Five-Year Retrospective Analysis of Superficial Fungal Infections: Insights from Hospital Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
Inclusion and Exclusion Criteria
2.2. Specimen Sampling
2.3. Species Identification
2.4. Statistical Analysis
3. Results
3.1. The Ratio of Taxa in Positive Samples per Year, Irrespective of Gender
3.2. Number of Taxa in Positive Samples per Month, Irrespective of Year or Gender
3.3. Gender-Based Abundances of Taxa
3.4. Ratio of Species by Body Part
3.5. Ratio of Taxa per Surface Type
3.6. “Non-MALDI-TOF” and MALDI-TOF-Detected Samples
3.7. Diversity Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casadevall, A.; Pirofski, L.A. What is a host? Incorporating the microbiota into the damage-response framework. Infect. Immun. 2014, 83, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte infections worldwide: Increase in incidence and associated antifungal resistance. Life 2023, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Inamadar, A.C.; Palit, A. The genus Malassezia and human disease. Indian. J. Dermatol. Venereol. Leprol. 2003, 69, 265–270. [Google Scholar]
- Kromann, C.B.; Graversgaard, C.; Riis, P.T.; Jemec, G.B.E.; Serup, J.; Saunte, D.M.L. Dermatophyte prevalence in tools of 43 hairdressing salons in Copenhagen. Acta Derm. Venereol. 2016, 96, 846–847. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N. Emerging drugs for the treatment of onychomycosis. Expert. Opin. Emerg. Drugs 2019, 24, 213–220. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, V.; Ballal, M. Distribution of Candida species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in southern India. J. Glob. Infect. Dis. 2011, 3, 4–8. [Google Scholar] [CrossRef]
- Kashem, S.W.; Kaplan, D.H. Skin immunity to Candida albicans. Trends Immunol. 2016, 37, 440–450. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, H.; Li, C.; Rajapakse, M.P.; Wong, W.C.; Xu, J.; Saunders, C.W.; Reeder, N.L.; Reilman, R.A.; Scheynius, A.; et al. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet. 2015, 11, e1005614. [Google Scholar] [CrossRef]
- Cabañes, F.J. Malassezia yeasts: How many species infect humans and animals? PLoS Pathog. 2014, 10, e1003892. [Google Scholar] [CrossRef]
- Scher, R.K. Onychomycosis: Therapeutic update. J. Am. Acad. Dermatol. 1999, 40 Pt 2, S99–S102. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cooper, E.A.; Paquet, M. Recurrences of dermatophyte toenail onychomycosis during long-term follow-up after successful treatments with mono- and combined therapy of terbinafine and itraconazole. J. Cutan. Med. Surg. 2013, 17, 201–206. [Google Scholar] [CrossRef]
- Foster, K.W.; Ghannoum, M.A.; Elewski, B.E. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J. Am. Acad. Dermatol. 2004, 50, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. Trends Sport. Sci. 2014, 1, 19–25. [Google Scholar]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 178. [Google Scholar]
- Shin, S.; Lim, S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J. Appl. Microbiol. 2004, 97, 1289–1296. [Google Scholar] [CrossRef]
- Otašević, S.; Momčilović, S.; Golubović, M.; Ignjatović, A.; Rančić, N.; Đorđević, M.; Ranđelović, M.; Hay, R.; Arsić-Arsenijević, V. Species distribution and epidemiological characteristics of superficial fungal infections in Southeastern Serbia. Mycoses 2019, 62, 458–465. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51 (Suppl. S4), 2–15. [Google Scholar] [CrossRef]
- Chen, S.C.A.; Slavin, M.A.; Sorrell, T.C. Echinocandin antifungal drugs in fungal infections: A comparison. Drugs 2011, 71, 11–41. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae—An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide—A Multidimensional Perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Susmita; Nguyen, H.C.; Liddy, A.; Economopoulos, V.; Wang, T. Terbinafine Resistance in Trichophyton rubrum and Trichophyton indotineae: A Literature Review. Antibiotics 2025, 14, 472. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and emerged Candida species: Beyond the Candida albicans paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar] [CrossRef] [PubMed]
- Gräser, Y. Species identification of dermatophytes by MALDI-TOF MS. Curr. Fungal Infect. Rep. 2014, 8, 193–197. [Google Scholar] [CrossRef]
- Philpot, C.M. Geographical distribution of the dermatophytes: A review. J. Hyg. 1978, 80, 301–313. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Mayser, P.; Koch, D.; Mütze, H.; Krüger, C.; Schulze, I.; Nenoff, P. Zoophilic dermatophytes during coronavirus pandemic in Germany. Dermatologie 2023, 74, 430–439. [Google Scholar] [CrossRef]
- Seebacher, C.; Bouchara, J.P.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef]
- Baranová, Z.; Kampe, T.; Dorko, E.; Rimárová, K. Epidemiological and clinical aspects of dermatophytoses in Eastern Slovakia: A retrospective three-year study. Cent. Eur. J. Public Health 2018, 26, S72–S75. [Google Scholar] [CrossRef]
- Pires, C.A.A.; da Cruz, N.F.S.; Lobato, A.M.; de Sousa, P.O.; Carneiro, F.R.O.; Mendes, A.M.D. Clinical, epidemiological, and therapeutic profile of dermatophytosis. An. Bras. Dermatol. 2014, 89, 259–264. [Google Scholar] [CrossRef]
- Women in Defence System—Serbia—2023—Balkan Defence Monitor. Available online: https://balkandefencemonitor.com/women-in-defence-system-serbia-2023/ (accessed on 21 August 2024).
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust. J. Gen. Pract. 2019, 48, 706–711. [Google Scholar] [CrossRef]
- Petrucelli, M.F.; Abreu, M.H.; Cantelli, B.A.; Segura, G.G.; Nishimura, F.G.; Bitencourt, T.A.; Marins, M.; Fachin, A.L. Epidemiology and diagnostic perspectives of dermatophytoses. J. Fungi 2020, 6, 268. [Google Scholar] [CrossRef] [PubMed]
- Moskaluk, A.E.; VandeWoude, S. Current topics in dermatophyte classification and clinical diagnosis. Pathogens 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Gaitanis, G.; Hay, R.J. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front. Cell Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Gawdzik, A.; Nowogrodzka, K.; Hryncewicz-Gwóźdź, A.; Maj, J.; Szepietowski, J.; Jankowska-Konsur, A. Epidemiology of dermatomycoses in southwest Poland, years 2011-2016. Postepy Dermatol. Alergol. 2019, 36, 604–608. [Google Scholar] [CrossRef]
- Das, S.; De, A.; Saha, R.; Sharma, N.; Khemka, M.; Singh, S.; Hesanoor Reja, A.H.; Kumar, P. The current Indian epidemic of dermatophytosis: A study on causative agents and sensitivity patterns. Indian J. Dermatol. 2020, 65, 118–122. [Google Scholar]
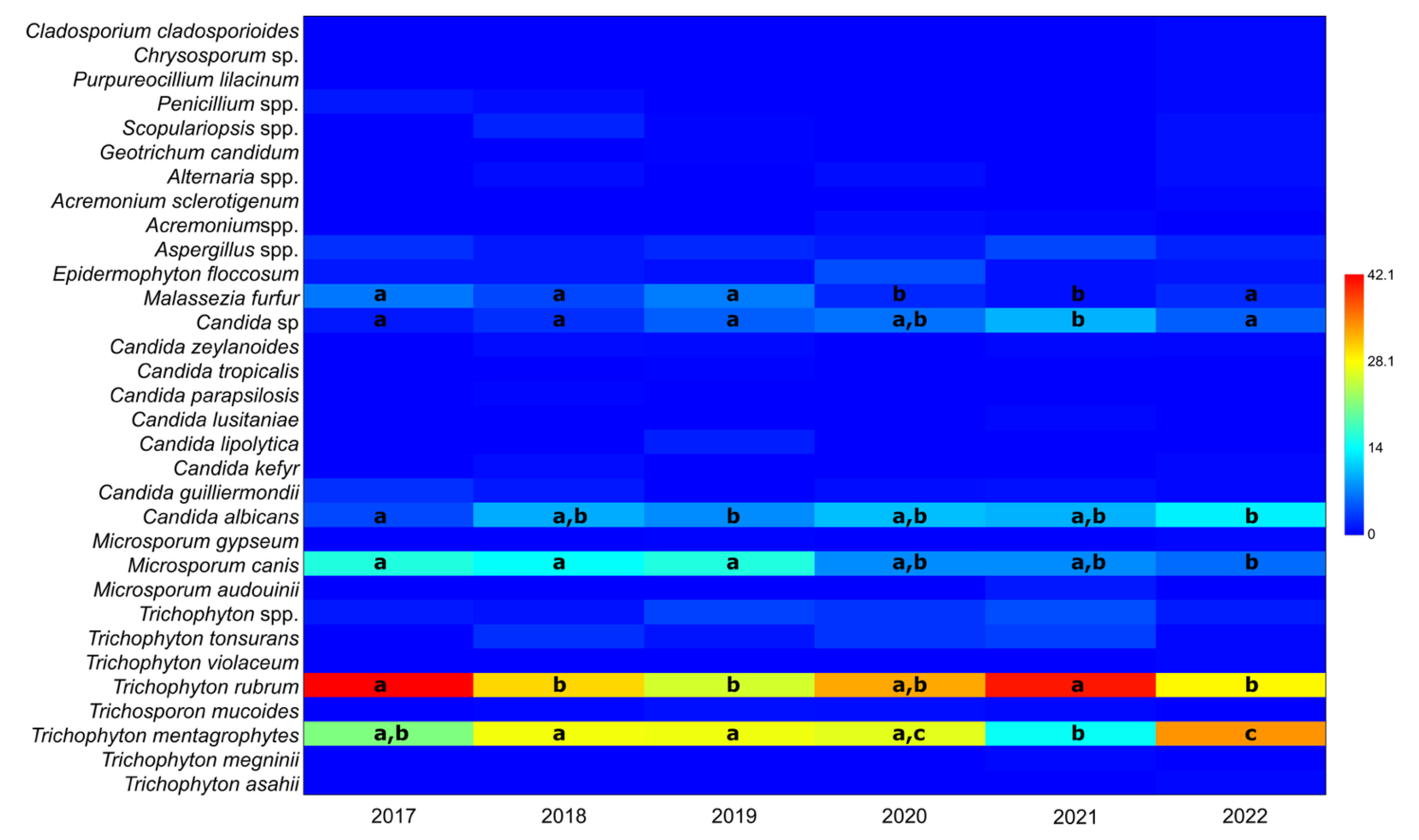
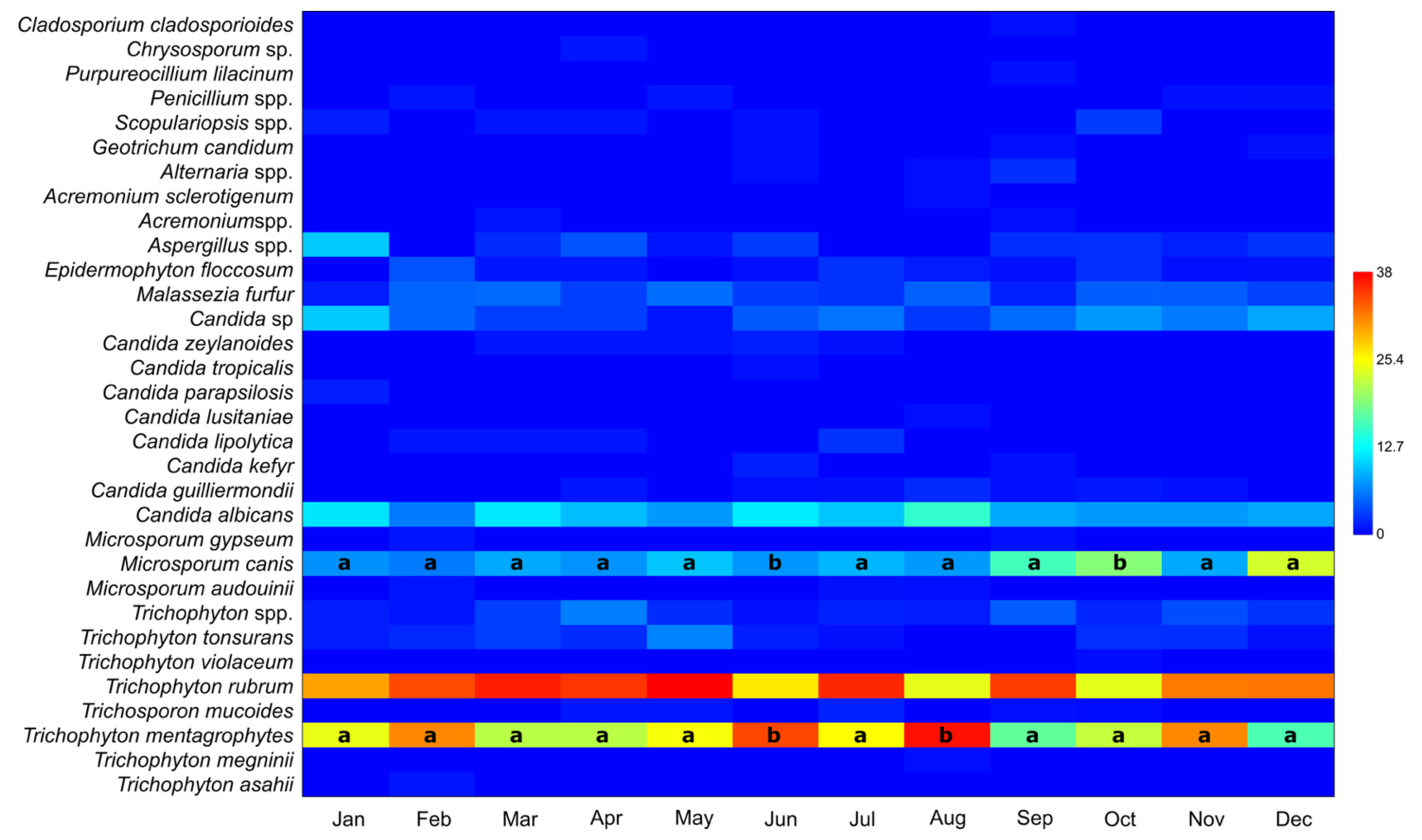
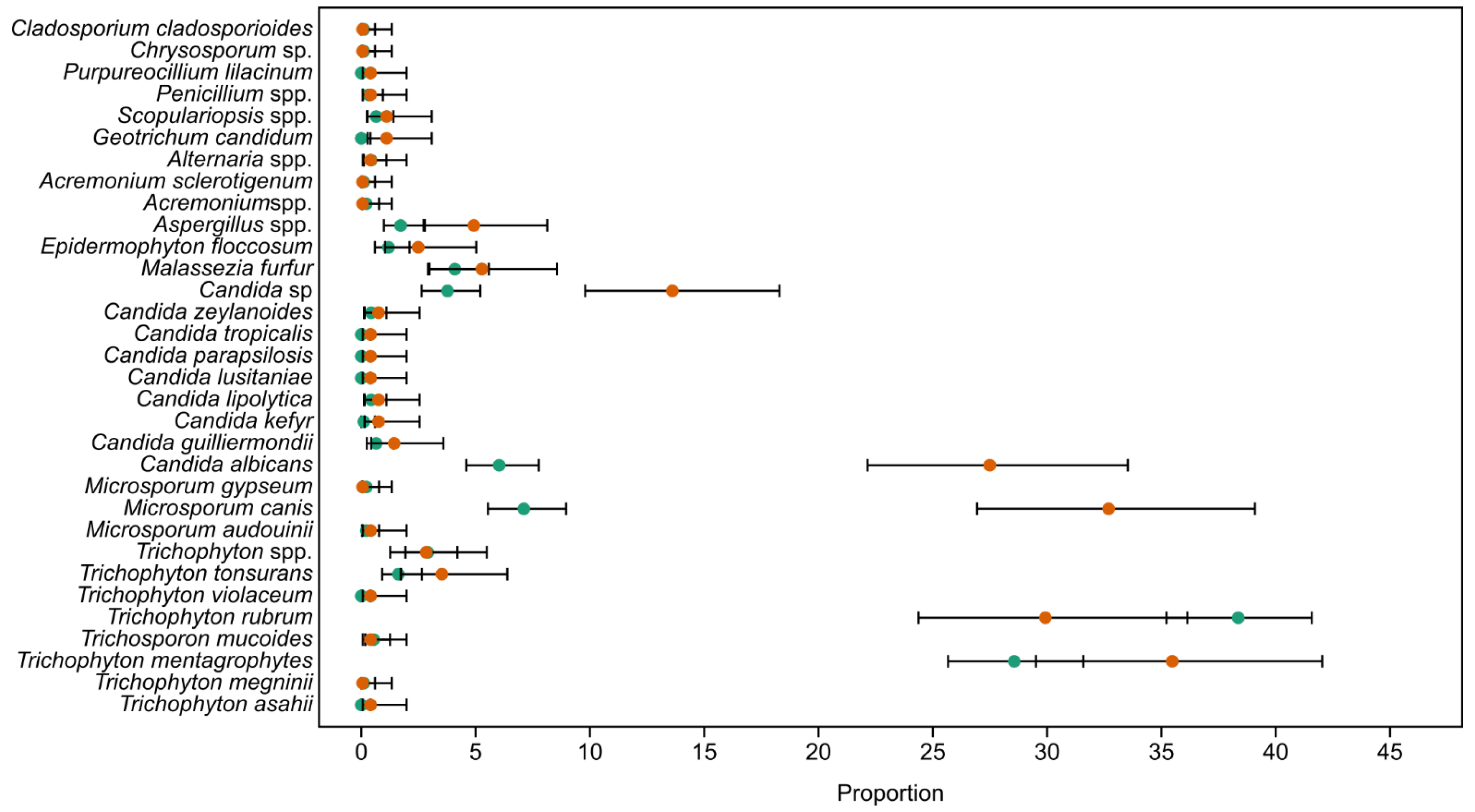


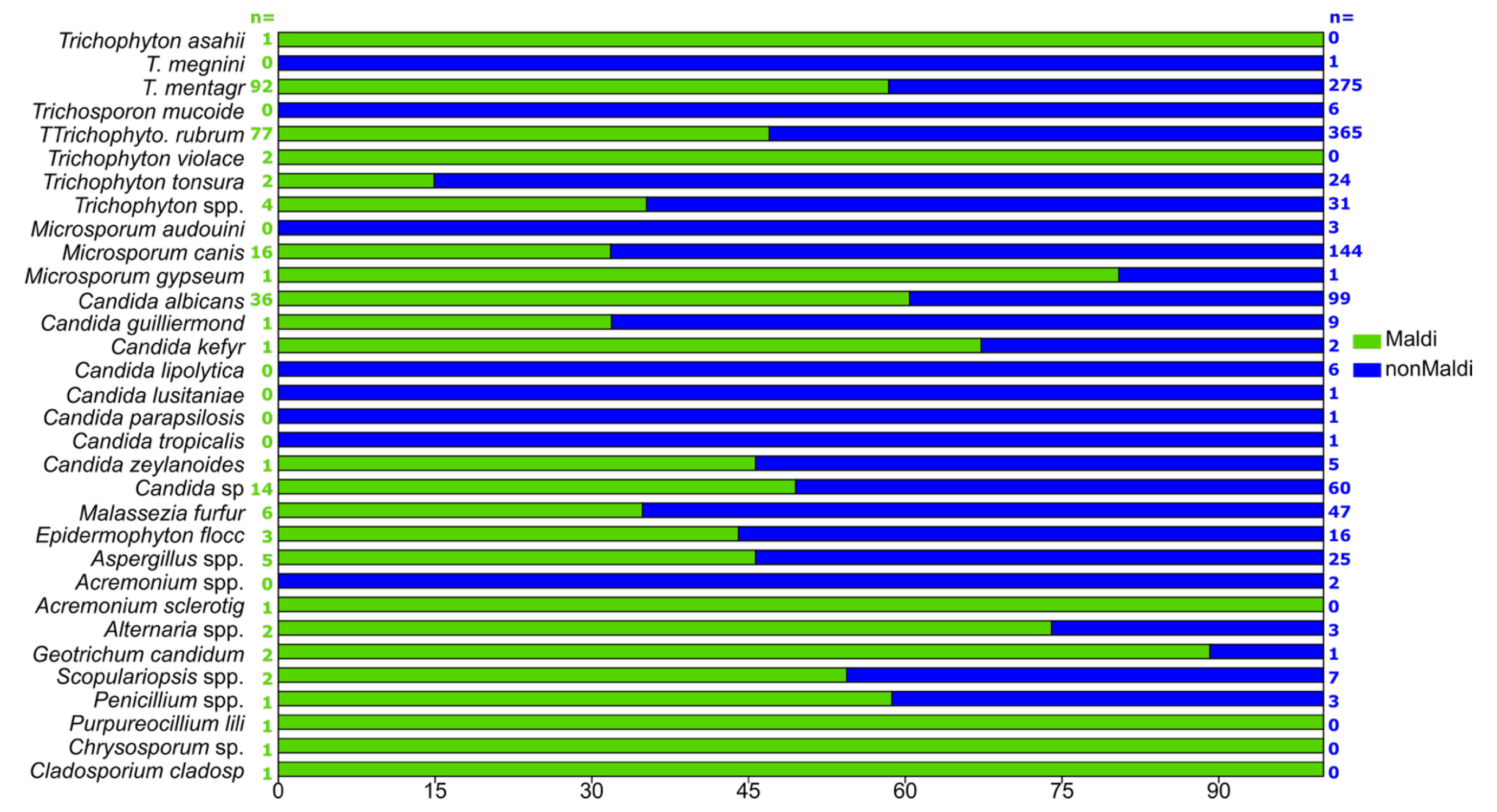
| Taxon | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|
| Trichophyton mentagrophytes | 21.1 (0.13–0.32) | 27.4 (0.23–0.33) | 27.2 (0.23–0.32) | 26.6 (0.19–0.35) | 14.5 (0.10–0.20) | 33.9 (0.28–0.40) |
| Trichophyton rubrum | 42.1 (0.31–0.54) | 30.3 (0.25–0.36) | 25.6 (0.21–0.30) | 32.9 (0.25–0.42) | 40.9 (0.35–0.48) | 28.4 (0.23–0.34) |
| Microsporum canis | 15.8 (0.08–0.26) | 14.2 (0.11–0.19) | 15.8 (0.12–0.2) | 7.7 (0.04–0.13) | 7.7 (0.04–0.12) | 5.9 (0.03–0.09) |
| Candida albicans | 3.9 (0.01–0.11) | 9.5 (0.06–0.13) | 7.6 (0.05–0.11) | 10.5 (0.06–0.17) | 9.8 (0.06–0.14) | 13.3 (0.09–0.18) |
| Malassezia furfur | 6.6 (0.02–0.15) | 3.8 (0.02–0.07) | 6.8 (0.04–0.10) | 2.1 (0.00–0.06) | 0.9 (0.00–0.03) | 2.2 (0.01–0.05) |
| Candida sp. | 1.3 (0.00–0.07) | 2.5 (0.01–0.05) | 5.2 (0.03–0.08) | 6.3 (0.03–0.12) | 9.8 (0.06–0.14) | 5.2 (0.03–0.09) |
| Taxon | χ2 | p | |
|---|---|---|---|
| Trichophyton mentagrophytes | 18.27 | 0.000978 | June/August diff. |
| Trichophyton rubrum | 10.23 | 0.14 | No diff. |
| Microsporum canis | 13.6 | 0.00000644 | Oct/Dec diff. |
| Candida albicans | 2.65 | 0.53 | No diff. |
| Candida sp. | 2.28 | 0.18 | No diff. |
| Malassezia furfur | 0.72 | 0.84 | No diff. |
| Taxon | X2 | p | ε2 | Mann–Whitney |
|---|---|---|---|---|
| Trichophyton mentagrophytes | 60.12 | 3.17 × 10−18 | 0.04 | More common in foot; foot differed from all other parts, except neck and arm |
| Trichophyton rubrum | 108.3 | 2.71 × 10−31 | 0.08 | More common in foot; foot differed from all but leg. Gluteus differed from all other parts by a low frequency. |
| Microsporium cannis | 140 | 1.32 × 10−92 | 0.10 | Almost all of the body parts showed statistically significant differences. |
| Microsporium gypseum | 0.08 | 2.98 × 10−2 | <0.001 | Only foot was different from leg and arm as it was present only in these body parts. No other differences. |
| Candida albicans | 61.85 | 2.28 × 10−45 | 0.04 | Only hand was different from all other body parts due to high frequency. |
| Candida zeylanoides | 0.34 | 1.6 × 10−3 | <0.001 | Only foot was different from hand and head. No other differences due to absence of detection on other body parts. |
| Candida sp. | 12.66 | 3.53 × 10−14 | 0.010 | Only hand was different from all other body parts due to higher frequency. |
| Malassezia furfur | 46.22 | 1.93 × 10−84 | 0.03 | Back, abdomen, and chest were different from all other parts and between each other due to varying and higher abundances. |
| Taxon | X2 | p | ε2 | Mann–Whitney |
|---|---|---|---|---|
| Trichophyton rubrum | 9.4 | 7.1 × 10−4 | 0.007 | Complete differentiation of all surfaces. |
| Microsporium cannis | 77.6 | 1.29 × 10−55 | 0.056 | Complete differentiation of all surfaces. |
| Candida albicans | 4.61 | 1.63 × 10−5 | 0.003 | Skin and nail were different from hair due to absence in hair. More commonly found on nail. |
| Candida sp. | 3.25 | 2.29 × 10−05 | 0.002 | Only skin and nail were different due to absence in hair. More commonly found on nail. |
| Malassezia furfur | 7.52 | 1.74 × 10−15 | 0.005 | Found only on skin. |
| Aspergillus spp. | 1.03 | 3.07 × 10−5 | <0.001 | Only skin and nail were different, with higher frequency on nail and due to absence in hair. |
| Male | Female | Abdomen | Arm | Back | Chest | Foot | Gluteus | Hand | Head | Leg | Neck | Skin | Nail | Hair | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of taxa | 24 | 26 | 10 | 9 | 6 | 4 | 23 | 6 | 19 | 11 | 11 | 1 | 15 | 28 | 3 |
| Individuals | 927 | 480 | 63 | 54 | 42 | 18 | 828 | 24 | 196 | 91 | 90 | 2 | 626 | 762 | 20 |
| Dominance | 0.242 | 0.151 | 0.203 | 0.266 | 0.3159 | 0.366 | 0.306 | 0.449 | 0.208 | 0.401 | 0.226 | 1 | 0.203 | 0.218 | 0.7211 |
| Simpson index | 0.758 | 0.850 | 0.797 | 0.735 | 0.684 | 0.634 | 0.694 | 0.551 | 0.792 | 0.599 | 0.774 | 0 | 0.797 | 0.782 | 0.2789 |
| Shannon index | 1.894 | 2.206 | 1.891 | 1.659 | 1.372 | 1.125 | 1.649 | 1.239 | 2.032 | 1.470 | 1.803 | 0 | 1.848 | 1.978 | 0.5682 |
| January | February | March | April | May | June | July | August | September | October | November | December | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of taxa | 11 | 14 | 14 | 16 | 12 | 17 | 14 | 14 | 17 | 14 | 12 | 12 |
| Individuals | 70 | 99 | 96 | 96 | 92 | 136 | 122 | 145 | 130 | 170 | 131 | 121 |
| Dominance | 0.176 | 0.223 | 0.1991 | 0.1877 | 0.2224 | 0.2076 | 0.2119 | 0.225 | 0.190 | 0.1578 | 0.216 | 0.194 |
| Simpson index | 0.824 | 0.777 | 0.8009 | 0.8123 | 0.7776 | 0.7924 | 0.7881 | 0.776 | 0.810 | 0.842 | 0.784 | 0.806 |
| Shannon index | 1.977 | 1.925 | 2.021 | 2.127 | 1.863 | 2.006 | 1.944 | 1.853 | 2.076 | 2.124 | 1.873 | 1.913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đorđevski, N.; Ristanović, E.; Ćirić, A.; Tomić, D.; Nikolić, B.; Rajčević, N.; Stojković, D. Five-Year Retrospective Analysis of Superficial Fungal Infections: Insights from Hospital Experience. J. Fungi 2025, 11, 474. https://doi.org/10.3390/jof11070474
Đorđevski N, Ristanović E, Ćirić A, Tomić D, Nikolić B, Rajčević N, Stojković D. Five-Year Retrospective Analysis of Superficial Fungal Infections: Insights from Hospital Experience. Journal of Fungi. 2025; 11(7):474. https://doi.org/10.3390/jof11070474
Chicago/Turabian StyleĐorđevski, Nikoleta, Elizabeta Ristanović, Ana Ćirić, Diana Tomić, Biljana Nikolić, Nemanja Rajčević, and Dejan Stojković. 2025. "Five-Year Retrospective Analysis of Superficial Fungal Infections: Insights from Hospital Experience" Journal of Fungi 11, no. 7: 474. https://doi.org/10.3390/jof11070474
APA StyleĐorđevski, N., Ristanović, E., Ćirić, A., Tomić, D., Nikolić, B., Rajčević, N., & Stojković, D. (2025). Five-Year Retrospective Analysis of Superficial Fungal Infections: Insights from Hospital Experience. Journal of Fungi, 11(7), 474. https://doi.org/10.3390/jof11070474








