Elicitor from Trichothecium roseum Activates the Disease Resistance of Salicylic Acid, Jasmonic Acid, and Ca2+-Dependent Pathways in Potato Tubers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection, Treatment, and Storage of Potato Tubers, Pathogens, and the Fungal Elicitor
2.3. In Vivo Inhibitory Activity Assessment of the Elicitor
2.4. The Rate of Superoxide Anion (O2·−) Production and Hydrogen Peroxide (H2O2) Content Assay
2.5. Detection of SA Content and Activities of Isochorismate Synthase (ICS) and PAL
2.6. Detection of JA Content and Activities of Allene Oxide Cyclase (AOC), Allene Oxide Synthase (AOS), and 13-Lipoxygenase (LOX)
2.7. Detection of Calmodulin (CaM) Content and Ca2+-ATPase Activity
2.8. Detection of SOD, CAT, PPO, Chitinase (CHI), and β-1,3-Glucanase (Glu) Activities
2.9. Gene Expression Analysis Using Quantitative Real-Time PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results
3.1. Effect of the Elicitor on Spore Germination and the Lesion Diameters of Dry Rot Disease of Potato Tubers In Vivo
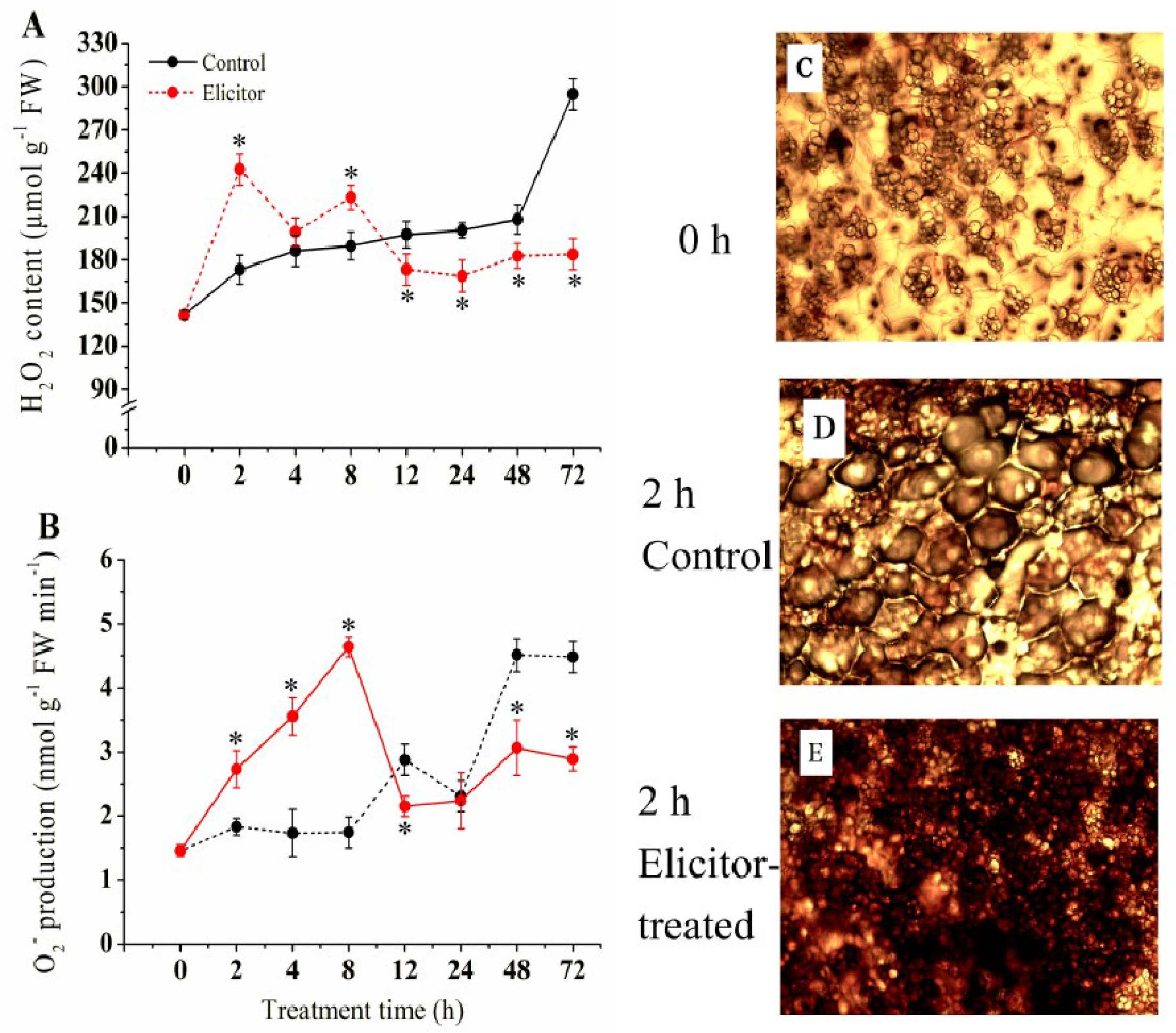
3.2. Effect of the Elicitor on the Generation of O2·− and the Content of H2O2
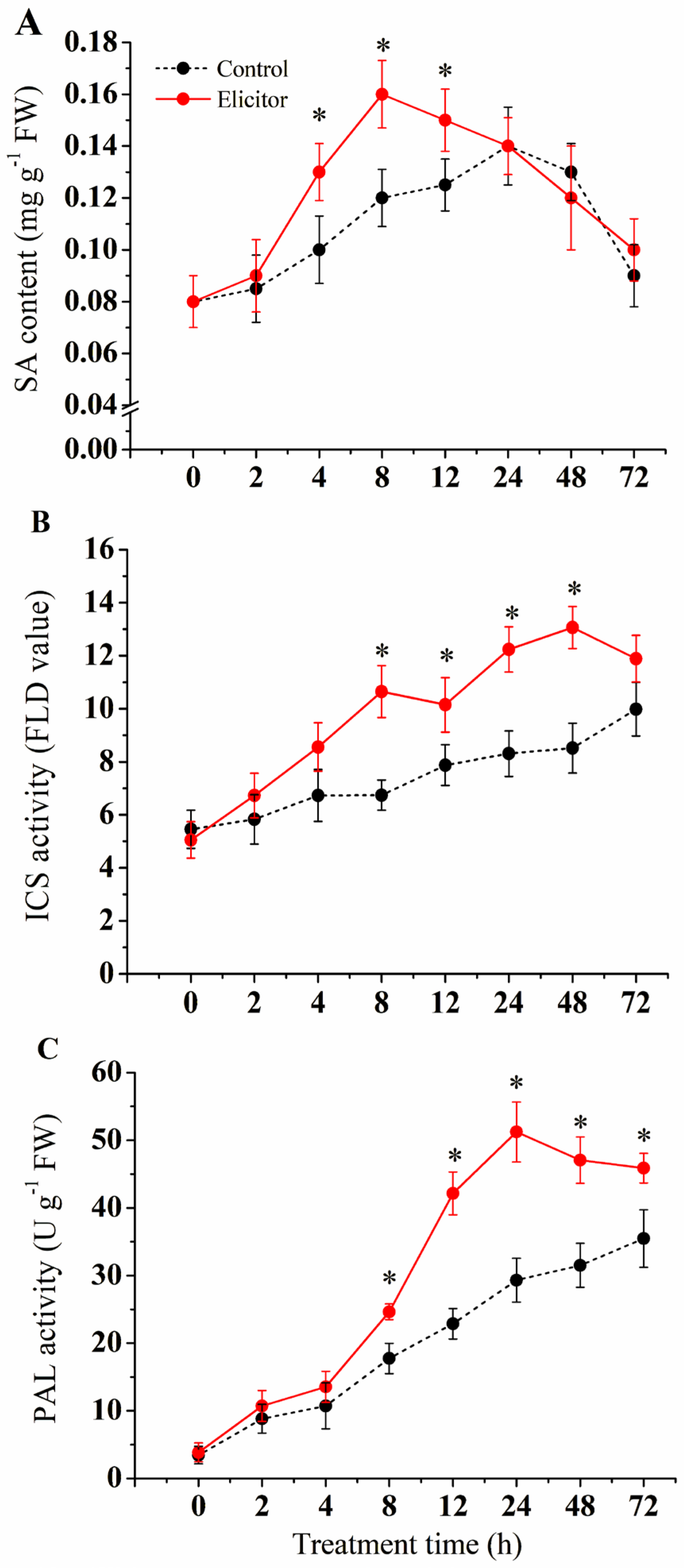
3.3. Effect of the Elicitor on SA Synthesis
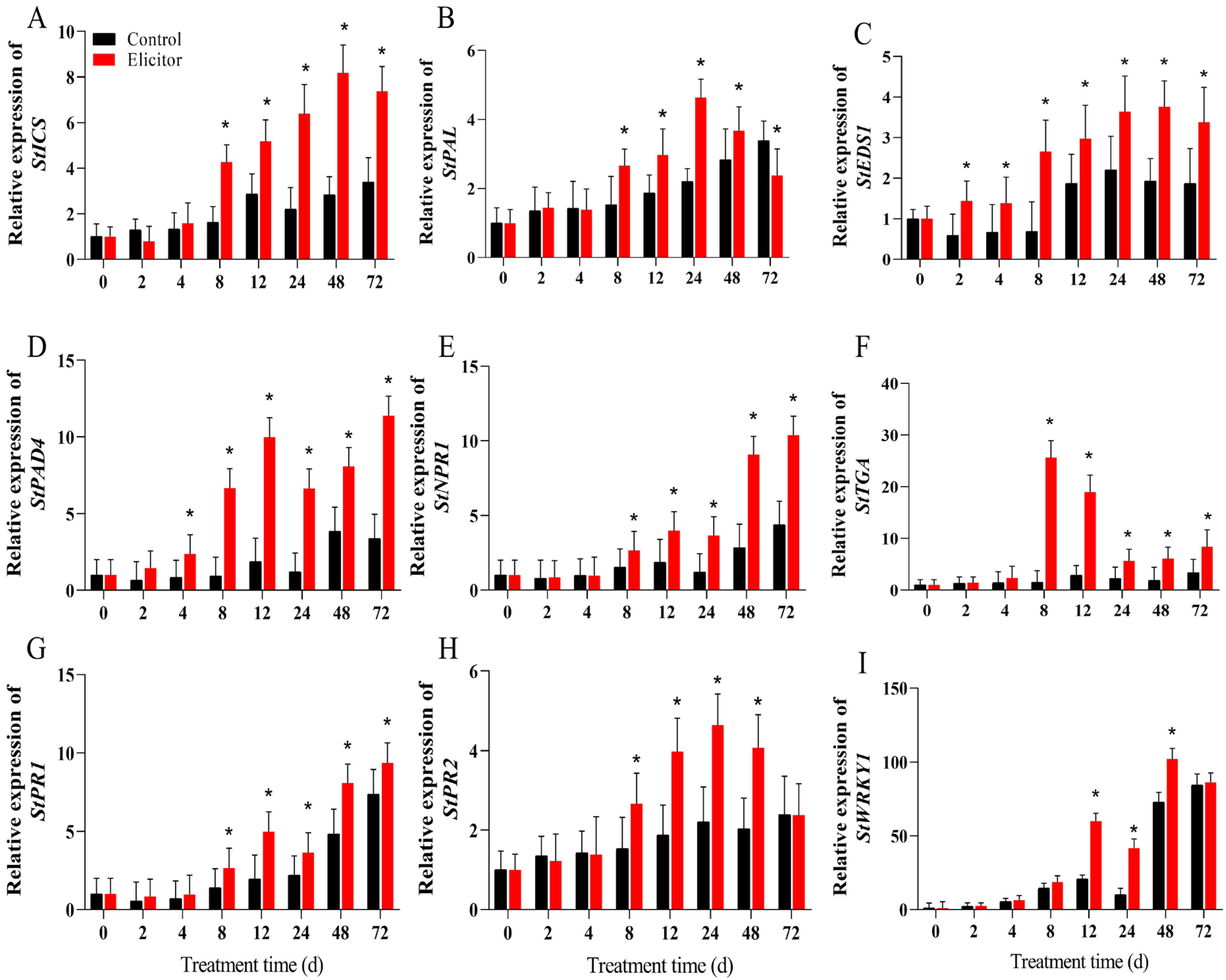
3.4. Effect of the Elicitor on the Expression of Key Genes Related to the SA Signaling Pathway
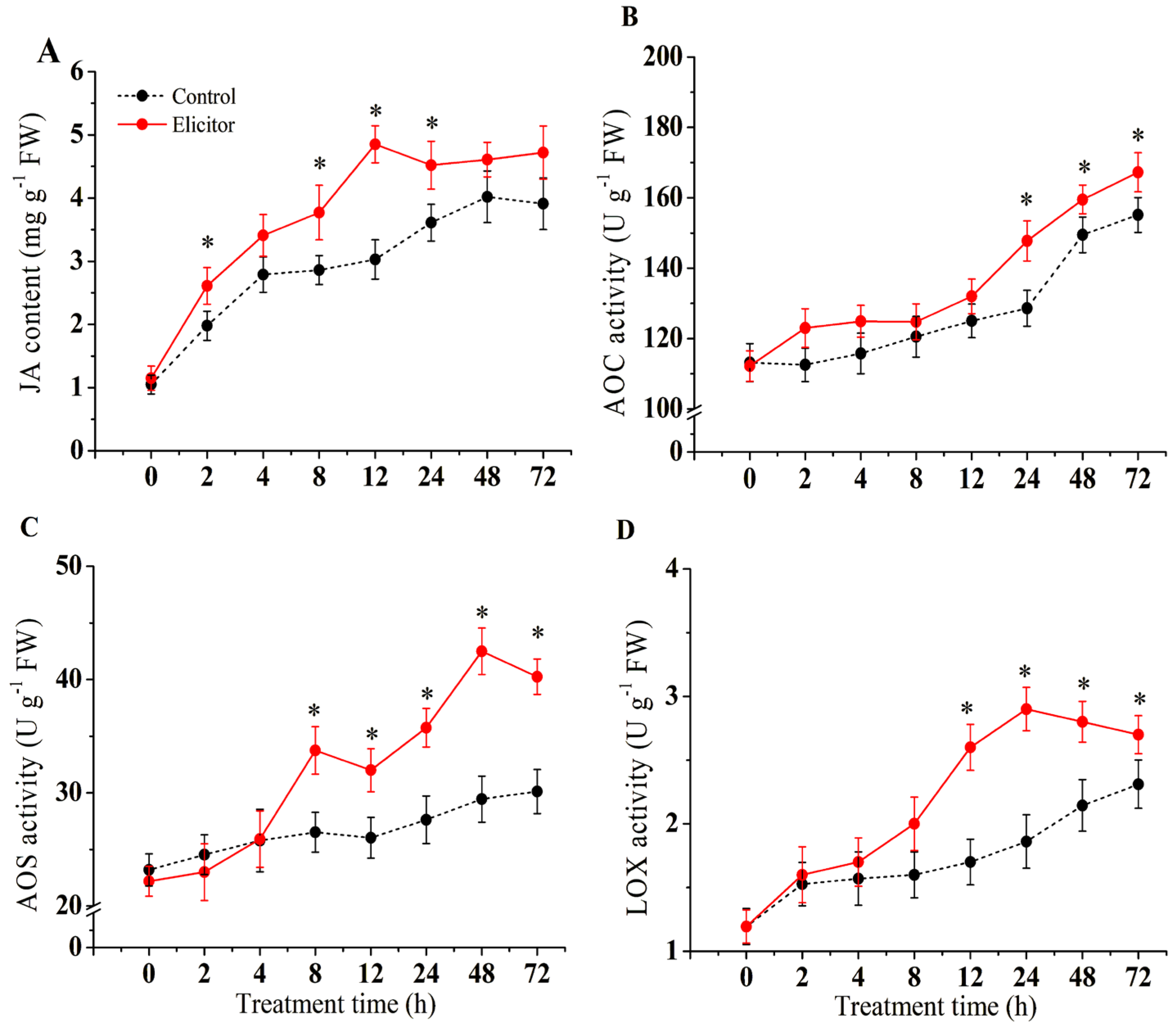
3.5. Effect of the Elicitor on JA
3.6. Effect of the Elicitor on the Expression of Key Genes Related to the JA Signaling Pathway
3.7. Effect of the Elicitor on the Calcium Signaling Pathway
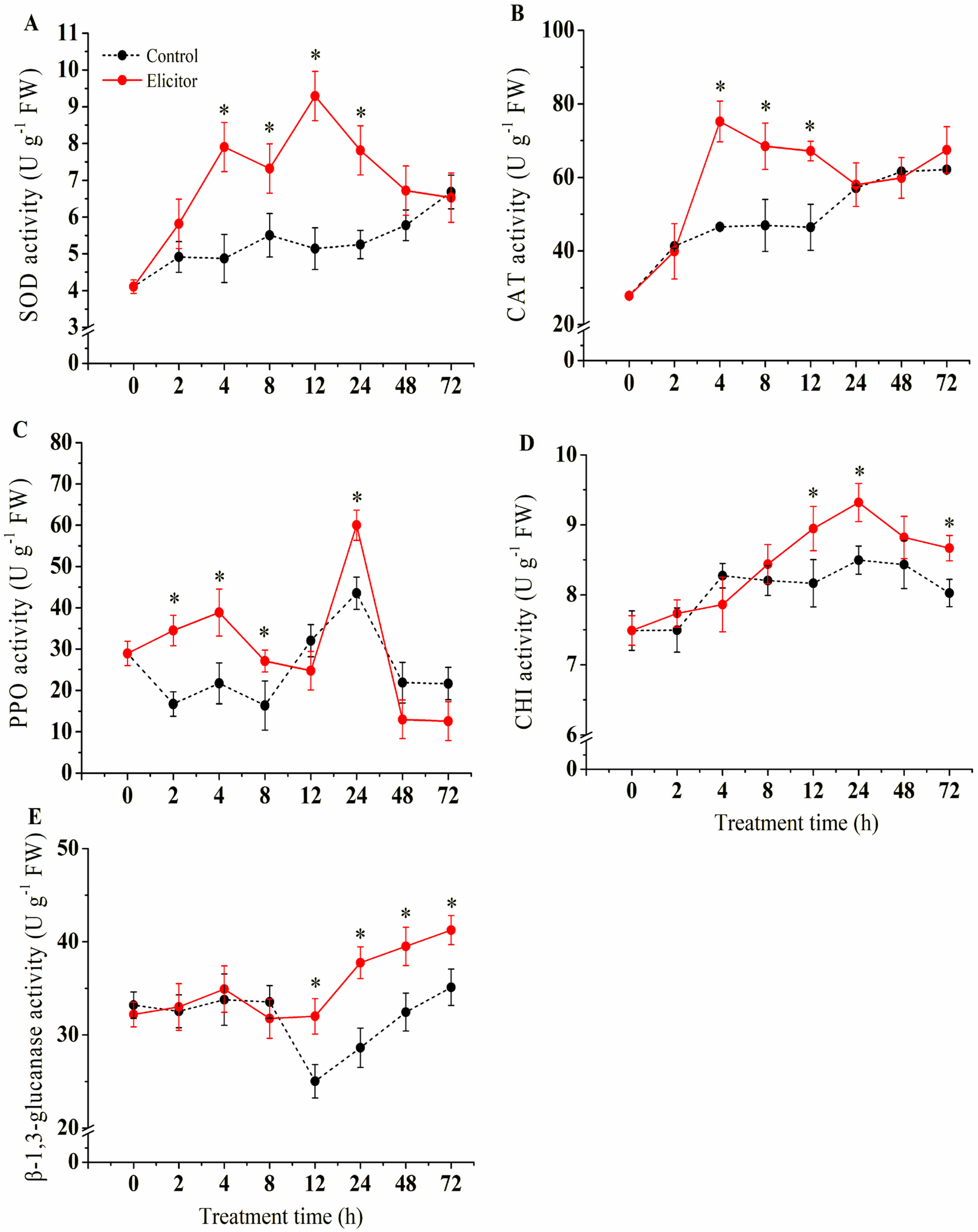
3.8. Effect of the Elicitor on the Activities of Defense-Related Enzymes
3.9. Effect of the Elicitor on the Gene Expression Levels of Defense-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Sagar, V.; Aggarwal, R.; Naga, K.C.; Lal, M.K.; Chourasia, K.N.; Kumar, D.; Kumar, M. Potato Dry Rot Disease: Current Status, Pathogenomics and Management. 3 Biotech 2020, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xue, H.; Bi, Y.; Zhang, R.; Kouasseu, C.J.; Liu, Q.; Nan, M.; Pu, L.; Prusky, D. Ozone Treatment Inhibits Dry Rot Development and Diacetoxyscirpenol Accumulation in Inoculated Potato Tuber by Influencing Growth of Fusarium sulphureum and Ergosterol Biosynthesis. Postharvest Biol. Technol. 2022, 185, 111796. [Google Scholar] [CrossRef]
- Xue, H.-L.; Bi, Y.; Tang, Y.-M.; Zhao, Y.; Wang, Y. Effect of Cultivars, Fusarium Strains and Storage Temperature on Trichothecenes Production in Inoculated Potato Tubers. Food Chem. 2014, 151, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Y.; Zhang, T.; Bi, Y.; Wang, Y.; Prusky, D. Exogenous Polyamines Enhance Resistance to Alternaria alternata by Modulating Redox Homeostasis in Apricot Fruit. Food Chem. 2019, 301, 125303. [Google Scholar] [CrossRef]
- De Britto, S.; Jogaiah, S. Priming with Fungal Elicitor Elicits Early Signaling Defense against Leaf Spot of Broccoli Underlying Cellular, Biochemical and Gene Expression. Microbiol. Res. 2022, 263, 127143. [Google Scholar] [CrossRef]
- Guarnizo, N.; Álvarez, A.; Oliveros, D.; Barbosa, O.; Joli, J.E.; Bermúdez-Cardona, M.B.; Murillo-Arango, W. Elicitor Activity of Curdlan and Its Potential Application in Protection of Hass Avocado Plants against Phytophthora cinnamomi Rands. Horticulturae 2022, 8, 646. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Jin, J.-H.; Sheng, G.-L.; Xing, Y.-P.; Liu, W.; Zhou, X.; Liu, Y.-Q.; Chen, X.-R. A Small Cysteine-Rich Phytotoxic Protein of Phytophthora capsici Functions as Both Plant Defense Elicitor and Virulence Factor. Mol. Plant Microbe Interact. 2021, 34, 891–903. [Google Scholar] [CrossRef]
- He, J.; Li, R.; Xu, C.; Chen, X.; Yao, J.; Li, Z.; Cheng, Y. Enhancing Fruit Resistance against Fungal Pathogens Using a Pathogen-Associated Molecular Pattern PdEIX. J. Agric. Food Chem. 2025, 73, 135–146. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, H.; Qiu, D.; Guo, L.; Yang, X.; Shi, H.; Zhou, T.; Zhao, J. Purification and Characterization of a Novel Hypersensitive Response-Inducing Elicitor from Magnaporthe oryzae That Triggers Defense Response in Rice. PLoS ONE 2012, 7, e37654. [Google Scholar] [CrossRef]
- Kulye, M.; Liu, H.; Zhang, Y.; Zeng, H.; Yang, X.; Qiu, D. Hrip1, a Novel Protein Elicitor from Necrotrophic Fungus, Alternaria tenuissima, Elicits Cell Death, Expression of Defence-related Genes and Systemic Acquired Resistance in Tobacco. Plant Cell Environ. 2012, 35, 2104–2120. [Google Scholar] [CrossRef]
- González, M.; Brito, N.; González, C. The Botrytis cinerea Elicitor Protein BcIEB1 Interacts with the Tobacco PR5-family Protein Osmotin and Protects the Fungus against Its Antifungal Activity. New Phytol. 2017, 215, 397–410. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.Y.; Xin, X.-F. Pattern-Recognition Receptors Are Required for NLR-Mediated Plant Immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI Crosstalk: An Integrative View of Plant Immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling Mechanisms in Pattern-Triggered Immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- El-Shetehy, M.; Wang, C.; Shine, M.B.; Yu, K.; Kachroo, A.; Kachroo, P. Nitric Oxide and Reactive Oxygen Species Are Required for Systemic Acquired Resistance in Plants. Plant Signal. Behav. 2015, 10, e998544. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Li, D.; Li, L.; Luo, Z. Effect of High Carbon Dioxide Treatment on Reactive Oxygen Species Accumulation and Antioxidant Capacity in Fresh-Cut Pear Fruit during Storage. Sci. Hortic. 2021, 281, 109925. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and Its Secondary Metabolites Improve Yield and Quality of Grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Synergistic Effects of Plant Defense Elicitors and Trichoderma harzianum on Enhanced Induction of Antioxidant Defense System in Tomato against Fusarium Wilt Disease. Bot. Stud. 2017, 58, 44. [Google Scholar] [CrossRef]
- Suleman, M.; Ma, M.; Ge, G.; Hua, D.; Li, H. The Role of Alternative Oxidase in Plant Hypersensitive Response. Plant Biol. J. 2021, 23, 415–419. [Google Scholar] [CrossRef]
- Zhang, P.; Jackson, E.; Li, X.; Zhang, Y. Salicylic Acid and Jasmonic Acid in Plant Immunity. Hortic. Res. 2025, 12, uhaf082. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Xu, J.; Liu, T.; Yang, D.; Wu, Z.; Shao, M. Application of Exogenous Salicylic Acid on Improving High Temperature Resistance of Nannochloropsis oceanica. Aquacult. Int. 2020, 28, 2235–2246. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, Y.; Ran, X.; Guo, H.; Yin, T.; Shen, Y.; Liu, W.; Ding, Y. Exogenous Application of Methyl Jasmonate at the Booting Stage Improves Rice’s Heat Tolerance by Enhancing Antioxidant and Photosynthetic Activities. Agronomy 2022, 12, 1573. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Alleviation of Field Low-Temperature Stress in Winter Wheat by Exogenous Application of Salicylic Acid. J. Plant Growth Regul. 2021, 40, 811–823. [Google Scholar] [CrossRef]
- Chen, M.; Guo, H.; Chen, S.; Li, T.; Li, M.; Rashid, A.; Xu, C.; Wang, K. Methyl Jasmonate Promotes Phospholipid Remodeling and Jasmonic Acid Signaling to Alleviate Chilling Injury in Peach Fruit. J. Agric. Food Chem. 2019, 67, 9958–9966. [Google Scholar] [CrossRef]
- Li, S.; Xiao, L.; Chen, M.; Cao, Q.; Luo, Z.; Kang, N.; Jia, M.; Chen, J.; Xiang, M. The Involvement of the Phenylpropanoid and Jasmonate Pathways in Methyl Jasmonate-Induced Soft Rot Resistance in Kiwifruit (Actinidia chinensis). Front. Plant Sci. 2022, 13, 1097733. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Li, M.; Zeng, P.; Zhang, Q.; Li, X.; Xu, Q.; Li, T.; Wang, X.; Kang, Z.; et al. TaBln1, a Member of the Blufensin Family, Negatively Regulates Wheat Resistance to Stripe Rust by Reducing Ca2+ Influx. Plant Physiol. 2022, 189, 1380–1396. [Google Scholar] [CrossRef]
- Song, P.; Wang, Y.; Hou, Y.; Mao, X.; Liu, Z.; Wei, M.; Yu, J.; Wang, B.; Qian, Y.; Yan, L.; et al. Crucial Role of Ca2+/CN Signalling Pathway in the Antifungal Activity of disenecioyl-cis-khellactone against Botrytis cinerea. Pest. Manag. Sci. 2022, 78, 4649–4659. [Google Scholar] [CrossRef]
- Yu, H.; Du, X. Differential Regulation of Calmodulin, Phenylalanine Ammonia-Lyase, and Salicylic Acid in Response to Botrytis cinerea Infection in Tomato with Different Ca2+ Concentrations. J. Plant Nutr. 2018, 41, 1104–1118. [Google Scholar] [CrossRef]
- Sun, X.; Pan, B.; Wang, Y.; Xu, W.; Zhang, S. Exogenous Calcium Improved Resistance to Botryosphaeria dothidea by Increasing Autophagy Activity and Salicylic Acid Level in Pear. Mol. Plant-Microbe Interact. 2020, 33, 1150–1160. [Google Scholar] [CrossRef]
- Bi, Y.; Tian, S.P.; Guo, Y.R.; Ge, Y.H.; Qin, G.Z. Sodium Silicate Reduces Postharvest Decay on Hami Melons: Induced Resistance and Fungistatic Effects. Plant Dis. 2006, 90, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bi, Y.; Yan, L.; Liu, X.; Wang, Y.; Shen, K.; Li, Y. Activation of Phenylpropanoid Pathway and PR of Potato Tuber against Fusarium sulphureum by Fungal Elicitor from Trichothecium roseum. World J. Microbiol. Biotechnol. 2016, 32, 142. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Lantos, E.; Nagyéri, G.; Janda, T. Comparative Study of Salicylic Acid Contents in Young Wheat and Rice Plants and Their Anticancer Activities in HepG2 and Caco-2 Cells. Biol. Futur. 2020, 71, 265–271. [Google Scholar] [CrossRef]
- Poulsen, C.; Van Der Heijden, R.; Verpoorte, R. Assay of Isochorismate Synthase from Plant Cell Cultures by High-Performance Liquid Chromatography. Phytochemistry 1991, 30, 2873–2876. [Google Scholar] [CrossRef]
- Li, S.; Ma, R.; Xu, J.; Shen, Z.; Yu, M. Effect of Melatonin Treatment on Improving Jasmonates Content and Cell Wall Stability Involved in Enhanced Chilling Tolerance of Peach Fruit during Cold Storage. Postharvest Biol. Technol. 2024, 213, 112957. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, H.; Liu, Z.; Yang, X.; Guo, L.; Qiu, D. Mutational Analysis of the Verticillium dahliae Protein Elicitor PevD1 Identifies Distinctive Regions Responsible for Hypersensitive Response and Systemic Acquired Resistance in Tobacco. Microbiol. Res. 2014, 169, 476–482. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of Postharvest Decay of Apple Fruit with Candida saitoana and Induction of Defense Responses. Phytopathology 2003, 93, 344–348. [Google Scholar] [CrossRef]
- Tian, S.; Wan, Y.; Qin, G.; Xu, Y. Induction of Defense Responses against Alternaria Rot by Different Elicitors in Harvested Pear Fruit. Appl. Microbiol. Biotechnol. 2006, 70, 729–734. [Google Scholar] [CrossRef]
- Muslim, A.; Hyakumachi, M.; Kageyama, K.; Suwandi, S. Indonesia Induction of Systemic Resistance in Cucumber by Hypovirulent Binucleate Rhizoctonia Against Anthracnose Caused by Colletotrichum orbiculare. Trop. Life Sci. Res. 2019, 30, 109–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zeng, H.; Guo, L.; Yuan, J.; Qiu, D. Fungal Elicitor Protein PebC1 from Botrytis cinerea Improves Disease Resistance in Arabidopsis thaliana. Biotechnol. Lett. 2014, 36, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, M.T.; Akita, M.; Frank, W.; Reski, R.; Valkonen, J.P.T. Involvement of a Class III Peroxidase and the Mitochondrial Protein TSPO in Oxidative Burst Upon Treatment of Moss Plants with a Fungal Elicitor. Mol. Plant Microbe Interact. 2012, 25, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bu, B.; Qiu, D.; Zeng, H.; Guo, L.; Yuan, J.; Yang, X. A Fungal Protein Elicitor PevD1 Induces Verticillium Wilt Resistance in Cotton. Plant Cell Rep. 2014, 33, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, P.; Jha, G. Alterations in Plant Sugar Metabolism: Signatory of Pathogen Attack. Planta 2019, 249, 305–318. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, Y.; Ritchie, E.S.; Macho, A.P.; Yu, F.; Wu, D. Manipulation of Plant Metabolism by Pathogen Effectors: More than Just Food. FEMS Microbiol. Rev. 2023, 47, fuad007. [Google Scholar] [CrossRef]
- Shine, M.B.; Yang, J.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative Functioning between Phenylalanine Ammonia Lyase and Isochorismate Synthase Activities Contributes to Salicylic Acid Biosynthesis in Soybean. New Phytol. 2016, 212, 627–636. [Google Scholar] [CrossRef]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A Core Function of EDS1 with PAD4 Is to Protect the Salicylic Acid Defense Sector in Arabidopsis Immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef]
- Fan, W.; Dong, X. In Vivo Interaction between NPR1 and Transcription Factor TGA2 Leads to Salicylic Acid–Mediated Gene Activation in Arabidopsis. Plant Cell 2002, 14, 1377–1389. [Google Scholar] [CrossRef]
- Fang, X.; Meng, X.; Zhang, J.; Xia, M.; Cao, S.; Tang, X.; Fan, T. AtWRKY1 Negatively Regulates the Response of Arabidopsis thaliana to Pst. DC3000. Plant Physiol. Biochem. 2021, 166, 799–806. [Google Scholar] [CrossRef]
- Han, Z.; Xiong, D.; Schneiter, R.; Tian, C. The Function of Plant PR1 and Other Members of the CAP Protein Superfamily in Plant–Pathogen Interactions. Mol. Plant Pathol. 2023, 24, 651–668. [Google Scholar] [CrossRef]
- Bueno, T.V.; Fontes, P.P.; Abe, V.Y.; Utiyama, A.S.; Senra, R.L.; Oliveira, L.S.; Brombini Dos Santos, A.; Ferreira, E.G.C.; Darben, L.M.; De Oliveira, A.B.; et al. A Phakopsora pachyrhizi Effector Suppresses PAMP-Triggered Immunity and Interacts with a Soybean Glucan Endo-1,3-β-Glucosidase to Promote Virulence. Mol. Plant-Microbe Interact. 2022, 35, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Hael-Conrad, V.; Abou-Mansour, E.; Díaz-Ricci, J.-C.; Métraux, J.-P.; Serrano, M. The Novel Elicitor AsES Triggers a Defense Response against Botrytis cinerea in Arabidopsis thaliana. Plant Sci. 2015, 241, 120–127. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Pandian, S.; Rakkammal, K.; Largia, M.J.V.; Thamilarasan, S.K.; Balaji, S.; Zoclanclounon, Y.A.B.; Shilpha, J.; Ramesh, M. Jasmonates in Plant Growth and Development and Elicitation of Secondary Metabolites: An Updated Overview. Front. Plant Sci. 2022, 13, 942789. [Google Scholar] [CrossRef]
- Valenzuela-Riffo, F.; Zúñiga, P.E.; Morales-Quintana, L.; Lolas, M.; Cáceres, M.; Figueroa, C.R. Priming of Defense Systems and Upregulation of MYC2 and JAZ1 Genes after Botrytis Cinerea Inoculation in Methyl Jasmonate-Treated Strawberry Fruits. Plants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Macioszek, V.K.; Jęcz, T.; Ciereszko, I.; Kononowicz, A.K. Jasmonic Acid as a Mediator in Plant Response to Necrotrophic Fungi. Cells 2023, 12, 1027. [Google Scholar] [CrossRef]
- Hanan, A.; Basit, A.; Nazir, T.; Majeed, M.Z.; Qiu, D. Anti-Insect Activity of a Partially Purified Protein Derived from the Entomopathogenic Fungus Lecanicillium lecanii (Zimmermann) and Its Putative Role in a Tomato Defense Mechanism against Green Peach Aphid. J. Invertebr. Pathol. 2020, 170, 107282. [Google Scholar] [CrossRef] [PubMed]
- Javed, K.; Qiu, D. Protein Elicitor PeBL1 of Brevibacillus laterosporus Enhances Resistance Against Myzus persicae in Tomato. Pathogens 2020, 9, 57. [Google Scholar] [CrossRef]
- Marcec, M.J.; Gilroy, S.; Poovaiah, B.W.; Tanaka, K. Mutual Interplay of Ca2+ and ROS Signaling in Plant Immune Response. Plant Sci. 2019, 283, 343–354. [Google Scholar] [CrossRef]
- Kuta, D.D.; Gaivaronskaya, L.M. Ca2+ and Reactive Oxygen Species Are Involved in the Defense Responses of Rice Callus Culture to Rice Blast Disease. Afr. J. Biotechnol. 2004, 3, 76–81. [Google Scholar] [CrossRef]
- Able, A.J.; Sutherland, M.W.; Guest, D.I. Production of Reactive Oxygen Species during Non-Specific Elicitation, Non-Host Resistance and Field Resistance Expression in Cultured Tobacco Cells. Funct. Plant Biol. 2003, 30, 91. [Google Scholar] [CrossRef]
- Gao, F.K.; Yong, Y.H.; Dai, C.C. Effects of endophytic fungal elicitor on two kinds of terpenoids production and physiological indexes in Euphorbia pekinensis suspension cells. J. Med. Plants Res. 2011, 5, 4418–4425. [Google Scholar]




| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Actin | CTGGGCAGAAGGAAAAGAGG | AATACTACGCAGGCTCATCAAAC |
| ICS | TGCTCGCTCTCGTCTTCACCTC | ATCTCTTGAATCGCCTTGGCATCC |
| PAL | GGTCACTGCCTCGGGTGAT | CCTGCCAGTGAGCAAACCA |
| EDS1 | CTTCTGCACTGGGAATAGGA | TTCGGAACTCAGTTGAGAGG |
| PAD4 | TTGCATTACCTTTGGCTCTC | CATGATGGGGAAAAGAACAG |
| NPR1 | GCACTTGAATCGGCTTAGGG | GCTTCTTCAGTTGACGCTCT |
| PR1 | GGCATCCCGAGCACAAAAT | CTGCACCGGAATCAAGT |
| PR2 | GTGAAGCTGGTTTGGGAAATG | TTGCCAATCAACGTCATGTCTAC |
| TGA | GGAGTATGGTCAGTGGGTGGA | GCTCAAAGTAGTGGTTCAGGCAA |
| WRKY1 | GAAGAATAAAGCCGGGTTCTTGG | CTTACACGATTTGATCACCTCATCC |
| AOC | CCCGATCTGCCATCTGAGTT | GCCCCATCCTCACAAGCTT |
| AOS | TTGAAACCCTAGATAAGGAAATGGC | AAGCCCCCAACGCCGACTTATCAA |
| LOX | TGGGTGGCTTCTGCTCTT | TTTGGAACTGGGCTGTGA |
| OPR3 | TGATTTCCCCGACTTCAGCT | CATGAGATGCACGACCAACA |
| JAZ1 | TTCATCATCGTCATCGTCGT | GGGGTTTTGTTTGTTGGCTA |
| COI1 | TTGGAGGAGTTTGGTGGTGG | GAGGGAAAACTAGTGCGGCAT |
| MYC2 | AAGAACAAGCGTAGAGCGTCGTC | CACCGCATCATCACCTCCACTTC |
| PFD1.2 | TCTTTTGCCTCGTCCTTGTT | TTGTGACCCCATGGTTTGTA |
| Ca2+-ATPase | CACTATGTTGCCAGCGGACTGC | GCCACCTCAGTTCCAGCGATTC |
| SOD | CTCCTGAAGATGAGGTGCGT | GAACCACAATAGAAGGGCAGAA |
| CAT | AGTCGGAGGAGCAAATCACAG | GCAGAGCCACAACAGCATCCC |
| PPO | TTGCCAATCAACGTCATGTCTAC | TGAACCGGGGTATGAGGGAT |
| CHI | ATTGGAAACGGATATGCTCCA | TCCTTACCTGAACGCCTGTCA |
| Glu | TCTTTTCCGCCTTCCTCTG | AACTTCGTTGGGGTTGTCTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, R.; Zhang, H.; Pei, Z.; Yu, X.; Ren, X.; Kong, Q. Elicitor from Trichothecium roseum Activates the Disease Resistance of Salicylic Acid, Jasmonic Acid, and Ca2+-Dependent Pathways in Potato Tubers. J. Fungi 2025, 11, 467. https://doi.org/10.3390/jof11070467
Wang D, Liu R, Zhang H, Pei Z, Yu X, Ren X, Kong Q. Elicitor from Trichothecium roseum Activates the Disease Resistance of Salicylic Acid, Jasmonic Acid, and Ca2+-Dependent Pathways in Potato Tubers. Journal of Fungi. 2025; 11(7):467. https://doi.org/10.3390/jof11070467
Chicago/Turabian StyleWang, Di, Rong Liu, Haijue Zhang, Zhifei Pei, Xiaoyan Yu, Xueyan Ren, and Qingjun Kong. 2025. "Elicitor from Trichothecium roseum Activates the Disease Resistance of Salicylic Acid, Jasmonic Acid, and Ca2+-Dependent Pathways in Potato Tubers" Journal of Fungi 11, no. 7: 467. https://doi.org/10.3390/jof11070467
APA StyleWang, D., Liu, R., Zhang, H., Pei, Z., Yu, X., Ren, X., & Kong, Q. (2025). Elicitor from Trichothecium roseum Activates the Disease Resistance of Salicylic Acid, Jasmonic Acid, and Ca2+-Dependent Pathways in Potato Tubers. Journal of Fungi, 11(7), 467. https://doi.org/10.3390/jof11070467






