Histoplasmosis in Immunocompromised and Immunocompetent Patients in Guadeloupe
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Ethical Considerations
2.3. Inclusion and Exclusion Criteria
- Confirmed histoplasmosis: Diagnoses were based on the clinical presentation and microbiological confirmation (positive culture, PCR, or direct microscopic examination). In immunocompromised patients, the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria [11] were applied, incorporating host factors, clinical features, and mycological evidence. For immunocompetent individuals, diagnoses were established based on compatible clinical symptoms and definitive microbiological evidence, even in the absence of EORTC/MSG-defined host factors.
- Age ≥ 18 years.
2.4. Data Collection
- Demographics: Age, gender, and nationality.
- Socioeconomic factors and risk factors for exposure: Employment status, housing conditions, recent travel history, and substance abuse.
- Clinical presentation: Symptoms and severity criteria.
- Coinfections.
- Laboratory and imaging results.
- Patients’ HIV status and viral load, if applicable.
- Immunosuppressive conditions.
- Treatments received: Antifungals and steroids.
- Outcomes: Survival at 30 days and relapse within 3 months.
2.5. Diagnostic Modalities
2.6. Definitions
2.6.1. Risk Factors for Exposure
2.6.2. Social Vulnerability
2.6.3. Severe Disease
- Pulmonary failure: The need for mechanical ventilation or high-flow oxygen therapy;
- Renal failure: Acute kidney injury stage III, according to the KDIGO criteria, and/or the need for acute kidney replacement therapy;
- Hemodynamic failure: A mean arterial pressure < 65 mmHg, lactate levels > 2 mmol/L, and/or the need for inotropic and/or vasopressor support (e.g., norepinephrine or dobutamine);
- Hematological failure: Disseminated intravascular coagulation (DIC), defined by a quick test (QT) result < 50% and platelet count < 50 G/L, and/or macrophage activation syndrome (MAS) requiring specific treatment (e.g., steroids or etoposide);
- Hepatic failure: A Factor V < 50% or symptomatic hypoglycemia requiring intravenous glucose;
- Neurological failure: A Glasgow Coma Scale score <8 or a persistent neurological deficit;
2.6.4. Use of Steroids in Histoplasmosis Treatment
2.6.5. Clinical Outcomes and Relapse
2.6.6. Statistical Analysis
3. Results
3.1. General Data
3.2. Immunosuppression
3.3. Environmental Exposure
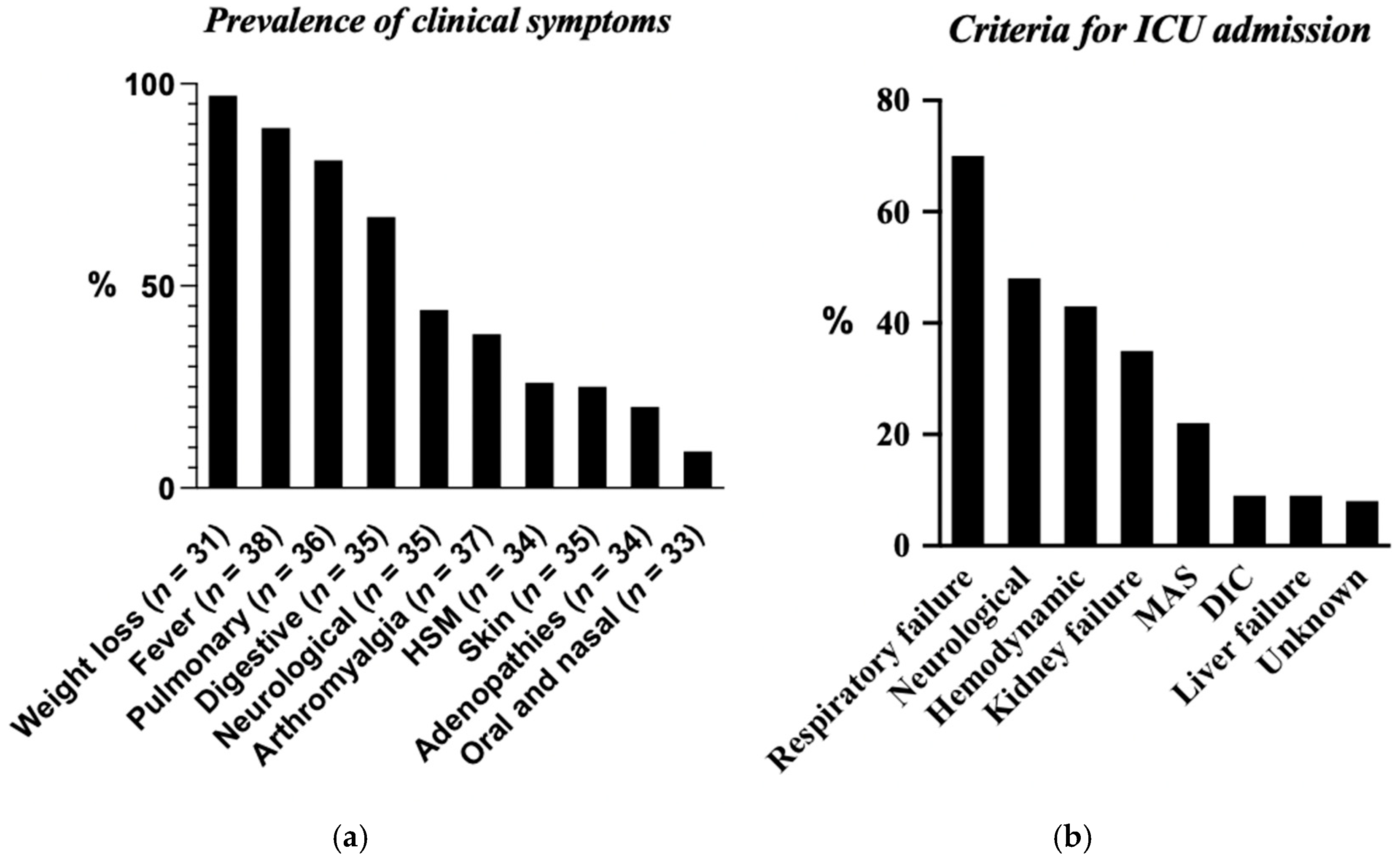
3.4. Clinical Symptoms
3.5. Biological Parameters
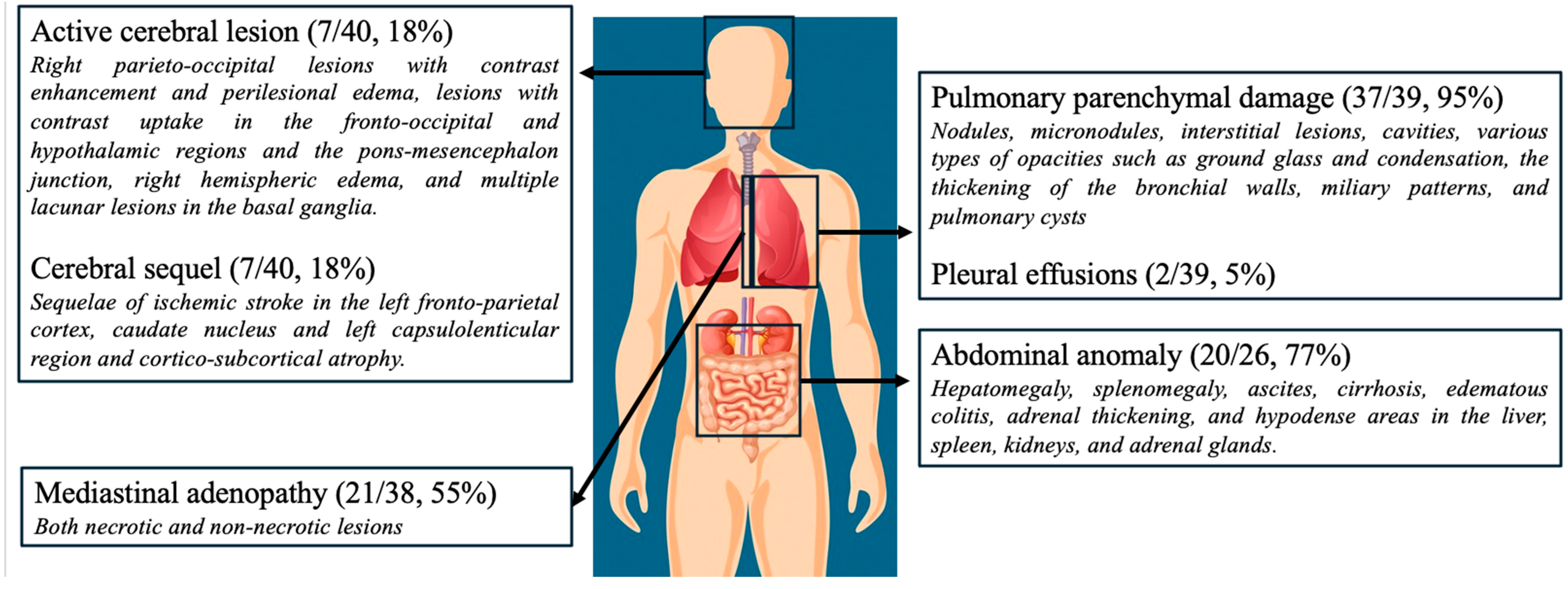
3.6. Imaging Data
3.7. Microbial Results
3.8. Initial Therapeutic Strategies
3.9. Maintenance Therapy and Follow-Up
3.10. Comparison of Most Relevant Results Across Three Immunosuppression Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| ATS | American Thoracic Society |
| AST | aspartate aminotransferase |
| aPTT | activated partial thromboplastin time |
| BAL | bronchoalveolar lavage |
| CMV | cytomegalovirus |
| CNS | central nervous system |
| CRP | C-reactive protein |
| CPK | creatinine phosphokinase |
| DIC | disseminated intravascular coagulation |
| EBV | Epstein–Barr virus |
| eGFR | estimated glomerular filtration rate |
| GGT | gamma-glutamyl transferase |
| HTLV1 | human T-lymphotropic virus 1 |
| HSM | hepato-splenomegaly |
| ICU | intensive care unit |
| IRIS | immune reconstitution inflammatory syndrome |
| LDH | lactate dehydrogenase |
| MAS | macrophage activation syndrome |
| NA | not applicable |
| PCR | polymerase chain reaction |
| QT | quick test |
| SD | standard deviation |
| SOT | solid organ transplant |
| WBC | white blood cell |
| HHV8 | human herpesvirus 8 |
Appendix A
| Viral reactivation |
|
| Bacterial and mycobacterial |
|
| Viral disease |
|
| Fungal |
|
| Parasitic |
|
References
- Barros, N.; Wheat, J.L.; Hage, C. Pulmonary histoplasmosis: A clinical update. J. Fungi 2023, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.L.; Reyes-Montes, M.d.R.; Estrada-Bárcenas, D.A.; Zancopé-Oliveira, R.M.; Rodríguez-Arellanes, G.; Ramírez, J.A.; Vives, M. Considerations about the geographic distribution of Histoplasma species. Appl. Environ. Microbiol. 2022, 88, e02010-21. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Couppié, P.; Epelboin, L.; Djossou, F.; Demar, M.; Adenis, A.; Chowdhary, A. Disseminated histoplasmosis: Fighting a neglected killer of patients with advanced HIV disease in Latin America. PLoS Pathog. 2020, 16, e1008449. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Neglected Histoplasmosis in Latin America Group. Disseminated histoplasmosis in Central and South America, the invisible elephant: The lethal blind spot of international health organizations. AIDS 2016, 30, 167–170. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Mariette, X.; Silva, J.T.; Benamu, E.; Calabrese, L.H.; Dumusc, A.; Smolen, J.S.; Aguado, J.M.; Fernández-Ruiz, M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (Soluble immune effector molecules [II]). Clin. Microbiol. Infect. 2018, 24 (Suppl. S2), S21–S40. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Chiller, T.M. Fungal infections and new biologic therapies. Curr. Rheumatol. Rep. 2016, 18, 29. [Google Scholar] [CrossRef]
- Moriane, E.; Pitot, S. Atlas des Populations Immigrées en Guadeloupe; INSEE: Paris, France, 2006. [Google Scholar]
- Garsaud, P.; Boisseau-Garsaud, A.M.; Desbois, N.; Verneuil, L.; Calès-Quist, D.; Hélénon, R.; Jouannelle, A.; Delord, J.M.; Sobesky, G.; Panelatti, G. Epidemiology of histoplasmosis in the French West Indies (Martinique). Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 264–267. [Google Scholar] [CrossRef]
- Nacher, M.; Adenis, A.; Mc Donald, S.; Gomes, M.D.S.M.; Singh, S.; Lima, I.L.; Leite, R.M.; Hermelijn, S.; Wongsokarijo, M.; Van Eer, M.; et al. Disseminated histoplasmosis in HIV-infected patients in South America: A neglected killer continues on its rampage. PLoS Negl. Trop. Dis. 2013, 7, e2319. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the EORTC/MSG Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Benedict, K.; Mody, R.K. Epidemiology of histoplasmosis outbreaks, United States, 1938–2013. Emerg. Infect. Dis. 2016, 22, 370–378. [Google Scholar] [CrossRef]
- Diaz, J.H. Environmental and wilderness-related risk factors for histoplasmosis: More than bats in caves. Wilderness Environ. Med. 2018, 29, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Li, Z.; Moqueet, N.; Moghadas, S.M.; Galvani, A.P.; Cooper, L.A.; Stranges, S.; Haworth-Brockman, M.; Pinto, A.D.; Asaria, M.; et al. Incorporating social determinants of health in infectious disease models: A systematic review of guidelines. Med. Decis. Making 2024, 44, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Kosmidis, C.; Rozaliyani, A.; Wahyuningsih, R.; Denning, D.W. Chronic pulmonary histoplasmosis—A scoping literature review. Open Forum Infect. Dis. 2020, 7, ofaa119. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization; World Health Organization. Diagnosing and Managing Disseminated Histoplasmosis Among People Living with HIV; PAHO; WHO: Washington, DC, USA, 2020. [Google Scholar]
- Franklin, A.D.; Larson, L.; Rauseo, A.M.; Rutjanawech, S.; Hendrix, M.J.; Powderly, W.G.; Spec, A. A comparison of presentations and outcomes of histoplasmosis across patients with varying immune status. Med. Mycol. 2021, 59, myaa112. [Google Scholar] [CrossRef]
- Casadevall, A. Immunity to invasive fungal diseases. Annu. Rev. Immunol. 2022, 40, 121–141. [Google Scholar] [CrossRef]
- American Thoracic Society. An official ATS statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am. J. Respir. Crit. Care Med. 2009, 180, 96–128. [Google Scholar] [CrossRef]
- Myint, T.; Anderson, A.M.; Sanchez, A.; Farabi, A.; Hage, C.; Baddley, J.W.; Jhaveri, M.; Greenberg, R.N.; Bamberger, D.M.; Rodgers, M.; et al. Histoplasmosis in patients with HIV/AIDS: Multicenter study of outcomes and factors associated with relapse. Medicine 2014, 93, e29. [Google Scholar] [CrossRef]
- Chiaruzzi, M.; Adenis, A.; Bonifay, T.; Djossou, F.; Bretagne, S.; Lanternier, F.; Epelboin, L.; France, O. Épidémiologie des infections à Histoplasma capsulatum diagnostiquées en France métropolitaine: Étude transversale multicentrique 2007–2018. Med. Mal. Infect. 2020, 50, S5. [Google Scholar] [CrossRef]
- Adenis, A.; Nacher, M.; Hanf, M.; Vantilcke, V.; Boukhari, R.; Blachet, D.; Demar, M.; Aznar, C.; Carme, B.; Couppie, P. HIV-associated histoplasmosis early mortality and incidence trends: From neglect to priority. PLoS Negl. Trop. Dis. 2014, 8, e3100. [Google Scholar] [CrossRef]
- Shankar, J.; Restrepo, A.; Clemons, K.V.; Stevens, D.A. Hormones and the resistance of women to paracoccidioidomycosis. Clin. Microbiol. Rev. 2011, 24, 296–313. [Google Scholar] [CrossRef]
- Vantilcke, V.; Boukhari, R.; Jolivet, A.; Vautrin, C.; Misslin, C.; Adenis, A.; Nacher, M. Fever in hospitalized HIV-infected patients in Western French Guiana: First think histoplasmosis. Int. J. STD AIDS 2014, 25, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Masson, E. Lymphopénie T CD4+ idiopathique: Revue systématique de la littérature. La Rev. Méd. Interne 2016, 37, A84. [Google Scholar] [CrossRef]
- Nacher, M.; Valdes, A.; Adenis, A.; Blaizot, R.; Abboud, P.; Demar, M.; Djossou, F.; Epelboin, L.; Drak Alsibai, K.; Misslin, C.; et al. Heterogeneity of clinical presentations and paraclinical explorations to diagnose disseminated histoplasmosis in patients with advanced HIV: 34 years of experience in French Guiana. J. Fungi 2020, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Couppié, P.; Herceg, K.; Bourne-Watrin, M.; Thomas, V.; Blanchet, D.; Alsibai, K.D.; Louvel, D.; Djossou, F.; Demar, M.; Blaizot, R.; et al. The broad clinical spectrum of disseminated histoplasmosis in HIV-infected patients: A 30 years’ experience in French Guiana. J. Fungi 2019, 5, 115. [Google Scholar] [CrossRef]
- Batista, J.M.; Martins, M.A.P.; Bertollo, C.M. Primary cutaneous histoplasmosis difficult to treat in an immunocompetent patient: Case report and literature review. Einstein 2021, 19, eRC5488. [Google Scholar] [CrossRef]
- Sepúlveda, V.E.; Márquez, R.; Turissini, D.A.; Goldman, W.E.; Matute, D.R.; Taylor, J.W. Genome sequences reveal cryptic speciation in the human pathogen Histoplasma Capsulatum. MBio 2017, 8, 10–128. [Google Scholar] [CrossRef]
- Nguyen, D.; Nacher, M.; Epelboin, L.; Melzani, A.; Demar, M.; Blanchet, D.; Blaizot, R.; Alsibai, K.D.; Abboud, P.; Djossou, F.; et al. Hemophagocytic lymphohistiocytosis during HIV infection in Cayenne Hospital 2012–2015: First think histoplasmosis. Front. Cell Infect. Microbiol. 2020, 10, 574584. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 10–128. [Google Scholar] [CrossRef]
- Caceres, D.H.; Knuth, M.; Derado, G.; Lindsley, M.D. Diagnosis of progressive disseminated histoplasmosis in advanced HIV: A meta-analysis of assay analytical performance. J. Fungi 2019, 5, 76. [Google Scholar] [CrossRef]
- Hove, M.G.; Woods, G.L. Duration of fungal culture incubation in an area endemic for Histoplasma capsulatum. Diagn. Microbiol. Infect. Dis. 1997, 28, 41–43. [Google Scholar] [CrossRef]
- Connolly, P.A.; Durkin, M.M.; LeMonte, A.M.; Hackett, E.J.; Wheat, L.J. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 2007, 14, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Françoise, U.; Nacher, M.; Bourne-Watrin, M.; Epelboin, L.; Thorey, C.; Demar, M.; Carod, J.-F.; Djossou, F.; Couppié, P.; Adenis, A. Development of a case fatality prognostic score for HIV-associated histoplasmosis. Int. J. Infect. Dis. 2023, 132, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ma, J.; Saliba, F.; Dupont, B.; Wingard, J.R.; Hachem, R.Y.; Mattiuzzi, G.N.; Chandrasekar, P.H.; Kontoyiannis, D.P.; Rolston, K.V.; et al. Drug-induced nephrotoxicity caused by amphotericin B lipid complex and liposomal amphotericin B: A review and meta-analysis. Medicine 2010, 89, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Breton, G.; Adle-Biassette, H.; Therby, A.; Ramanoelina, J.; Choudat, L.; Bissuel, F.; Huerre, M.; Dromer, F.; Dupont, B.; Lortholary, O. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. AIDS 2006, 20, 119–121. [Google Scholar] [CrossRef]
- Melzani, A.; Michel, R.d.R.d.S.; Ntab, B.; Djossou, F.; Epelboin, L.; Nacher, M.; Blanchet, D.; Demar, M.; Couppie, P.; Adenis, A. Incidence and trends in immune reconstitution inflammatory syndrome associated with Histoplasma capsulatum among people living with HIV: A 20-year case series and literature review. Clin. Infect. Dis. 2020, 70, 643–652. [Google Scholar] [CrossRef]
- Li, Z.; Denning, D.W. The impact of corticosteroids on the outcome of fungal disease: A systematic review and meta-analysis. Curr. Fungal Infect. Rep. 2023, 17, 54–70. [Google Scholar] [CrossRef]
- Adenis, A.A.; Valdes, A.; Cropet, C.; McCotter, O.Z.; Derado, G.; Couppie, P.; Chiller, T.; Nacher, M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: A modelling study. Lancet Infect. Dis. 2018, 18, 1150–1159. [Google Scholar] [CrossRef]
| Biological Parameters | Overall Cohort (N = 42) | HIV-Positive (N = 20) | HIV-Negative, with Other Cause of Immunosuppression (N = 12) | HIV-Negative, Presumed Immunocompetent (N = 10) |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 2 | 2 | 2 | |
| Platelets (G/L) | 120 | 105 | 80 | 140 |
| WBCs (G/L) | 2.5 | 3 | 2 | 2 |
| Lymphocytes (G/L) | 0.6 | 0.5 | 0.5 | 0.7 |
| Neutrophils (G/L) | 2 (n = 41) | 2 | 2 (n = 11) | |
| QT (%) | 15 (n = 41) | 18 | 12 (n = 11) | 12 |
| aPTT | 0.3 (n = 41) | 0.3 | 0.3 (n = 11) | 0.1 |
| AST | 11 (n = 41) | 16 | 2.4 (n = 11) | 0.8 |
| ALT | 3 (n = 41) | 4 | 1 (n = 11) | 1.7 |
| GGT | 4 (n = 41) | 5 | 3 (n = 11) | 4 |
| ALP | 12 (n = 41) | 17 | 1.4 (n = 11) | 1.5 |
| mol/L) | 16.5 (n = 41) | 20 | 12 (n = 11) | 14 |
| CRP (mg/L) | 100 | 118 | 57 | 107 |
| eGFR (ml/min) | 32 (n = 41) | 31 (n = 19) | 32 | 28 |
| CPK | 0.5 (n = 34) | 0.7 (n = 15) | 0.3 (n = 11) | 0 (n = 8) |
| Albuminemia (g/L) | 8 (n = 38) | 7 (n = 18) | 10 (n = 8) | |
| LDH | 6 (n = 37) | 7 (n = 19) | 1.4 (n = 10) | 0.7 (n = 8) |
| Triglycerides (mmol/L) | 4 (n = 31) | 1 (n = 18) | 7.6 (n = 9) | 1.3 (n = 4) |
| Natremia (mmol/L) | 4 | 4 | ||
| Ferritinemia (ng/mL) | 17,120 (n = 36) | 17,008 (n = 19) | 16,852 (n = 11) | 1061 (n = 6) |
| Fibrinogen (g/L) | 2 (n = 32) | 1.7 (n = 16) | 1.8 (n = 9) | 1.7 (n = 7) |
| Microbial Results | Total n/N (%) | Specimen-Level Positivity (n/N, %) | Multiple Positive Samples in a Single Patient |
|---|---|---|---|
| Direct microscopy | 31/50 (62%) |
|
|
| Culture | 75/111 (68%) |
|
|
| PCR | 14/24 (58%) |
|
|
| Serum GM | 6/25 (24%) | - | - |
| Histoplasma serology | 23/29 (79%) | - | - |
| Contamination with other molds observed in direct examination or culture results | 17/92 (18%) |
| - |
| HIV-Positive (n = 20) | HIV-Negative, with Other Cause of Immunosuppression (n = 12) | HIV-Negative, Presumed Immunocompetent (n = 10) | |
|---|---|---|---|
| Age (years) | |||
| Sex (M/F) | 16 (80%)/4 (20%) | 7 (58%)/5 (42%) | 7 (70%)/3 (30%) |
| Social vulnerability | 16 (80%) | 1 (8%) | 1 (10%) |
| Severity criteria | 18 (90%) | 9 (75%) | 4 (40%) |
| ICU admission | 18 (90%) | 11 (92%) | 4 (40%) |
| Coinfections (per patient) | n = 18) | n = 10) | n = 9) |
| Positive BAL | 14 (70%) | 9 (75%) | 9 (90%) |
| Positive bone marrow cultures | 16 (80%) | 9 (75%) | 3 (30%) |
| Positive blood cultures | 15 (75%) | 6 (50%) | 2 (20%) |
| Positive direct examination | 13 (65%) | 11 (92%) | 4 (40%) |
| Positive cultures (any site) | 18 (90%) | 11 (92%) | 8 (80%) |
| Distinct positive culture samples | |||
| Liposomal amphotericin B as 1st-line therapy | 18 (90%) | 12 (100%) | 9 (90%) |
| Concurrent steroid use | 12 (60%) | 10 (83%) | 9 (90%) |
| Mortality | 8 (40%) | 2 (17%) | 2 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahuna, C.; Dequidt, T.; Postel-Vinay, P.; Peugny, S.; Haboub, M.; Markowicz, S.; Nicolas, M. Histoplasmosis in Immunocompromised and Immunocompetent Patients in Guadeloupe. J. Fungi 2025, 11, 462. https://doi.org/10.3390/jof11060462
Lahuna C, Dequidt T, Postel-Vinay P, Peugny S, Haboub M, Markowicz S, Nicolas M. Histoplasmosis in Immunocompromised and Immunocompetent Patients in Guadeloupe. Journal of Fungi. 2025; 11(6):462. https://doi.org/10.3390/jof11060462
Chicago/Turabian StyleLahuna, Constance, Tanguy Dequidt, Pierre Postel-Vinay, Sandrine Peugny, Marwan Haboub, Samuel Markowicz, and Muriel Nicolas. 2025. "Histoplasmosis in Immunocompromised and Immunocompetent Patients in Guadeloupe" Journal of Fungi 11, no. 6: 462. https://doi.org/10.3390/jof11060462
APA StyleLahuna, C., Dequidt, T., Postel-Vinay, P., Peugny, S., Haboub, M., Markowicz, S., & Nicolas, M. (2025). Histoplasmosis in Immunocompromised and Immunocompetent Patients in Guadeloupe. Journal of Fungi, 11(6), 462. https://doi.org/10.3390/jof11060462






