossRef]
- Chang, E.Y.; Fatima, S.; Balan, S.; Bhyravabhotla, K.; Erickson, M.; Chan, A.; Ivonye, C.; Bradley, C. Candida dubliniensis abscess: A clinical case and a review of the literature. Med. Mycol. Case Rep. 2018, 21, 41–43. [Google Scholar] [CrossRef]
- High time to tackle drug-resistant fungal infections. Nature 2025, 640, 569. [CrossRef]

| Type of Study | Main Findings | Reference |
|---|---|---|
| In vivo and in vitro | MPSSS from Lentinus edodes reduced MDSCs in tumor-bearing mice, enhanced CD4⁺ T-cell activation, and altered MDSC signaling, promoting a stronger immune response. | [33] |
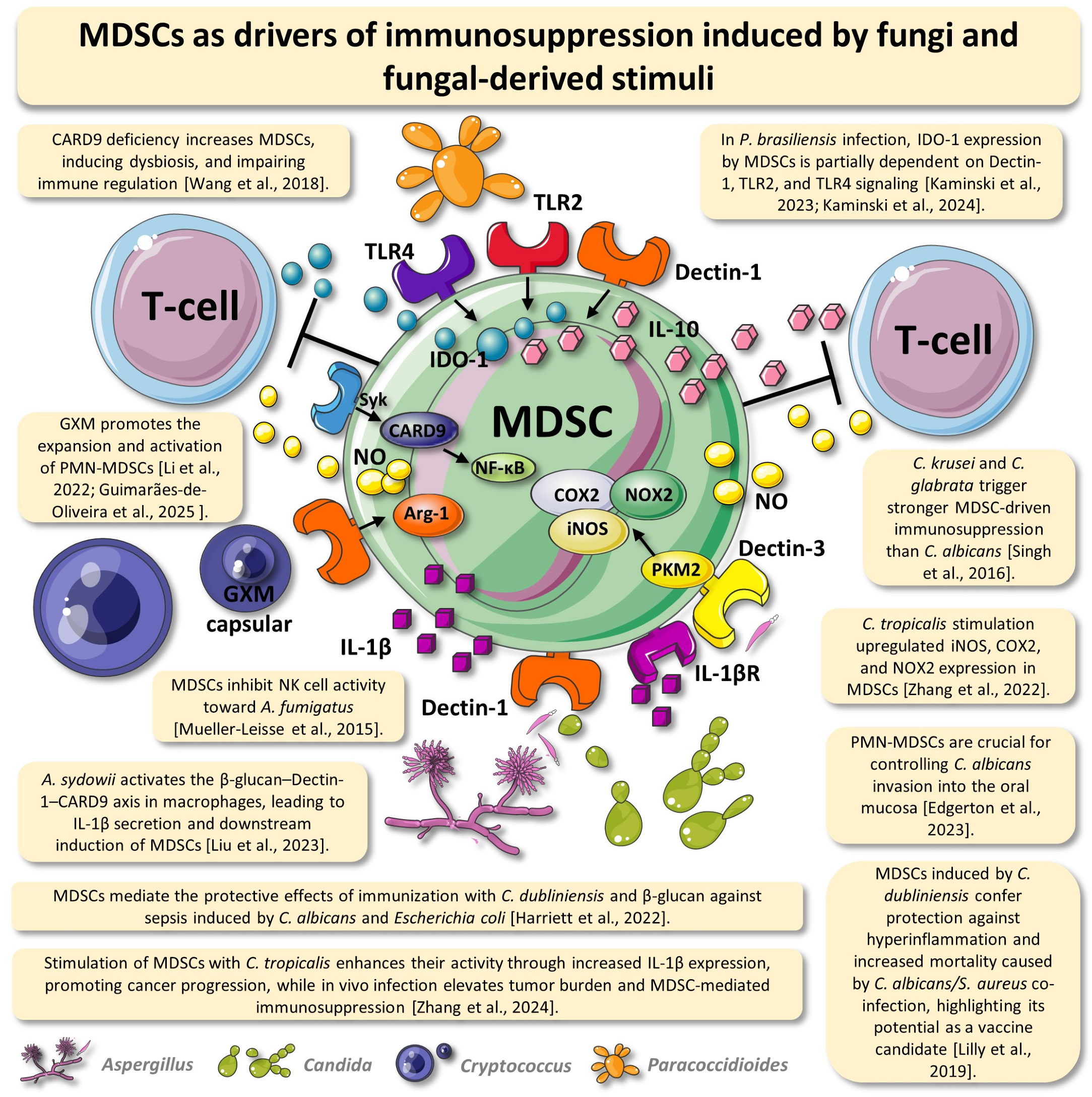
| In vivo and in vitro | A. fumigatus and C. albicans induced MDSC recruitment, which suppressed T-lymphocytes and NK cells. MDSC recruitment was mediated by Dectin-1 and IL-1β. In addition, MDSC transfer improved survival in C. albicans infection but not in A. fumigatus. | [14] |
| In vivo | Polysaccharides from Agaricus blazei Murill promoted the differentiation of MDSCs from an M2 to M1 phenotype, helping to inhibit tumor immune evasion via the TLR2 pathway. | [34] |
| In vitro | Granulocytic MDSCs and polymorphonuclear neutrophils inhibited NK cell activity toward Aspergillus fumigatus. | [35] |
| In vitro | Compared to C. albicans, C. krusei and C. glabrata more potently induced MDSC-mediated immunosuppression via Dectin-1. MDSC recruitment was driven by GM-CSF and IL-1β, resulting in NK cell inhibition. | [36] |
| In vivo | Co-infection with C. albicans and Staphylococcus aureus led to high mortality due to excessive inflammation. In contrast, previous infection with low-virulence C. dubliniensis protected against lethality by expanding and activating MDSCs, suggesting a potential vaccine strategy. | [37] |
| In vivo | CARD9 deficiency promoted immunosuppression, tumor progression, and colorectal cancer susceptibility by increasing MDSCs, inducing dysbiosis, and impairing immune regulation. | [38] |
| In vivo | Ganoderma lucidum polysaccharides (GLPs) boosted antitumor immune responses by modulating the differentiation and suppression of MDSCs through the CARD9–NF-κB–IDO signaling pathway. | [39] |
| In vivo | Innate immune training induced by vaccination with low-virulence Candida species conferred protection against diverse forms of fungal sepsis through Ly6G⁺ Gr-1⁺ myeloid cells (which the authors referred to as putative MDSCs). | [40] |
| In vivo | Treatment with GMI, an immunomodulatory peptide from Ganoderma microsporum, enhanced T-cell function and modulated MDSC activity, improving the immune response against G. microsporum infection. | [41] |
| In vivo and in vitro | Candida tropicalis stimulation enhanced iNOS, COX2, and NOX2 expression in MDSCs, with iNOS-derived nitric oxide activating their glycolytic metabolism; the inhibition of PKM2 reduced these markers, underscoring the role of C. tropicalis and PKM2 in MDSC metabolic activation. | [42] |
| In vivo | The inhibition of arginase-1 production by MDSCs enhanced T-cell-based immunotherapy against C. neoformans infection. | [32] |
| In vivo | Immunization with C. dubliniensis and β-glucan protected against sepsis induced by C. albicans and Escherichia coli. Such protection was mediated by Gr-1+ cells (MDSCs). In addition, Gr-1+ cell depletion increased mortality, with varying β-glucan efficacy depending on the pathogen. | [43] |
| In vivo | PMN-MDSCs associated with neutrophil swarms played a key role in resolving oropharyngeal candidiasis by preventing deep invasion of C. albicans into the oral mucosa. | [44] |
| In vivo | Monocytic and polymorphonuclear MDSCs infiltrated the lungs during Paracoccidioides brasiliensis infection. Partial depletion of MDSCs through anti-Gr1 therapy enhanced Th1/Th17 responses, resulting in disease regression, reduced fungal burden, less lung pathology, and lower mortality compared to the control group. | [20] |
| In vivo | In P. brasiliensis infection, IDO-1 expression by MDSCs regulated T-cell proliferation. IDO-1 production by MDSCs partially depended on Dectin-1, TLR2, and TLR4 signaling during murine paracoccidioidomycosis. | [30] |
| In vivo | In lung adenocarcinoma, Aspergillus sydowii promoted MDSC accumulation through IL-1β induction, contributing to an immunosuppressive microenvironment and tumor progression. | [45] |
| In vivo and in vitro | β-glucan impaired the immunosuppressive function of MDSCs in neonates, reducing ROS and arginase-1 levels. It also decreased the frequency and ROS levels in PMN-MDSCs in vitro. | [46] |
| In vivo | White button mushroom (WBM) inhibited tumor growth in prostate cancer murine models by reducing PMN-MDSCs and inhibiting the STAT3/IRF1 and TGFβ pathways. In patients, treatment decreased PMN-MDSCs and increased CD8⁺ T-cells and NK cells. WBM enhanced anti-PD-1 antibody efficacy. | [47] |
| In vivo and in vitro | Stimulation of MDSCs with C. tropicalis increased IL-1β expression, enhancing their activity and promoting cancer progression. In vivo, C. tropicalis infection increased tumor burden and elevated the MDSC levels and activity. | [48] |
| In vivo | A polysaccharide from Grifola frondosa reduced the frequency of MDSCs in the tumor microenvironment and inhibited tumor growth in breast cancer models. The treatment also enhanced T-cell responses, potentiating immune activity against tumor cells. | [49] |
| In vivo and in vitro | In P. brasiliensis infection, IDO-1 expression by MDSCs regulated T-cell proliferation, with IDO-1 production being partially dependent on Dectin-1, TLR2, and TLR4 signaling. | [50] |
| In vivo | In P. brasiliensis infection, treatment with 5-FU depleted MDSCs, improving the immune response by increasing T-cell activity and enhancing pro-inflammatory cytokine production, reducing disease severity. | [51] |
| In vivo | In C. neoformans infection, MDSCs induced by the B3501 strain exhibited T-cell suppressive activity, whereas those associated with the CAP67 strain lacked this function. | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminski, V.d.L.; Menezes, A.L.O.; de Lima, K.G.; de Almeida, S.L.; da Silva, D.V.A.; Franco, F.N.; Preite, N.W.; Loures, F.V. Immunoregulation in Fungal Infections: A Review and Update on the Critical Role of Myeloid-Derived Suppressor Cells. J. Fungi 2025, 11, 496. https://doi.org/10.3390/jof11070496
Kaminski VdL, Menezes ALO, de Lima KG, de Almeida SL, da Silva DVA, Franco FN, Preite NW, Loures FV. Immunoregulation in Fungal Infections: A Review and Update on the Critical Role of Myeloid-Derived Suppressor Cells. Journal of Fungi. 2025; 11(7):496. https://doi.org/10.3390/jof11070496
Chicago/Turabian StyleKaminski, Valéria de Lima, Ana Luiza Oliveira Menezes, Kauan Gonçalves de Lima, Stephani Leonelo de Almeida, Diego Vinícius Alves da Silva, Filipe Nogueira Franco, Nycolas Willian Preite, and Flávio Vieira Loures. 2025. "Immunoregulation in Fungal Infections: A Review and Update on the Critical Role of Myeloid-Derived Suppressor Cells" Journal of Fungi 11, no. 7: 496. https://doi.org/10.3390/jof11070496
APA StyleKaminski, V. d. L., Menezes, A. L. O., de Lima, K. G., de Almeida, S. L., da Silva, D. V. A., Franco, F. N., Preite, N. W., & Loures, F. V. (2025). Immunoregulation in Fungal Infections: A Review and Update on the Critical Role of Myeloid-Derived Suppressor Cells. Journal of Fungi, 11(7), 496. https://doi.org/10.3390/jof11070496






