New Therapeutic Options for Fusariosis: A Patent Review (2008–2023)
Abstract
1. Introduction
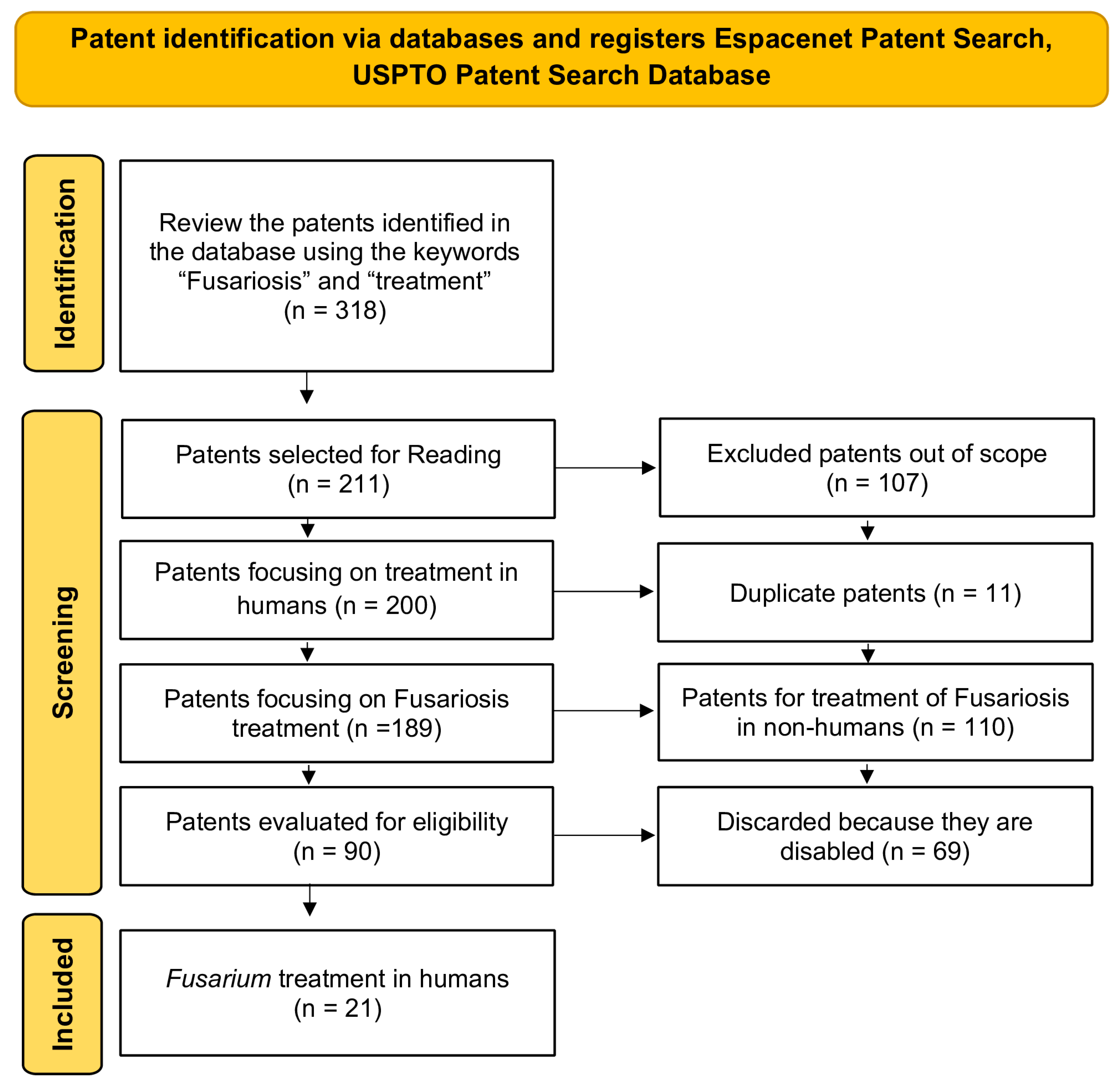
2. Materials and Methods
Study Design
3. Results and Discussion
3.1. Data Extraction
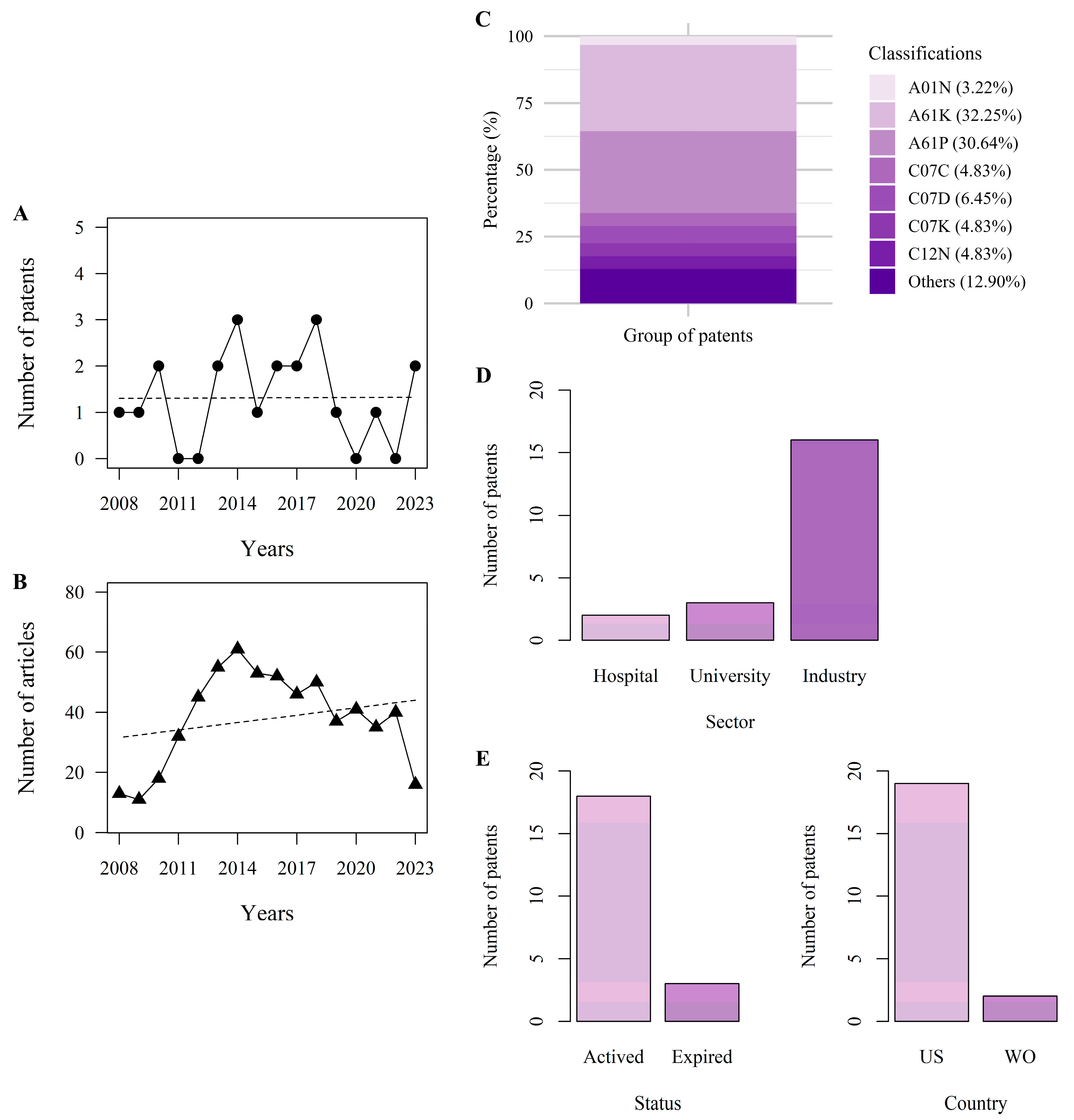
3.2. Patent History
3.3. Patents per Classification
3.4. Patent Market Trends
3.5. Status of the Patents
3.6. Patents Description
3.7. Drug Delivery
3.8. Gene Expression
3.9. Immunotherapy
3.10. Modified Drugs and Association
3.11. New Compounds
3.12. Peptides and Polypeptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hatmi, A.M.S.; Bonifaz, A.; Ranque, S.; de Hoog, G.S.; Verweij, P.E.; Meis, J.F. Current antifungal treatment of fusariosis. Int. J. Antimicrob. Agents 2018, 51, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Dabas, Y.; Bakhshi, S.; Xess, I. Fatal Cases of Bloodstream Infection by Fusarium solani and Review of Published Literature. Mycopathologia 2016, 181, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Audonneau, N.C.; Machouart, M.; Debourgogne, A. Fusarium infections: Epidemiological aspects over 10 years in a university hospital in France. J. Infect. Public Health 2020, 13, 1089–1093. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.-D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20 (Suppl. S3), 27–46. [Google Scholar] [CrossRef]
- Al Yazidi, L.S.; Al-Hatmi, A.M.S. Fusariosis: An update on therapeutic options for management. Expert Opin. Orphan Drugs 2021, 9, 95–103. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium Infections in Immunocompromised Patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Batista, B.G.; de Chaves, M.A.; Reginatto, P.; Saraiva, O.J.; Fuentefria, A.M. Human fusariosis: An emerging infection that is difficult to treat. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200013. [Google Scholar] [CrossRef]
- Chandorkar, A.; Simkins, J. Emerging Fungal Cutaneous Infections in Immunocompromised Patients. Curr. Fungal Infect. Rep. 2020, 14, 217–224. [Google Scholar] [CrossRef]
- Taj-Aldeen, S.J.; Salah, H.; Al-Hatmi, A.M.; Hamed, M.; Theelen, B.; van Diepeningen, A.D.; Boekhout, T.; Lass-Flörl, C. In vitro resistance of clinical Fusarium species to amphotericin B and voriconazole using the EUCAST antifungal susceptibility method. Diagn. Microbiol. Infect. Dis. 2016, 85, 438–443. [Google Scholar] [CrossRef]
- Taj-Aldeen, S.J. Reduced Multidrug Susceptibility Profile Is a Common Feature of Opportunistic Fusarium Species: Fusarium Multi-Drug Resistant Pattern. J. Fungi 2017, 3, 18. [Google Scholar] [CrossRef]
- Zhao, B.; He, D.; Wang, L. Advances in Fusarium drug resistance research. J. Glob. Antimicrob. Resist. 2021, 24, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.; Carneiro, H.; Coleman, J.; Mylonakis, E. The challenge of managing fusariosis. Virulence 2011, 2, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.; Zaragoza, O. Antifungal Efficacy during Candida krusei Infection in Non-Conventional Models Correlates with the Yeast In Vitro Susceptibility Profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Crespi, G.; D’este, P.; Fontana, R.; Geuna, A. The impact of academic patenting on university research and its transfer. Res. Policy 2011, 40, 55–68. [Google Scholar] [CrossRef]
- Wu, Y.; Welch, E.W.; Huang, W.-L. Commercialization of university inventions: Individual and institutional factors affecting licensing of university patents. Technovation 2015, 36–37, 12–25. [Google Scholar] [CrossRef]
- WIPO. World Intellectual Property Indicators 2022; World Intellectual Property Organization: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Ahlert, I.B.; Camara, E.G. Patentes: Proteção na lei de Propriedade Industrial; Atlas: São Paulo, Brazil, 2019. [Google Scholar]
- Labruine, J. Direito de Patentes Condições Legais de Obtenção e Nulidades; Manole: Barueri, Brazil, 2006. [Google Scholar]
- Pinto, E.C. A Crise Americana: Dívida, Desemprego E Política. In Boletim de Economia e Política Internacional; Viana, A.R., Oliveira, I.T.M., Eds.; Diretoria de Estudos e Relações Econômicas e Políticas Internacionais: São Paulo, Brazil, 2011; Volume 8, pp. 7–25. [Google Scholar]
- Harper, L.; Kalfa, N.; Beckers, G.; Kaefer, M.; Nieuwhof-Leppink, A.; Fossum, M.; Herbst, K.; Bagli, D. The impact of COVID-19 on research. J. Pediatr. Urol. 2020, 16, 715–716. [Google Scholar] [CrossRef]
- Hall, B.H. Patents and patent policy. Oxf. Rev. Econ. Policy 2007, 23, 568–587. [Google Scholar] [CrossRef]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current applications and prospects of nanoparticles for antifungal drug delivery. EXCLI J. 2021, 20, 562–584. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, I.R.; Lemon, C.P.; Wang, Y. Binding Ligand Linked Drug Delivery Conjugates of Tubulysins. U.S. Patent 9,555,139, 31 January 2017. [Google Scholar]
- Nel, A.E.; Meng, H.; Liu, X. Mesoporous Silica Nanoparticles with Lipid Bilayer Coating for Cargo Delivery. U.S. Patent 10,143,660, 4 December 2018. [Google Scholar]
- Andersson, B.S.; Tarrand, J.; Valdez, B.C. Azole Pharmaceutical Formulations for Parenteral Administration and Methods for Preparing and Using the Same as Treatment of Diseases Sensitive to Azole Compounds. U.S. Patent 2,019,240,216, 16 December 2010. [Google Scholar]
- Subedi, L.; Song, S.-Y.; Jha, S.K.; Lee, S.-H.; Pangeni, R.; Koo, K.-T.; Kim, B.J.; Cho, S.-S.; Park, J.W. Preparation of Topical Itraconazole with Enhanced Skin/Nail Permeability and In Vivo Antifungal Efficacy against Superficial Mycosis. Pharmaceutics 2021, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Leverett, C.A.; Sukuru, S.C.K.; Vetelino, B.C.; Musto, S.; Parris, K.; Pandit, J.; Loganzo, F.; Varghese, A.H.; Bai, G.; Liu, B.; et al. Design, Synthesis, and Cytotoxic Evaluation of Novel Tubulysin Analogues as ADC Payloads. ACS Med. Chem. Lett. 2016, 7, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, A.P.; Baum, B.J. Gene Therapy: Some History, Applications, Problems, and Prospects. Toxicol. Pathol. 2008, 36, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2022, 7, e10258. [Google Scholar] [CrossRef]
- Chellappan, S.K.; Hormann, R.E.; Shulman, I. Boron-Containing Diacylhydrazines. U.S. Patent 9,127,024, 8 September 2015. [Google Scholar]
- Romero-Aguilar, K.S.; Arciniega-Martínez, I.M.; Farfán-García, E.D.; Campos-Rodríguez, R.; Reséndiz-Albor, A.A.; Soriano-Ursúa, M.A. Effects of boron-containing compounds on immune responses: Review and patenting trends. Expert Opin. Ther. Patents 2019, 29, 339–351. [Google Scholar] [CrossRef]
- Hormann, R.E.H.; De Paul, S.M.; Hilfiker, R.; Rödel, E.; Shulman, I. Crystalline Diacylhydrazine and the Use Thereof. U.S. Patent 8,946,294, 3 February 2015. [Google Scholar]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/patent/search/family/055129476/publication/WO2017109028A1?q=WO%202017109028%20A1 (accessed on 10 July 2024).
- Sandini, S.; La Valle, R.; Deaglio, S.; Malavasi, F.; Cassone, A.; De Bernardis, F. A highly immunogenic recombinant and truncated protein of the secreted aspartic proteases family (rSap2t) of Candida albicans as a mucosal anticandidal vaccine. FEMS Immunol. Med Microbiol. 2011, 62, 215–224. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.J.; Edwards, J.E.; Yue, F.U. Vaccine Compositions and Methods for Treatment of Mucormycosis and Other Fungal Diseases. U.S. Patent 8,444,985, 21 May 2013. [Google Scholar]
- Stanford, F.A.; Voigt, K. Iron Assimilation during Emerging Infections Caused by Opportunistic Fungi with emphasis on Mucorales and the Development of Antifungal Resistance. Genes 2020, 11, 1296. [Google Scholar] [CrossRef]
- Tonmoy, M.I.Q.; Ahmed, S.F.; Hami, I.; Shakil, S.K.; Verma, A.K.; Hasan, M.; Al Reza, H.; Bahadur, N.M.; Rahaman, M.; Hossain, S. Identification of novel inhibitors of high affinity iron permease (FTR1) through implementing pharmacokinetics index to fight against black fungus: An in silico approach. Infect. Genet. Evol. 2022, 106, 105385. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Josef Wieser, K.; Arthur Pichler, K.; Andreas Hotter, K.; Ulrich Griesser, I.; Christoph Langes, I.; Christian Laschober, K. Pharmaceutical Compositions Containing a Crystalline Form of Posaconazole. U.S. Patent 8,563,555, 22 October 2013. [Google Scholar]
- Lykouras, M.; Fertaki, S.; Orkoula, M.; Kontoyannis, C. Sample Preparation of Posaconazole Oral Suspensions for Identification of the Crystal Form of the Active Pharmaceutical Ingredient. Molecules 2020, 25, 6032. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.X.; Shrestha, S.K.; Garneau-Tsodikova, S. Identification of Ebsulfur Analogues with Broad-Spectrum Antifungal Activity. ChemMedChem 2016, 11, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Heimbecher, S.K.; Pipkin, J.D.; Monteith, D. Posaconazole Intravenous Solution Formulations Stabilized by Substituted β-Cyclodextrin. Patent EP3391890B1, 25 August 2021. [Google Scholar]
- Chandrika, N.T.; Shrestha, S.K.; Ngo, H.X.; Garneau-Tsodikova, S. Synthesis and investigation of novel benzimidazole derivatives as antifungal agents. Bioorganic Med. Chem. 2016, 24, 3680–3686. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.A.; Mueller, S.W.; Rower, J.E.; Washburn, T.; Kiser, T.H. Evaluation of Sulfobutylether-β-Cyclodextrin Exposure in a Critically Ill Patient Receiving Intravenous Posaconazole While Undergoing Continuous Venovenous Hemofiltration. Antimicrob. Agents Chemother. 2015, 59, 6653–6656. [Google Scholar] [CrossRef] [PubMed]
- Ogura, H.; Tatsumi, Y.; Fukamizu, Y. Azolylmethylidenehydrazine Derivative and Use Thereof. U.S. Patent 8,022,072, 20 September 2011. [Google Scholar]
- Lv, Q.-Z.; Ni, T.-J.; Li, L.-P.; Li, T.; Zhang, D.-Z.; Jiang, Y.-Y. A New Antifungal Agent (4-phenyl-1,3-thiazol-2-yl) Hydrazine Induces Oxidative Damage in Candida albicans. Front. Cell. Infect. Microbiol. 2020, 10, 578956. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Woosley, L.N.; Messer, S.A.; Jones, R.N.; Castanheira, M. Significance of Molecular Identification and Antifungal Susceptibility of Clinically Significant Yeasts and Moulds in a Global Antifungal Surveillance Programme. Mycopathologia 2012, 174, 259–271. [Google Scholar] [CrossRef]
- Pannu, J.; McCarthy, A.; Martin, A.; Hamouda, T.; Ciotti, S.; Fothergill, A.; Sutcliffe, J. NB-002, a Novel Nanoemulsion with Broad Antifungal Activity against Dermatophytes, Other Filamentous Fungi, and Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 3273–3279. [Google Scholar] [CrossRef]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/patent/search/family/041669134/publication/US8722727B2?q=US%208722727%20B2 (accessed on 15 July 2024).
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/patent/search/family/062063223/publication/US2018325919A1?q=US%202018325919%20A1 (accessed on 20 July 2024).
- Lamoth, F.; Alexander, B.D. Antifungal Activities of SCY-078 (MK-3118) and Standard Antifungal Agents against Clinical Non-Aspergillus Mold Isolates. Antimicrob. Agents Chemother. 2015, 59, 4308–4311. [Google Scholar] [CrossRef]
- Mitsuyama, J.; Nomura, N.; Hashimoto, K.; Yamada, E.; Nishikawa, H.; Kaeriyama, M.; Kimura, A.; Todo, Y.; Narita, H. In Vitro and In Vivo Antifungal Activities of T-2307, a Novel Arylamidine. Antimicrob. Agents Chemother. 2008, 52, 1318–1324. [Google Scholar] [CrossRef]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef]
- Dow, G.S. Methods for the Treatment and Prevention of Lung Infections Caused by Gram-Positive Bacteria, Fungus, or Virus by Administration of Tafenoquine. U.S. Patent 11,633,391, 25 April 2023. [Google Scholar]
- Dow, G.; Smith, B. Tafenoquine exhibits broad spectrum antifungal activity at clinically relevant concentrations in vitro and decreases lung fungal burden in an invasive pulmonary model of Rhizopus in vivo. New Microbes New Infect. 2022, 45, 100964. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol. Ther. 2019, 195, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/patent/search/family/061831552/publication/US2019381038A1?q=US%202019381038%20A1 (accessed on 22 July 2024).
- Umemura, M.; Okamoto, M.; Nakayama, K.-I.; Sagane, K.; Tsukahara, K.; Hata, K.; Jigami, Y. GWT1 Gene Is Required for Inositol Acylation of Glycosylphosphatidylinositol Anchors in Yeast. J. Biol. Chem. 2003, 278, 23639–23647. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Dasgupta, A.; Chopra, S. Fosmanogepix. GPI-anchored wall transfer protein 1 (Gwt1) (fungal) inhibitor, Treatment of invasive fungal infections. Drugs Futur. 2021, 46, 253–271. [Google Scholar] [CrossRef]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/patent/search/family/049769718/publication/US9505735B2?q=US%209505735%20B2 (accessed on 25 July 2024).
- Lufton, M.; Bustan, O.; Eylon, B.; Shtifman-Segal, E.; Croitoru-Sadger, T.; Shagan, A.; Shabtay-Orbach, A.; Corem-Salkmon, E.; Berman, J.; Nyska, A.; et al. Living Bacteria in Thermoresponsive Gel for Treating Fungal Infections. Adv. Funct. Mater. 2018, 28, 1801581. [Google Scholar] [CrossRef]
- McLellan, C.A.; Whitesell, L.; King, O.D.; Lancaster, A.K.; Mazitschek, R.; Lindquist, S. Inhibiting GPI Anchor Biosynthesis in Fungi Stresses the Endoplasmic Reticulum and Enhances Immunogenicity. ACS Chem. Biol. 2012, 7, 1520–1528. [Google Scholar] [CrossRef]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/patent/search/family/065633645/publication/US11633434B2?q=US%2011633434%20B2 (accessed on 26 July 2024).
- Matejuk, A.; Leng, Q.; Begum, M.D.; Woodle, M.; Scaria, P.; Chou, S.-T.; Mixson, A. Peptide-based antifungal therapies against emerging infections. Drugs Futur. 2010, 35, 197. [Google Scholar] [CrossRef]
- Dal Mas, C.; Rossato, L.; Shimizu, T.; Oliveira, E.B.; da Silva Junior, P.I.; Meis, J.F.; Colombo, A.L.; Hayashi, M.A.F. Effects of the Natural Peptide Crotamine from a South American Rattlesnake on Candida auris, an Emergent Multidrug Antifungal Resistant Human Pathogen. Biomolecules 2019, 9, 205. [Google Scholar] [CrossRef]
- Souza, G.H.d.A.d.; Rossato, L.; de Oliveira, A.R.; Simionatto, S. Antimicrobial peptides against polymyxin-resistant Klebsiella pneumoniae: A patent review. World J. Microbiol. Biotechnol. 2023, 39, 86. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145. [Google Scholar] [CrossRef]
- de Ullivarri, M.F.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/patent/search/family/037891189/publication/US2010184696A1?q=US2010184696A1 (accessed on 28 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccin, I.D.; Salomé, T.M.; de Almeida de Souza, G.H.; da Costa Xavier, L.; Alves, I.A.; Lima, V.C.F.; Lucini, F.; Simionatto, S.; Rossato, L. New Therapeutic Options for Fusariosis: A Patent Review (2008–2023). J. Fungi 2025, 11, 463. https://doi.org/10.3390/jof11060463
Faccin ID, Salomé TM, de Almeida de Souza GH, da Costa Xavier L, Alves IA, Lima VCF, Lucini F, Simionatto S, Rossato L. New Therapeutic Options for Fusariosis: A Patent Review (2008–2023). Journal of Fungi. 2025; 11(6):463. https://doi.org/10.3390/jof11060463
Chicago/Turabian StyleFaccin, Izadora Dillis, Túlio Máximo Salomé, Gleyce Hellen de Almeida de Souza, Leonardo da Costa Xavier, Izabel Almeida Alves, Vanessa Castro Felix Lima, Fabíola Lucini, Simone Simionatto, and Luana Rossato. 2025. "New Therapeutic Options for Fusariosis: A Patent Review (2008–2023)" Journal of Fungi 11, no. 6: 463. https://doi.org/10.3390/jof11060463
APA StyleFaccin, I. D., Salomé, T. M., de Almeida de Souza, G. H., da Costa Xavier, L., Alves, I. A., Lima, V. C. F., Lucini, F., Simionatto, S., & Rossato, L. (2025). New Therapeutic Options for Fusariosis: A Patent Review (2008–2023). Journal of Fungi, 11(6), 463. https://doi.org/10.3390/jof11060463






