Streptomyces-Based Bioformulation to Control Wilt of Morchella sextelata Caused by Pestalotiopsis trachicarpicola
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening of Antagonistic Actinomycetes
2.2. Identification of Antagonistic Actinomycetes
2.3. Assessment of Antimicrobial Efficacy of Volatile Compounds
2.4. Suppression of Conidial Germination in P. trachicarpicola by Fermentation Supernatant
2.5. Biocontrol of the Fermentation Filtrate of Antagonistic Actinomycetes Against P. trachicarpicola in M. sextelata Fruiting Bodies
2.6. Field Cultivation Assay
3. Results
3.1. Isolation of Antagonistic Actinomycetes
3.2. Identification and Biochemical Characteristics of Antagonistic Actinomycetes
3.3. Determination of Antifungal Effects of Volatile Substances from Isolates F16 and F19 Against P. trachicarpicola
3.4. Determination of the Inhibitory Effects of Fermentation Filtrates of Isolates F16 and F19 Against P. trachicarpicola
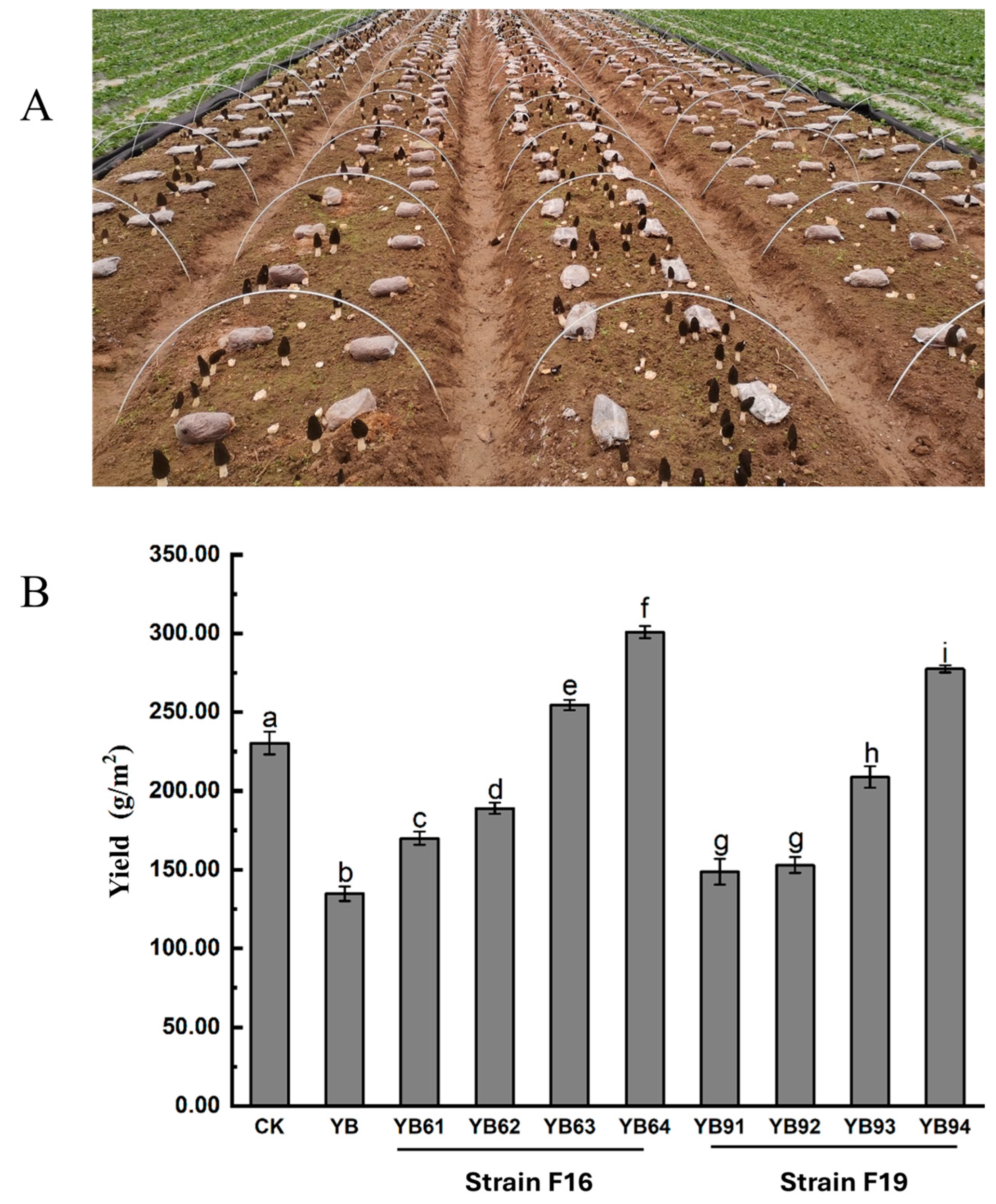
3.5. Field Experiment for Evaluating the Efficacy of the Isolates F16 and F19 on P. trachicarpicola in M. sextelata
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDA | Potato dextrose agar medium |
| NCBI | National Center of Biotechnology Information |
References
- Du, X.H.; Wu, D.; Kang, H.; Wang, H.; Xu, N.; Li, T.; Chen, K. Heterothallism and potential hybridization events inferred for twenty-two yellow morel species. IMA Fungus 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Zhu, Y.; Tang, Y.; Lu, N.; Song, J.L.; Yuan, W.D.; Jia, Y. Non-volatile taste components of different cultivated mushrooms at mycelia, primordium, and fruit body cultivation stages. Int. J. Food Prop. 2016, 19, 1938–1948. [Google Scholar] [CrossRef]
- Vieira Gomes, D.C.; de Alencar, M.V.O.B.; dos Reis, A.C.; de Lima, R.M.T.; de Oliveira Santos, J.V.; da Mata, A.M.O.F.; Soares Dias, A.C.; da Costa, J.S.; de Medeiros, M.d.G.F.; Paz, M.F.C.J.; et al. Antioxidant, anti-inflammatory and cytotoxic/antitumoral bioactives from the phylum Basidiomycota and their possible mechanisms of action. Biomed. Pharmacother. 2019, 112, 108643. [Google Scholar] [CrossRef]
- Shi, X.; Liu, D.; He, X.; Liu, W.; Yu, F. Epidemic identification of fungal diseases in morchella cultivation across China. J. Fungi 2022, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Liu, T.; Yu, Y.; Tang, J.; Jiang, L.; Martin Francis, M.; Peng, W. Morel production related to soil microbial diversity and evenness. Microbiol. Spectr. 2021, 9, e00229-21. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.P.; Chen, K.; Wang, G.Z.; Bian, Y.B. First report of stipe rot disease on morchella importuna caused by Fusarium incarnatum—F. equiseti species complex in China. Plant Dis. 2016, 100, 2530. [Google Scholar] [CrossRef]
- Lan, Y.F.; Cong, Q.Q.; Wang, Q.W.; Tang, L.N.; Li, X.M.; Yu, Q.W.; Cui, X.; An, X.R.; Yu, C.X.; Kong, F.H.; et al. First report of Cladobotryum protrusum causing cobweb disease on cultivated Morchella importuna. Plant Dis. 2019, 104, 977. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, T.; Liu, L.; Chen, Y.; Tang, J.; Peng, W.; Tan, H. Application of the mushroom volatile 1-octen-3-ol to suppress a morel disease caused by Paecilomyces penicillatus. Appl. Microbiol. Biotechnol. 2022, 106, 4787–4799. [Google Scholar] [CrossRef]
- He, X.L.; Peng, W.H.; Miao, R.Y.; Tang, J.; Chen, Y.; Liu, L.X.; Wang, D.; Gan, B.C. White mold on cultivated morels caused by Paecilomyces penicillatus. FEMS Microbiol. Lett. 2017, 364, fnx037. [Google Scholar] [CrossRef]
- He, P.; Li, C.; Cai, Y.; Zhang, Y.; Bian, Y.; Liu, W. First report of pileus rot disease on cultivated Morchella importuna caused by Diploöspora longispora in China. J. Gen. Plant Pathol. 2018, 84, 65–69. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, Y.; Chen, X.; Song, L.; Chen, Y.; Lv, B. First report of Aspergillus niger causing rot of Morchella sextelata in China. Plant Dis. 2024, 108, 804. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, Y.; Zhao, K.; Li, L.; Jiang, S. First report of Pestalotiopsis trachicarpicola causing apothecium wither on cultivated Morchella sextelata in Sichuan, China. Plant Dis. 2023, 107, 3631. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef] [PubMed]
- João Frederico, M.; dos Passos, P.B.D.C.; Murilo, D.; Costa, G.R.; Zaffari, G.N.; José Itamar Boneti, A.M.R.; de Oliveira, L.M.P. Cultivable bacteria isolated from apple trees cultivated under different crop systems: Diversity and antagonistic activity against Colletotrichum gloeosporioides. Genet. Mol. Biol. 2014, 37, 560–572. [Google Scholar]
- Bhat, P.S.; Kumar, N.R.P.; Ranganath, H.R.; Saroja, S. Pests and their management in cucurbits. In Trends in Horticultural Entomology; Mani, M., Ed.; Springer Nature: Singapore, 2022; pp. 1013–1030. [Google Scholar]
- Bondarenko, S.V.; Stankevych, S.V.; Matsyura, A.V. Major cucumber diseases and the crop immunity. Ukr. J. Ecol. 2021, 11, 46–54. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Lv, Y.; Zhao, L.; Jiang, W.; Lv, J.; Xu, X.; Yu, Y.; Liu, Y.; Chen, X.; et al. Screening and identification of Paenibacillus polymyxa GRY-11 and its biological control potential against apple replant disease. Folia Microbiol. 2024, 70, 475–487. [Google Scholar] [CrossRef]
- Yang, D.; Luo, J.; Zhou, Y.; Zhou, S.; Liu, X.; Liu, C. Identification and biological characterization of pathogen causing sooty blotch of Ardisia crispa (Thunb.) A.DC. PeerJ 2025, 13, e19130. [Google Scholar] [CrossRef]
- Conville Patricia, S.; Brown-Elliott Barbara, A.; Smith, T.; Zelazny Adrian, M. The complexities of Nocardia taxonomy and identification. J. Clin. Microbiol. 2017, 56, 10–1128. [Google Scholar]
- Zhu, X.; Ma, K.; Sun, M.; Zhang, J.; Liu, L.; Niu, S. Isolation and identification of pathogens of Morchella sextelata bacterial disease. Front. Microbiol. 2023, 14, 1231353. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Sun, L.; Bonito, G.; Guo, Y.; Li, Y.; Fu, Y. Genome sequencing of Paecilomyces penicillatus provides insights into its phylogenetic placement and mycoparasitism mechanisms on morel mushrooms. Pathogens 2020, 9, 834. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Chao, S.; Song, L.; Wu, G.; Sun, Y.; Chen, Y.; Lv, B. Biocontrol potential of endophytic Bacillus subtilis A9 against rot disease of Morchella esculenta. Front. Microbiol. 2024, 15, 1388669. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Geng, X.; Cai, Y.; Yu, Y.; Hou, Y.; Liu, Y.; Ya, C.; Liu, Q. Identification, fermentation optimization, and biocontrol efficacy of actinomycete YG-5 for the prevention of Alternaria leaf spot disease in star anise. Sci. Rep. 2024, 14, 18621. [Google Scholar] [CrossRef]
- Zhong, J.; Sui, W.W.; Bai, X.Y.; Qiu, Z.L.; Li, X.G.; Zhu, J.Z. Characterization and biocontrol mechanism of Streptomyces olivoreticuli as a potential biocontrol agent against Rhizoctonia solani. Pestic. Biochem. Physiol. 2023, 197, 105681. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a promising biological control agents for plant pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef]
- Sudha, A.; Durgadevi, D.; Archana, S.; Muthukumar, A.; Suthin Raj, T.; Nakkeeran, S.; Poczai, P.; Nasif, O.; Ansari, M.J.; Sayyed, R.Z. Unraveling the tripartite interaction of volatile compounds of Streptomyces rochei with grain mold pathogens infecting sorghum. Front. Microbiol. 2022, 13, 923360. [Google Scholar] [CrossRef]
- Liu, W.; He, P.; Zhang, J.; Wu, L.; Er, L.; Shi, X.; Gu, Z.; Yu, F.; Pérez-Moreno, J. Ultrastructure and physiological characterization of Morchella mitospores and their relevance in the understanding of the morel life cycle. Microorganisms 2023, 11, 345. [Google Scholar] [CrossRef]
- Shao, J.; Pei, Z.; Jing, H.; Wang, L.; Jiang, C.; Du, X.; Jiang, C.; Lou, Z.; Wang, H. Antifungal activity of myriocin against Fusarium graminearum and its inhibitory effect on deoxynivalenol production in wheat grains. Physiol. Mol. Plant Pathol. 2021, 114, 101635. [Google Scholar] [CrossRef]
- Kamil, F.H.; Saeed, E.E.; El-Tarabily, K.A.; AbuQamar, S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using Streptomycete and non-streptomycete Actinobacteria in the united arab emirates. Front. Microbiol. 2018, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Šantrić, L.; Ivana, P.; Ljiljana, R.; Gajić, U.J.; Emil, R.; Bojan, D.; Svetlana, M. Impact of a native Streptomyces flavovirens from mushroom compost on green mold control and yield of Agaricus bisporus. J. Environ. Sci. Health Part B 2018, 53, 677–684. [Google Scholar] [CrossRef] [PubMed]
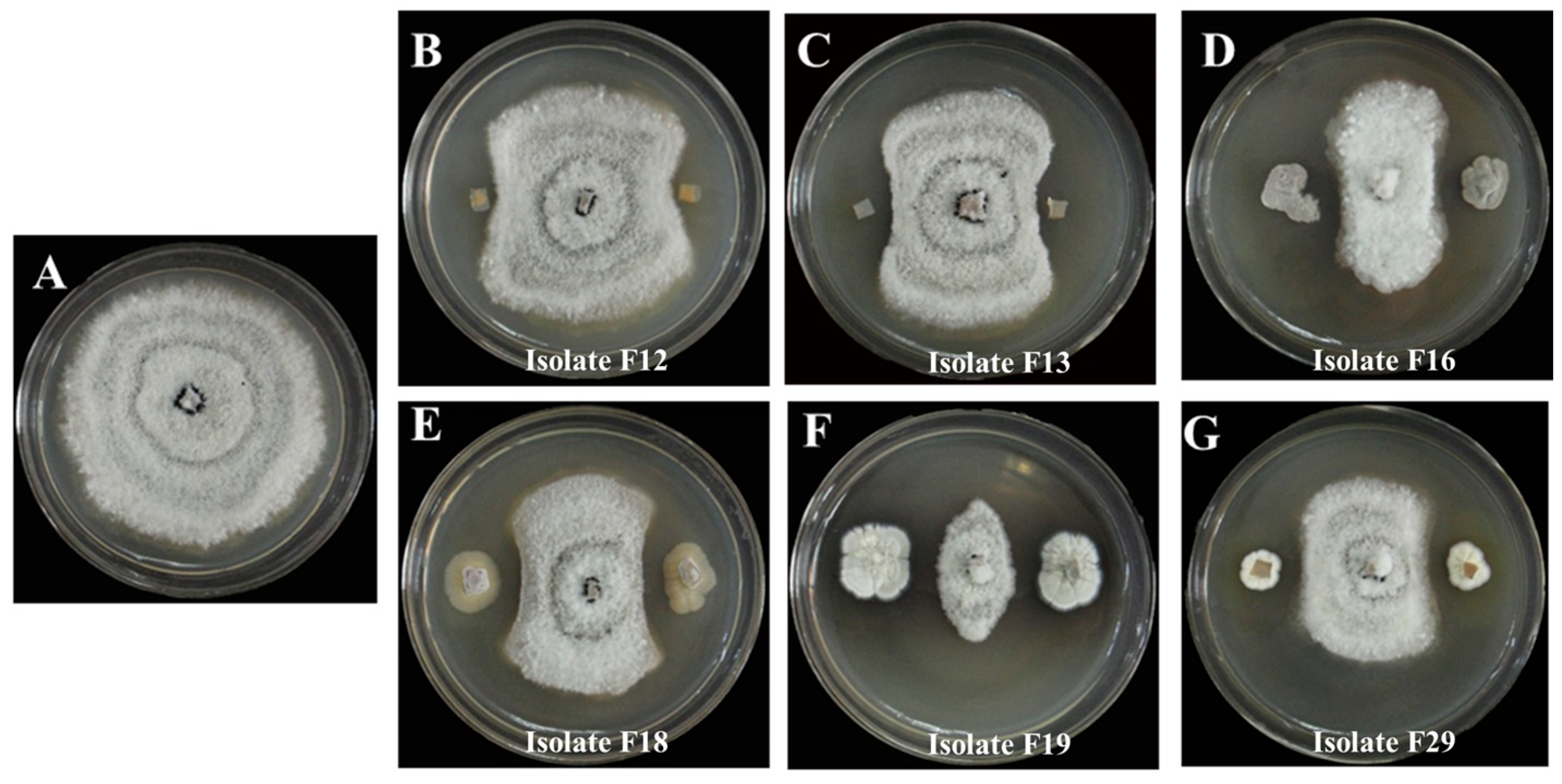
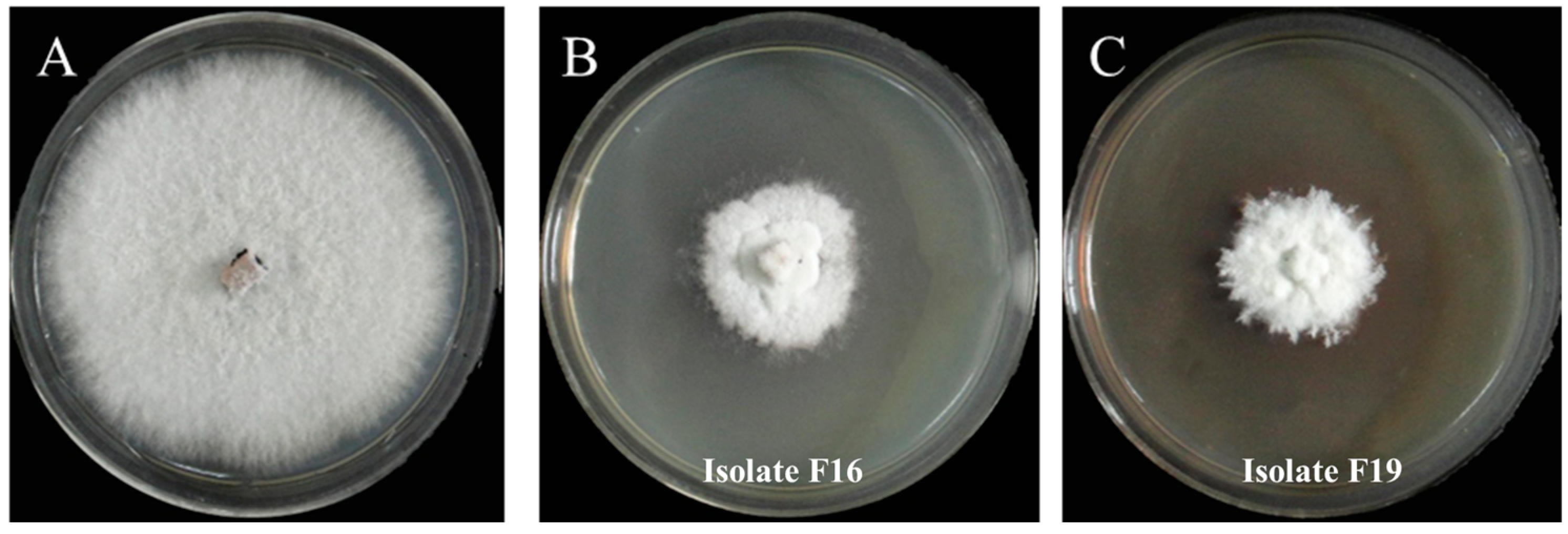

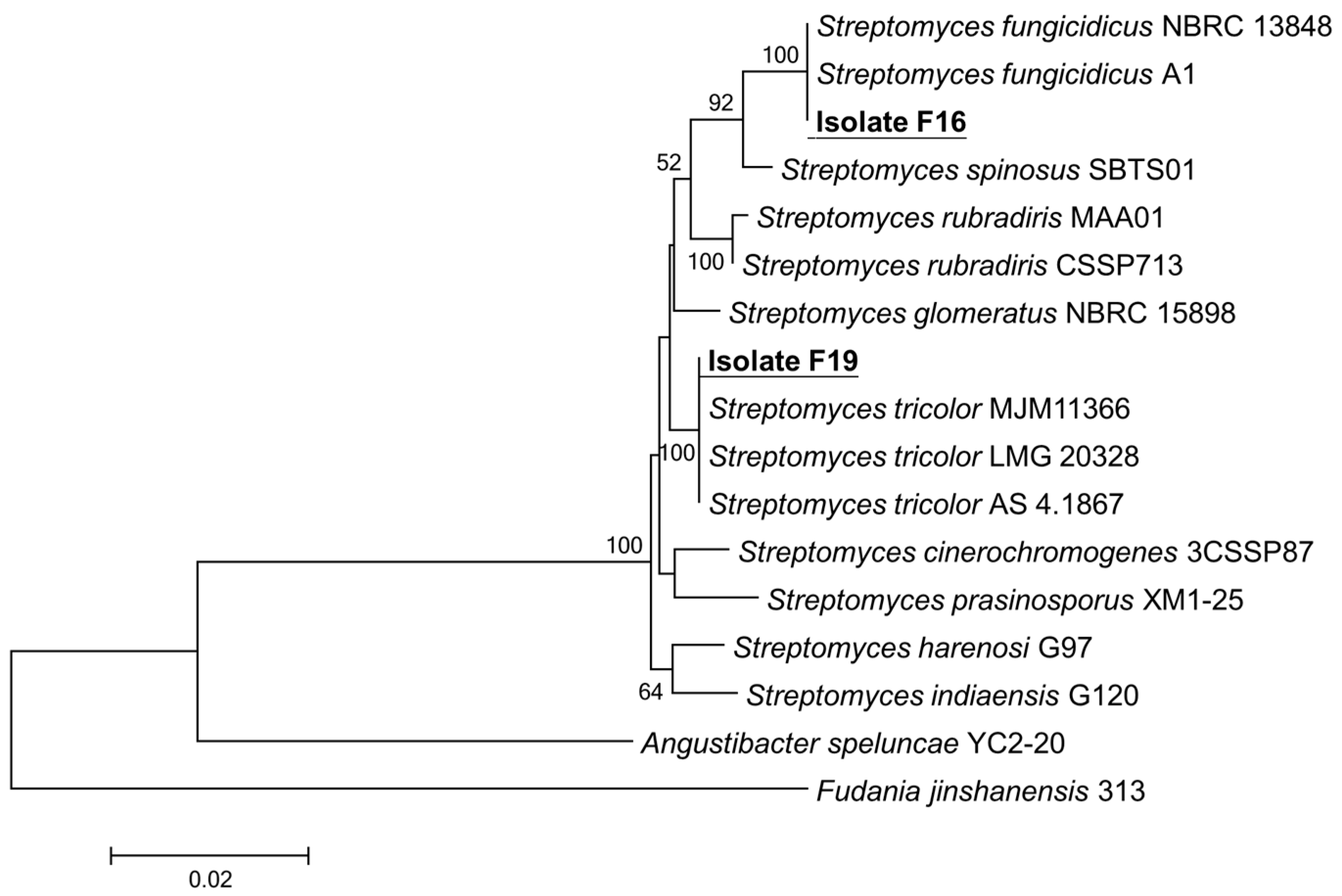
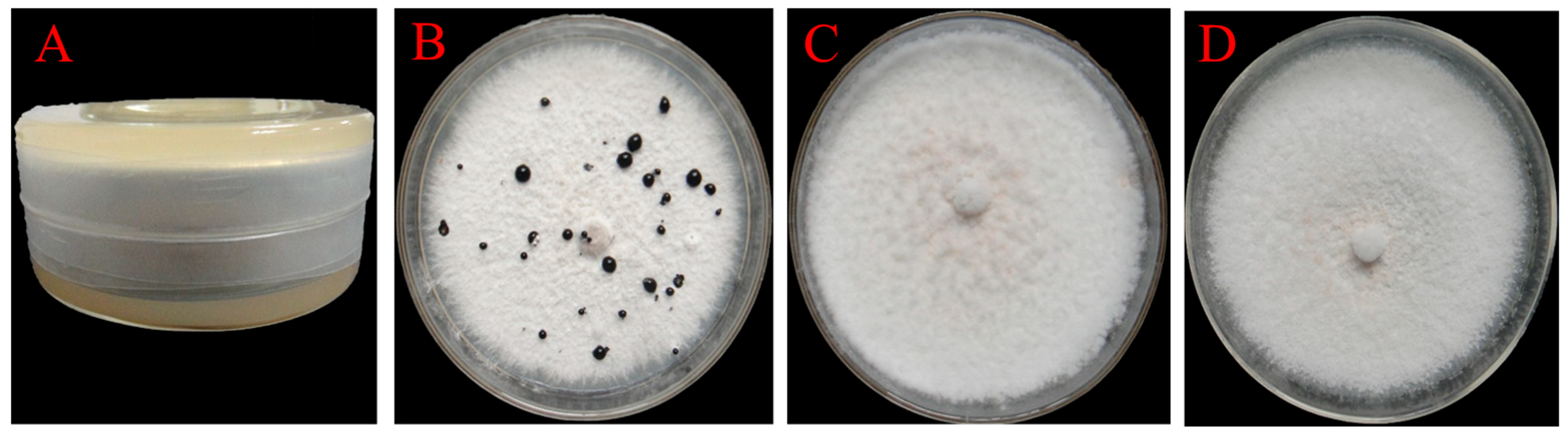


| Isolate Number | Colony Diameter (cm) | Antifungal Rate (%) |
|---|---|---|
| CK | 8.42 ± 0.08 a | — |
| F12 | 6.47 ± 0.15 b | 24.66% ± 0.02 a |
| F13 | 6.87 ± 0.18 c | 19.61% ± 0.02 b |
| F16 | 3.47 ± 0.15 d | 62.54% ± 0.02 c |
| F18 | 6.45 ± 0.15 b | 24.87% ± 0.02 a |
| F19 | 3.65 ± 0.14 e | 60.23% ± 0.02 d |
| F29 | 4.82 ± 0.12 f | 45.50% ± 0.01 e |
| Dilution Times | Inhibition Rate of Conidial Germination (%) | |
|---|---|---|
| Streptomyces sp. F16 | Streptomyces sp. F19 | |
| 0 | 99.81% ± 0.00 a | 97.86% ± 0.01 a |
| 5 | 99.22% ± 0.01 a | 94.94% ± 0.01 a |
| 10 | 88.91% ± 0.02 b | 79.96% ± 0.01 b |
| 20 | 78.40% ± 0.03 c | 66.73% ± 0.03 c |
| 50 | 66.93% ± 0.05 d | 49.22% ± 0.03 d |
| 100 | 36.96% ± 0.03 e | 21.21% ± 0.04 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Liu, Y.; Mao, A.; Hu, Z.; Li, J. Streptomyces-Based Bioformulation to Control Wilt of Morchella sextelata Caused by Pestalotiopsis trachicarpicola. J. Fungi 2025, 11, 452. https://doi.org/10.3390/jof11060452
Li B, Liu Y, Mao A, Hu Z, Li J. Streptomyces-Based Bioformulation to Control Wilt of Morchella sextelata Caused by Pestalotiopsis trachicarpicola. Journal of Fungi. 2025; 11(6):452. https://doi.org/10.3390/jof11060452
Chicago/Turabian StyleLi, Binghan, Yue Liu, Aihua Mao, Zhong Hu, and Jin Li. 2025. "Streptomyces-Based Bioformulation to Control Wilt of Morchella sextelata Caused by Pestalotiopsis trachicarpicola" Journal of Fungi 11, no. 6: 452. https://doi.org/10.3390/jof11060452
APA StyleLi, B., Liu, Y., Mao, A., Hu, Z., & Li, J. (2025). Streptomyces-Based Bioformulation to Control Wilt of Morchella sextelata Caused by Pestalotiopsis trachicarpicola. Journal of Fungi, 11(6), 452. https://doi.org/10.3390/jof11060452





