Abstract
The fungus Alternaria alternata, which causes tobacco brown spot disease, poses a serious threat to the tobacco industry. Beneficial microorganisms and their secondary metabolites have emerged as a promising green strategy for disease management. This study recovered 16 endophytic bacterial strains from Mentha haplocalyx Briq., a therapeutic herb. The study revealed that strain B5, with an inhibition rate of 82.76%, exhibited the highest antifungal activity against A. alternata. This strain exhibited broad-spectrum antifungal activity, with inhibition rates ranging from 66.34% to 87.23%. Phylogenetic analysis of 16S rDNA and gyrA gene sequences identified it as Bacillus velezensis (GenBank: PV168970 and PV173738). Further characterization revealed that strain B5 can secrete cell wall-degrading enzymes, produce IAA, and synthesize siderophores. The growth of mycelium in A. alternata was greatly reduced by both the ethyl acetate extract and the filtered liquid from the sterile fermentation, resulting in marked morphological abnormalities. Multiple antifungal active substances were identified through liquid LC-MS analysis. Greenhouse experiments demonstrated that the B5 fermentation broth effectively suppressed the occurrence of tobacco brown spot disease, achieving a relative control efficacy of 60.66%, comparable to that of 10% difenoconazole water dispersible granule (WDG). Additionally, strain B5 enhances plant disease resistance by activating the activities of key defense enzymes. B. velezensis B5 serves as a safe alternative to chemical fungicides and is highly effective at controlling tobacco brown spot disease.
1. Introduction
Brown spot disease in tobacco (Nicotiana tabacum) is believed to be associated with the fungus Alternaria alternata. Throughout the tobacco plant’s growth cycle, this pathogen adversely impacts the plant by inducing pathological conditions, primarily in the leaves. In addition to causing leaf infections, A. alternata can further exacerbate plant health issues, affecting stems, peduncles, and capsules as the infestation progresses [1]. Within the realm of agricultural pathology, the species A. alternata is notorious for infecting a wide range of crops. It causes diseases that degrade crop quality and reduce yield, thereby incurring substantial economic losses [2].
In China, the majority of tobacco cultivation areas currently suffer from a lack of adequate expertise in disease prevention and management strategies among local cultivators. Tobacco makers have suffered large financial losses as a result of a rising incidence of tobacco brown spot disease caused by this deficit. Chemical control is the use of chemicals, such as insecticides and fungicides, to manage plant diseases and pests. Key chemicals used in this approach include fludioxonil and cyprodinil, among others [3]. Chemical control offers advantages, such as ease of preparation and rapid effectiveness [4]. However, it also has significant drawbacks, including the development of disease resistance, the disruption of soil microbial ecosystems, and pesticide residue issues that have not yet been adequately addressed by current technologies [5,6].
Due to the numerous drawbacks associated with chemical control methods, environmentally friendly and less health-threatening biological control strategies have emerged as a focal point in research aimed at managing tobacco brown spot disease. Biological control involves the utilization of beneficial organisms and their bioactive capabilities, including their mechanisms of action and natural interactions, to suppress the development of pathogenic species [7]. In this process, biocontrol agents, particularly antagonistic microorganisms, play a pivotal role by establishing dominant microbial communities that contribute to disease suppression.
Endophytic bacteria serve as potential biocontrol agents, with numerous studies documenting their ability to enhance plant growth and fortify plant health through various mechanisms [8,9]. These endophytes are essential components of the plant’s internal microbiota, capable of inducing the expression of defense-related genes, producing secondary metabolites that inhibit or eradicate pathogens, competing with pathogens for resources and spatial niches, and disrupting pathogen virulence [10]. During long-term coevolution, the microbiome of medicinal plants regulates the production of secondary metabolites. Their endophytes can synthesize antimicrobial compounds that effectively inhibit pathogens [11,12]. For example, Sahu et al. [13] isolated eight strains of Bacillus spp. from Ocimum tenuiflorum, all of which were able to reduce the incidence of sheath rot disease to varying degrees. Iantas et al. [14] investigated the antimicrobial potential of endophytic fungi from the leaves and petioles of two Brazilian medicinal plants against phytopathogens (three fungal pathogens: C. abscissum, P. citricarpa, F. graminearum; and one bacterial strain: X. citri subsp. citri). From 1304 fungal isolates, they screened and identified strains exhibiting dual antifungal and antibacterial activity and further analyzed these strains. The results demonstrated that endophytic fungi from medicinal plants produce secondary metabolites with bioactivity against both plant pathogenic fungi and bacteria. The endophytes of medicinal plants possess certain antimicrobials that are important resources for screening antagonistic microorganisms against plant pathogens. In this study, we focused on the medicinal plant Mentha spp. (mint) as a potential source of biocontrol agents (BCAs).
Mentha haplocalyx Briq. has been utilized in traditional folklore medicine for over 250 years [15]. The essential oil of M. haplocalyx is highly valued commercially due to its high content of menthol (30–55%) and menthone (14–32%) [16]. Extracts from M. haplocalyx have long played a significant role in traditional medical systems for treating various ailments [17]. Notably, menthol has demonstrated a 50% growth inhibition effect on fungal pathogens, such as Aspergillus flavus and A. parasiticus [18]. In another study, menthol was shown to reduce the levels of cell wall-degrading enzymes secreted by pathogenic fungi in legumes affected by root rot pathogens, thereby contributing to a decrease in disease incidence [19]. These findings highlight the potential of Mentha haplocalyx. extracts as natural biocontrol agents in modern agricultural practices, offering a sustainable alternative to chemical fungicides.
The biocontrol mechanisms of biocontrol bacteria typically encompass antibiosis, competition, hyperparasitism, and the induction of resistance. Some antagonistic bacteria primarily rely on a single mechanism; whereas, many others exhibit multiple antagonistic mechanisms. For instance, Bacillus spp. control other organisms through multiple mechanisms, such as 1. producing cell wall-degrading enzymes (e.g., chitinase, β-1,3-glucanase, protease) to degrade fungal cell wall components (e.g., chitin, glucan, proteins); 2. synthesizing antimicrobial compounds (e.g., antibiotics, siderophores) to inhibit pathogens; 3. generating plant growth hormones (e.g., indole-3-acetic acid) to enhance plant vigor; and 4. inducing systemic plant resistance to strengthen defense against infections [20]. Proteases, β-1,3-glucanases, and chitinases are enzymes that degrade crucial components of a pathogen’s cell wall, thereby weakening it and inhibiting the growth of plant diseases [21,22]. Xu et al. [23] have found that Bacillus strains SG08-09 and SG09-12 can produce proteases and exhibit strong efficacy against tomato gray mold. Additionally, Bacillus spp. can inhibit the growth of cellulose-containing phytopathogenic Oomycete, such as Phytophthora, by secreting cellulases [24,25]. The secondary metabolites produced by endophytes include various antibiotics, such as lipopeptides (surfactins, iturins, and fengycins), polyketides (macrolactins, bacillaene, and difficidin), and aminoglycosides (butirosin), which have the potential to disrupt the structure of the cell membrane and impede the proliferation of pathogenic bacteria and fungi [26]. Understanding these multifaceted mechanisms is crucial for optimizing the application of biocontrol bacteria in sustainable agricultural practices and developing effective strategies against plant pathogens.
In this study, we targeted tobacco brown spot disease. A bacterial strain exhibiting resistance to A. alternata was isolated and screened from the medicinal plant Mentha haplocalyx. Furthermore, the strain was identified, its antifungal activity assessed, and its control spectrum determined. We also explored the strain’s antagonistic mechanism. This research offers novel microbial resources for the biological management of tobacco brown spot disease. Our research contributes to the advancement of sustainable agricultural practices by providing an environmentally benign alternative to chemical control methods for the management of tobacco brown spot disease. To our knowledge, this is the first report of an endophytic Bacillus velezensis strain isolated from the medicinal plant Mentha haplocalyx Briq., exhibiting strong antifungal activity against A. alternata.
2. Materials and Methods
2.1. Endophytic Bacteria Were Isolated from M. haplocalyx
Mentha spp. samples were collected from the West Campus of Yangtze University, Hubei, China. The samples were surface-sterilized using the following procedure: The samples were initially rinsed in sterile distilled water for 30 s. Subsequently, the specimen was immersed in 70% ethanol for 2 min, treated with 2.5% sodium hypochlorite for 2 min, and then immersed in 70% ethanol for an additional 2 min. Finally, the specimen was rinsed three times with sterile distilled water. A mortar and pestle were employed to grind the sterilized Mentha tissues, and the resulting suspension was serially diluted. Aliquots of the 10−4, 10−5, and 10−6 dilutions were incubated at 28 °C for 48 h on LB. The single colonies that arose were selected and purified by successive streaking on fresh LB agar plates. Subsequently, each purified colony was stored in glycerol depots at −80 °C [27].
2.2. Screening of BCAs Antagonistic to Tobacco Brown Spot Disease In Vitro
The plate confrontation method was employed to assess the antagonistic activity of the bacterial strains against fungal pathogens. The fungi to be tested were inoculated onto potato dextrose agar (PDA) plates. Mycelial discs with a diameter of 6 mm were transferred to the center of each plate after two days of incubation at 28 °C. (The fungal strains were obtained from the Fungal Specimen Room at Yangtze University, Jingzhou, Hubei, China). At four symmetrical points, each 25 mm away from the fungal plug, a paper filter disc with a circumference of 6 mm was immersed in the prepared bacterial culture solution. The controls consisted of paper filters that had been immersed in sterile distilled water. The mycelial diameter and inhibition rate were subsequently assessed after the dishes were incubated at 28 °C for two days. The entire experiment was repeated three times to ensure reproducibility, and each treatment group underwent three replicate experiments [13].
Inhibition rate (%) = [(Colony diameter of control − Colony diameter of treatment)/(Colony diameter of control − Initial colony diameter)] × 100.
2.3. Evaluation of the Antimicrobial Spectrum of Strains
The methodology employed in this study follows the procedures described in Section 2.2. Specifically, the mycelial growth inhibition rate of the most effective antagonistic strain, B5, was assessed against six pathogenic fungi: Phytophthora nicotianae, Fusarium fujikuroi, F. verticillioides (formerly F. moniliforme), F. graminearum, F. culmorum, and Bipolaris maydis. All fungal strains were maintained in the Fungal Herbarium at Yangtze University, Jingzhou, Hubei Province, China. To guarantee the reproducibility and reliability of the results, each experimental treatment was implemented in triplicate.
2.4. Identification of the Antagonistic Strain B5
2.4.1. Morphological Identification
Strain B5 was inoculated onto a Luria agar (LA) plate and incubated at 25 °C for 7 d. The morphological characteristics of the mycelium and spores were subsequently observed under a light microscope (manufactured by Ningbo Shunyu Optical Technology (Group) Co., Ltd., Ningbo, China) [28].
2.4.2. Biochemical and Physiological Indicators
The physiological and biochemical characteristics of the antagonistic bacteria were analyzed using the Manual of Systematic Identification of Common Bacteria and Berger’s Manual of Bacterial Identification as reference [29]. The tests conducted included Gram staining, enzyme hydrolysis, gelatin liquefaction, sodium citrate utilization, the indole test, the V-P test, the methyl red test, and the salt tolerance test.
2.4.3. Molecular Identification
To identify strain B5, we employed 16S rDNA and gyrA sequence amplification followed by phylogenetic analysis. The strain was inoculated into LB media and incubated overnight at 28 °C, shaking at 140 rpm. DNA was extracted using the TIANamp Bacterial DNA Kit (Tiangen Biotechnology Co., Ltd., Beijing, China). PCR amplification was carried out using universal primers that focused on the 16S rDNA [30] and gyrA [31]. genes. Following PCR, the amplified products were electrophoresed on a 1.0% agarose gel and sequenced at Wuhan’s Huazhong University of Science and Technology. The sequencing results were corrected, and a preliminary BLAST alignment was performed on the NCBI database (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 25 October 2024). Based on the alignment results, the sequences of type isolates that are closely related were downloaded. (Table 1). Using BioEdit 7.0 software, the sequences were corrected, and Escherichia coli isolate L1 E925 was designated as the outgroup. The maximum likelihood (ML) method was implemented to generate a multi-gene phylogenetic tree with MEGA 5.0 software [32].

Table 1.
Strains used in phylogenetic analysis and their corresponding GenBank accession numbers.
2.5. Effect of B5 on Tobacco Red Star Disease Control in the Greenhouse
A pot experiment was also conducted to assess the control effects of strain B5 on tobacco brown spot disease [33]. When flue-cured tobacco plants had 8 to 10 leaves, they were treated as follows: A spray method was used to evenly apply a fermentation broth containing viable strain B5 cells at a concentration of 1 × 108 CFU/mL to the top of the tobacco leaves, ensuring that the liquid droplets did not drip [34]. After drying, 6 mm-diameter mycelial discs of A. alternata were inoculated onto each leaf, with four discs per leaf. The experiment included four treatments—CK1 (Tobacco plants are sprayed with sterile Nutrient Broth (NB) medium), CK2 (Tobacco plants sprayed with sterile NB medium and then inoculated with the pathogen), CK3 (Tobacco plants sprayed with the B5-containing fermentation broth and then inoculated with the pathogen), and CK4 (Tobacco plants sprayed with 10% difenoconazole WDG at a 2000-fold dilution and then inoculated with the pathogen). In each treatment, there were three replicates, each containing ten tobacco plants. The pot trials were carried out in a greenhouse (June–September 2024) under the following conditions: 12 h light/12 h dark cycle, 28 °C constant temperature, and RH maintained at >80% (typically >90%). The disease investigation was conducted according to the grading standard for brown spot disease once obvious disease spots appeared on CK2. The incidence, severity index, and relative control efficacy were then calculated [35].
Incidence = (number of diseased leaves/total number of investigated leaves) ×100%
Disease index =∑ (number of diseased leaves at all levels × disease progression)/(total number of surveyed leaves × highest disease progression) × 100
Relative control effect = (disease index of the control group − disease index of the treatment group)/disease index of the control group × 100%
2.6. Determination of Biological Characteristics
2.6.1. Extracellular Enzyme Detection
The plate method was employed to evaluate the activity of extracellular enzymes in strain B5, which includes protease, chitinase, amylase, glucanase, and cellulase. After being inoculated into LB liquid medium, the bacteria that showed positive antagonistic effects were shaken at 37 °C for the entire night at 200 rpm. Five microliters of the bacterial suspension were then poured into the middle of each matching medium. To observe the formation of a transparent zone (dissolution circle) surrounding the bacterial colonies, which is indicative of enzyme activity, the plates were subsequently incubated at 37 °C for 24 h [36].
2.6.2. Siderophore Production Assessment
As previously mentioned, CAS agar plates were used to measure the bacterial strain’s generation of siderophores [37]. One microliter of bacterial fermentation broth, which had been agitated beforehand, was spotted over the center of each CAS agar plate within a laminar flow cabinet. As a control, sterile distilled water was applied to several plates. A constant–temperature incubator was then utilized to incubate the CAS plates at 28 °C. The appearance of an orange halo around the bacterial colonies indicated siderophore production. We carefully measured and recorded the diameter of the halo for further analysis.
2.6.3. IAA Synthesis Capability Test
As previously mentioned, the Salkowski reagent colorimetric method was used to evaluate strain B5’s capacity to produce IAA [38]. The specific steps were as follows: Strain B5 was inoculated into LB medium that was enriched with 1 g/L tyrosine and incubated at the appropriate temperature for 3 d. The negative control was LB medium without inoculum. After 48 h of cultivation at 28 °C, 2 mL of supernatant was obtained from each culture. Each supernatant sample was then supplemented with 4 mL of Salkowski reagent and 2 droplets of orthophosphoric acid. The mixed solution was subsequently allowed to remain in the dark for 30 min to detect any color change that would suggest the presence of IAA.
2.6.4. Determination of the Ability to Form Biofilm
The biofilm-forming capability of strain B5 was evaluated using glass test tubes, adhering to the protocol outlined by Sun et al. [39]. The strain was initially cultivated in LB medium at 28 °C with shaking at 130 rpm for 24 h. The bacterial suspension that resulted was subsequently attenuated to various optical densities (OD600 = 0, 0.2, 0.4, 0.6, 0.8, and 1.0) using fresh LB medium. Four microliters of the suspension was pipetted into triplicate test containers for each concentration and incubated at 28 °C for 48 h. The crystal violet staining method was employed to evaluate the formation of biofilm. In each tube, 5 mL of a 0.1% (w/v) crystal violet solution was added after incubation, and the tubes were permitted to stain for 15 min at 28 °C. Excess stain was eliminated by decanting and thoroughly washing the containers with sterile water. The presence of a purple rim on the inner wall of the tube suggested biofilm formation. To dissolve the biofilms, each tube was treated with 5 mL of 95% (v/v) ethanol, which was then gently oscillated. The absorbance of the dissolved solution was then measured at 570 nm using a UV-visible spectrophotometer (METASH, Shanghai, China), with a sample of LB medium that had no bacteria as the control.
2.7. Study on Non-Volatile Compounds Produced by Strain B5
2.7.1. Impact of Bacterial Culture Filtrate on A. alternata Mycelial Growth
Detection of Antifungal Activity in Cell-Free Fermentation Broth
After being injected into 50 mL of LB medium, strain B5 was grown for seven days at 28 °C, while being shaken at 130 rpm on a rotary shaker. Centrifugation was used to gather the supernatant at 10,000 rpm for 15 min at 4 °C. The bacterial culture filtrate (BCF) of strain B5 was obtained by filtering the supernatant through a 0.22 µm microporous membrane. To create PDA plates with various sterile filtrate concentrations, different amounts of BCF were added to PDA medium, resulting in final concentrations of 5%, 10%, 15%, 20%, and 25% (v/v), respectively, as described in [40]. The control group received an equivalent volume of uninoculated NB medium. Three duplicates of each treatment were prepared. The center of each plate was injected with the pathogen responsible for tobacco brown spot disease, and the plates were subsequently incubated for three days at a consistent temperature of 28 °C. The inhibition rate was determined by measuring the size of the pathogen’s colony in both the treatment and control groups, following the procedures outlined in Section 2.2.
Observation of Hyphal Morphology by Scanning Electron Microscopy (SEM)
Following the method by Wu et al. [41], the fungal hyphae were treated with a 2.5% glutaraldehyde solution to fix them in a 1,4-dithioctane-2,3-diol-pentanoic acid (PIPES) buffer (NeoCide GB02009, Suzhou, China) overnight. After double-layer blotting paper filtration, it was rinsed twice more with the aforementioned solution and then soaked in 1% osmium tetroxide for fixation for 2 h. The fixed fungal hyphae were dehydrated in ethanol solutions of 30%, 50%, 70%, 80%, 90%, and 100% for 15 to 20 min, respectively, placed in a culture dish, and dried in a freeze dryer. The dried samples were fixed onto carbon conductive tape and subjected to ion sputter coating with gold, followed by observation under a scanning electron microscope (Tescan Vega3, Prague, Czech Republic).
2.7.2. Extraction and Antifungal Activity Determination of Strain B5
Regarding the approach outlined by Mandal et al. [42], an equivalent volume of ethyl acetate was combined with 300 milliliters of sterile BCF. After being moved to a separatory funnel and given a thorough shake, the mixture was left to stand until the solution had fully separated into layers. The upper organic phase was collected after 2–3 repeated extractions, while the remaining lower aqueous phase was further extracted with ethyl acetate for a total of three extractions. The organic and aqueous phases were collected separately. A rotary evaporator was used to completely evaporate the solvent from the combined organic phases of the extractions. The dry extract that resulted was then diluted in one milliliter of HPLC-grade methanol. The ethyl acetate and aqueous fractions of the crude extract (10%, v/v) were incorporated into PDA medium. Each plate was inoculated with 6 mm diameter mycelial discs of the pathogen, which were then incubated for 7 d at 25 °C. We used PDA media that had the pathogen but not the extract as a control.
2.7.3. LC-MS Analysis of Lipopeptide Active Substances of Strain B5
A high-resolution LC-MS instrument was used to further identify and evaluate the composition of the lipopeptide active compounds generated by strain B5, following their separation. The crude extract obtained after rotary evaporation was dissolved in mass spectrometry-grade methanol and subsequently diluted to 5 ppm before being dispensed into sample vials for detection. Using an LC-MS-grade acetonitrile as mobile phase B and ultrapure water as mobile phase A, a Kinetex® F5 column (100 mm × 2.1 mm, 2.6 μm particle size) was used for the chromatographic separation. The LC-MS analysis of strain B5 was performed according to the protocols established by Jin et al. [43], Chen et al. [44], and Fan et al. [45]. Data interpretation and detailed analysis were conducted using SCIEX OS software version 1.7.0. The primary compositional profile of the lipopeptide active substances produced by strain B5 was determined by comparing the obtained data with existing entries in the relevant database.
2.8. Identification of Enzyme Activity Related to Plant Defense
In a controlled greenhouse environment, following inoculation with A. alternata, a total of three collections were conducted, with the third to sixth expanded leaves of each tobacco plant being sampled at 48 h intervals, counting from the top. The leaves were promptly kept at −80 °C to conduct subsequent analytical procedures. In the leaves that were subjected to various interventions, the activity levels of several defense enzymes, such as phenylalanine ammonia-lyase (PAL), peroxidase (POD), cathode (CAT), phenol oxidase (PPO), and superoxide dismutase (SOD), were assessed. Pathogen inoculation achieved the disease control, while nutrient broth (NB) inoculation achieved the healthy control. The extraction of the defense enzyme solutions and the determination of their activity were performed using a kit (Solarbio, Beijing, China) with strict adherence to the manufacturer’s instructions [46].
2.9. Statistical Analysis
One-way ANOVA was used to analyze the experimental data using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) [47]. The mean values of the various treatment groups were compared using Duncan’s Multiple Range Test. A significance level of p ≤ 0.05 was used to establish statistical significance [48].
3. Results and Analysis
3.1. Screening of Antagonistic Bacteria and Determination of Antifungal Spectrum
From the medicinal plant Mentha (mint), 16 types of endophytic bacteria were identified. The strain that had the most antagonistic impact was chosen and given the designation B5 through plate confrontation cultivation. In the plate confrontation assay, B5 demonstrated an inhibition rate of 82.76% against A. alternata (Figure 1). To evaluate the antifungal activity of strain B5 against various pathogens, six different fungal pathogens were chosen. As illustrated in Figure 2, with inhibition rates ranging from 66.34% to 87.23%, strain B5 showed antifungal activity against each of the six investigated pathogens. Notably, B5 exhibited the highest antifungal activity against the maize spot pathogen, achieving an inhibition rate of 75.23%; in contrast, its antifungal activity against the wheat scab pathogen was relatively weaker, with an inhibition rate of 59.7%.
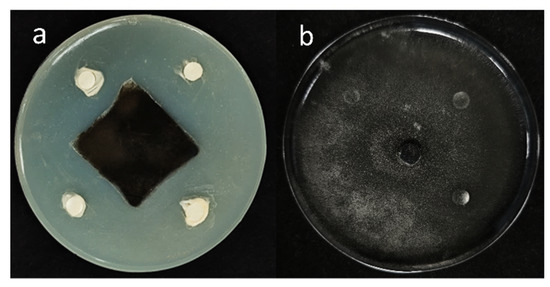
Figure 1.
Antifungal activity of B5 against A. alternata. A 6 mm-diameter mycelial disc of A. alternata was centrally inoculated on PDA plates. Filter paper discs impregnated with B5 bacterial suspension (a) or sterile water (negative control, (b)) were aseptically placed at four equidistant positions. Significant inhibition zones surrounding B5-treated discs (a) demonstrated antifungal activity; whereas, confluent mycelial growth in control discs (b) confirmed the absence of inhibition.
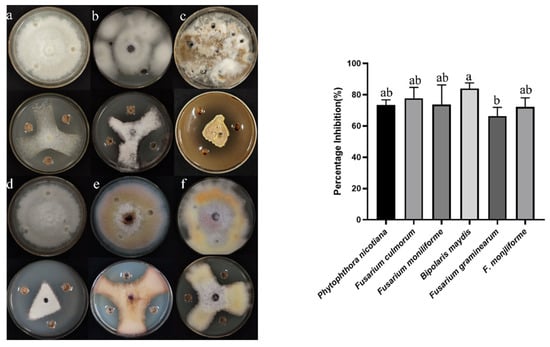
Figure 2.
Inhibitory effects of B5 on six plant pathogenic fungi on PDA: (a) P. nicotianae, (b) F. culmorum, (c) F. fujikuroi, (d) B. maydis, (e) F. graminearum, (f) F. verticillioides. Control cultures are displayed adjacent to each test plate. Error bars indicate the standard error. Columns marked with the same letter are not significantly different (p ≤ 0.05, t-test).
3.2. Antagonistic Bacteria B5 Identification
3.2.1. The Morphology of Colonies as Well as Their Physiological and Biochemical Traits
Observations of colony morphology revealed that after 1 day of streak cultivation on LB medium, strain B5 exhibited a milky white appearance on the plate, with a smooth colony surface and wrinkled edges (Figure 3a). Gram staining of strain B5 was positive (Figure 3b). Under electron microscopy, the bacterial cells appeared rod-shaped, measuring approximately 1–2 μm in length (Figure 3c). In terms of physiological and biochemical characteristics, strain B5 exhibits normal mycelial growth within the pH range of 4.5 to 8.5. Additionally, this strain can grow normally in a culture medium containing 0–10% NaCl. Strain B5 was capable of decomposing gelatin, starch, D-xylose, and D-mannitol but not L-arabinose. The Voges–Proskauer (V-P) reaction and the propionate reaction were negative for strain B5; whereas, the citrate utilization test and nitrate reduction reactions were positive (Table 2).
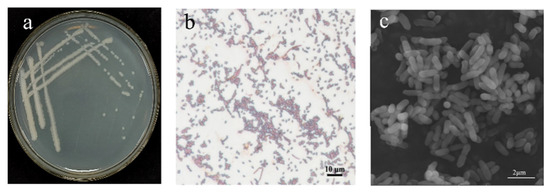
Figure 3.
Morphological characteristics of strain B5: (a) colony morphology of B5; (b) Gram-stained cells of B5 (scale bar: 10 μm); (c) scanning electron microscope (SEM) images of B5 (scale bar: 2 μm/nm).

Table 2.
Physiological and biochemical characteristics of antagonistic strain B5.
3.2.2. Phylogenetic Analysis
The 16S rDNA and gyrA gene sequences were used to create a phylogenetic tree for strain B5. (Figure 4) The results indicate that strain B5 clusters with Bacillus velezensis B-001, with 100% bootstrap support. Considering its morphological characteristics, strain B5 is identified as Bacillus velezensis based on its physiological and biochemical characteristics, as well as findings from a phylogenetic study. The sequences of the 16S rDNA and gyrA genes of strain B5 have been deposited in the GenBank database under accession numbers PV168970 and PV173738, respectively.
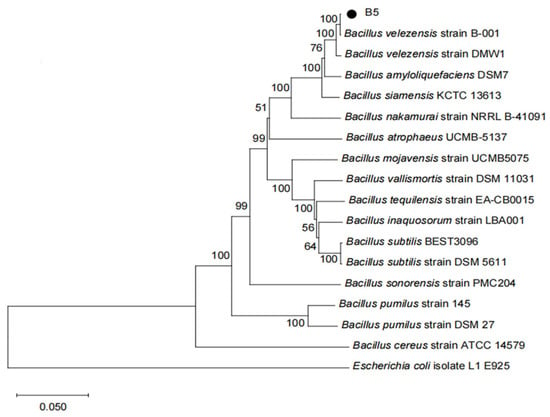
Figure 4.
A phylogenetic tree was constructed using the neighbor-joining method based on 16S rDNA and gyrA gene sequences. Bootstrap values (1000 replicates) are shown at branch nodes. Escherichia coli isolate L1 E925 was used as the outgroup. The scale bar indicates nucleotide substitutions per site.
3.3. Control Effect of B5 on Tobacco Brown Spot Disease
The results of the pot experiment (Figure 5, Table 3) demonstrate that the fermentation broth with strain B5 (1 × 108 CFU/mL) exhibits a significant control effect on tobacco brown spot disease. Specifically, treatment with the fermentation broth containing viable strain B5 cells (1 × 108 CFU/mL) resulted in substantial reductions in both the incidence and disease index of tobacco plants compared to the control group. The incidence of tobacco brown spot disease was reduced from 100.00% in the untreated control (CK2: NB+ A. alternata) to 64.81% in the B5-treated group, while the disease index was reduced from 32.92% in the control to 12.96% in the B5-treated group. This treatment achieved a relative efficacy of 60.66%, which was considerably greater than that of the untreated control group, and there was no significant difference in the relative control efficacy compared with 10% difenoconazole WDG.
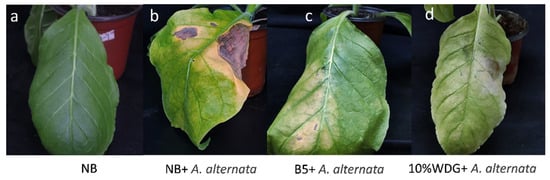
Figure 5.
Efficacy of strain B5 against tobacco brown spot disease in a greenhouse setting. (a) Sterile Nutrient Broth (NB) medium control; (b) tobacco plants treated with sterile Nutrient Broth (NB) and then inoculated with A. alternata; (c) tobacco plants sprayed with the fermentation broth with strain B5 (1 × 108 CFU/mL) followed by inoculation with A. alternata; (d) tobacco plants sprayed with 10% difenoconazole WDG at a 2000-fold dilution followed by inoculation with A. alternata. The images depict the comparative disease symptoms on tobacco leaves under different treatment conditions.

Table 3.
Control Effect of B5 on tobacco brown spot disease.
3.4. Identification of the Biological Features of Strain B5
The assessment of strain B5 using selective media demonstrated its capacity for extracellular enzyme production, siderophore synthesis, and indole-3-acetic acid (IAA) biosynthesis. Distinct hydrolysis zones on starch, protease, glucanase, and cellulase plates confirmed the activities of amylase, protease, glucanase, and cellulase, respectively. Characteristic yellow halos around colonies on CAS medium after 7-day incubation indicated siderophore production. Additionally, pink coloration developed in the Salkowski’s reagent assay verified IAA biosynthesis. This assay involved mixing 2 mL of supernatant with 4 mL of reagent and two drops of orthophosphoric acid, following 3-day cultivation in tryptophan-supplemented PDB medium with a 30 min dark incubation (Figure S1). Using a colorimetric assay, the capacity of strain B5 to form biofilms was identified. The results demonstrated that biofilm formation was indeed possible. The amount of biofilm adhering to the tube wall increased dramatically with increasing bacterial suspension concentration, reached a peak, and then declined rapidly. The maximum biofilm formation was observed when the absorbance value of the strain B5 suspension at 600 nm was 0.6 (Figure 6).
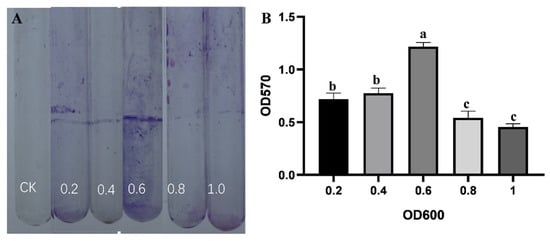
Figure 6.
Biofilm formation analysis. (A) Visualization of biofilms formed on glass tubes using 0.1% crystal violet staining. CK is sterile LB broth. (B) Quantitative analysis of biofilm biomass under different culture densities (OD600 = 0.2, 0.4, 0.6, 0.8, 1.0) with LB medium as control. Error bars represent the standard error of the mean, and the letter denotes significant differences at p ≤ 0.05.
3.5. Effect of Sterile Filtrate (BCF) on Mycelial Growth of A. alternata
The BCF of strain B5 significantly suppressed the development of A. alternata, with increasing efficacy at higher concentrations (Figure 7, Table 4). Notably, at a BCF concentration of 20%, the colony diameter of A. alternata was dramatically reduced to 5.88 mm, corresponding to an inhibition rate of 91%. At 25% BCF, fungal growth was completely suppressed, with no visible colonies and an inhibition rate of 100%. After treatment with BCF, changes in the structure of the fungal mycelia of A. alternata were looked at more closely using SEM (Figure 8). The control group’s mycelia maintained their healthy, typical structure. In contrast, mycelia treated with BCF exhibited significant morphological alterations. The SEM images revealed extensive structural deformations, including collapses, depressions, folds, and creases in the treated samples (Figure 8H). These observations suggest that the BCF inhibits fungal growth and causes severe morphological damage to the mycelia.

Figure 7.
Antifungal activity of sterile filtrates against A. alternata at different concentrations: blank control, 5%, 10%, 15%, 20%, and 25% sterile filtrates.

Table 4.
The inhibition rate of different concentrations of aseptic filtrate to A. alternata. The pathogenic fungus was grown on PDA mixed with culture filtrates for 2 days, followed by measurement of colony diameters. Values are the mean ± SD of three replicates, and those followed by the same letter are not significantly different at P ≤ 0.05 according to Duncan’s Multiple Range Test.
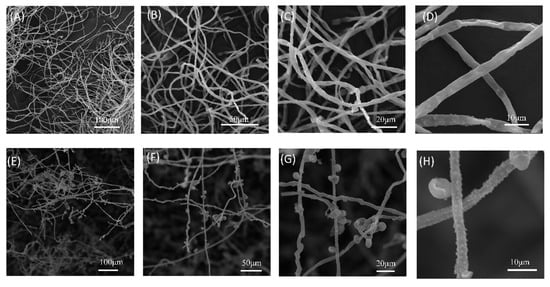
Figure 8.
Scanning electron micrographs showing morphological alterations in A. alternata hyphae treated with sterile filtrate from strain B5 (1:10, v/v). (A–D) Untreated control hyphae exhibit normal morphology. (E–H) B5 filtrate-treated hyphae displaying severe structural deformations. Fungal colonies were inoculated on PDA plates, with hyphal samples collected from colony margins after 5 days of incubation at 25 °C.
3.6. Determination of Antagonistic Activity of B5 Crude Extract
The ethyl acetate and aqueous fractions of strain B5 fermentation broth were prepared and subjected to plate assays. The findings showed that the growth of A. alternata was strongly suppressed by the ethyl acetate crude extract (60 mg/mL). In contrast, the aqueous fraction remaining after ethyl acetate extraction showed no inhibitory effect on A. alternata in the plate assay (Figure S2). Consequently, the bioactive compounds responsible for the antifungal properties are likely contained within the ethyl acetate crude extract, necessitating additional research.
3.7. Identification of the Constituents in Strain B5 Extracts Using LC-MS
Using SCIEX OS 1.7.0 software for LC-MS analysis, we identified various chemical components in the crude extract of strain B5. The following compounds exhibited high matching degrees and minor mass errors: Betaine (peak area = 51,550, retention time = 26.46 min, mass error = −5.0 ppm), Uridine (peak area = 436.7, retention time = 9.18 min, mass error = 0.1 ppm), Proline–Leucine Cyclopeptide (peak area = 43,080, retention time = 4.56 min, mass error = −0.5 ppm), Iturin A-7 (peak area = 3732, retention time = 7.76 min, mass error = −1.5 ppm), and Surfactin C (peak area = 415,000, retention time = 10.64 min, mass error = −0.4 ppm) (Figure S3, Table 5).

Table 5.
Liquid chromatography mass spectrometry (LC-MS) analysis for component identification in strain B5 crude extract.
3.8. Impact of Strain B5 on Tobacco Defense-Related Enzyme Activity
After treatment with B5 fermentation broth, Figure 9 illustrates the variations in the activities of POD, PPO, PAL, CAT, and SOD in tobacco leaves. The activities of POD, PPO, PAL, and CAT all exhibited a rising-then-falling pattern. After using different amounts of B5 fermentation broth, the levels of these defense enzymes were much higher than those in the sick control and the healthy control. On the fourth day after root irrigation, the activities of PAL and CAT peaked; on the sixth day, however, the POD, PPO, and SOD activity peaked. In comparison to the disease control and healthy control, the PAL activity of the tobacco leaves treated with 108 CFU/mL B5 fermentation broth was 101.8 U·g−1·min−1·FW, which was 35.56% and 89.50% higher, respectively. The CAT activity was 3.35 × 103 U·g−1·min−1·FW, which was 135.2% and 77.24% higher than that of the disease control and healthy control groups, respectively. In comparison to the disease control and healthy control, the POD activity was 6.18 × 103 U·g−1·min−1·FW, which was 114.3% and 40.13% higher, respectively. When compared to the disease control and healthy control, the PPO activity increased by 158.3% and 33.98%, respectively, to 132.47 U·g−1·min−1·FW. Finally, 138.09 U·g−1·min−1·FW was the SOD activity, which was 56.56% and 67.34% greater than the disease control and healthy control, respectively. The increase in plant defense enzyme activities with the rise in bacterial solution concentration demonstrates that the bacteria can stimulate plant defense responses, thereby facilitating the plants’ resistance to pathogen infection.
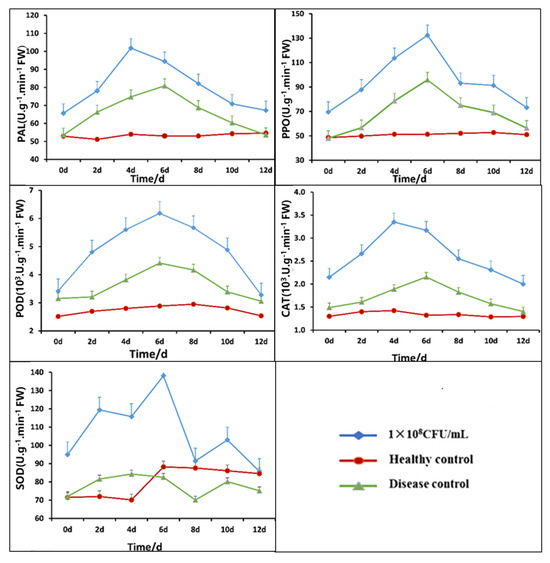
Figure 9.
Time-course changes in tobacco defense enzymes (PAL, PPO, POD, CAT, SOD) following treatment with Bacillus velezensis. 1 × 108 CFU/mL: 1 × 108 CFU/mL B5 fermentation broth treatment; healthy control: NB treatment; diseased control: pathogenic fungi treatment.
4. Discussion
Biological control, which utilizes microorganisms to mitigate plant diseases, is considered beneficial for plant disease management. The production and buildup of secondary compounds in the host have been greatly impacted by the microbiome of medicinal plants over long-term coevolution [49]. Therefore, bioactive substances that possess antagonistic qualities may be found in endophytes associated with therapeutic plants. For instance, Kim et al. [50] isolated the endophytic fungus Phoma sp. PF2 from the leaves of the Korean traditional medicinal plant Artemisia argyi, and after structural determination via NMR and MS, they found that phomalide A exhibited moderate antifungal activity against Staphylococcus aureus, while phomalide B showed weak cytotoxicity against HeLa cells, indicating that Phoma sp. PF2 is a potential source of bioactive secondary metabolites for drug development. This investigation identified 16 distinct endophytic bacterial strains from Mentha haplocalyx Briq, the source of the bacterial endophytes. Among them, strain B5, which exhibited the strongest antagonistic effect, was screened out and demonstrated antifungal activity against all six pathogenic fungi tested. The identification of strain B5 as B. velezensis was confirmed through morphological observation, 16S rDNA gene sequencing, and gyrA gene sequence analysis, aligning with the identification of B. velezensis BAC03. Antagonistic bacterial strains with biocontrol potential typically exhibit a wide range of antifungal activity [51,52]. Tan et al. [53] identified a strain of B. subtilis HZ-72 from flax rhizosphere soil [54], which inhibited the growth of seven distinct pathogenic fungi by more than 65% and demonstrated the effective prevention of flax seedling blight in potting tests. The growth of six different plant pathogenic fungi was also considerably inhibited by strain B5 in this study, with inhibition rates higher than 55%. Based on these experimental findings, strain B5 holds promise for the management of various plant diseases.
The purpose of this study was to evaluate the efficacy of live strain B5-containing fermentation broth (1 × 108 CFU/mL) in controlling tobacco brown spot disease under greenhouse conditions. The results demonstrated that this fermentation broth treatment effectively reduced disease severity. These findings confirm its potential as a biocontrol agent for disease management. In a separate investigation, Xie et al. [55] reported that strain LZ88 dramatically decreased the severity of tobacco brown spot disease in greenhouse seedlings by 78.64% compared to untreated control plants. The differences in indoor preventive effectiveness observed between these studies may be attributed to the varying sources of B. subtilis utilized in the tests.
Strain B5 does not secrete chitinase or cellulase, but it does produce cell wall lytic enzymes, proteases, amylases, and glucosidases. Additionally, B5 can release siderophores. Similarly, B. velezensis F21, a biocontrol bacterium effective against watermelon wilt disease, lacks chitinase production but may secrete proteases, glucosidases, and other hydrolytic enzymes. In contrast, B. velezensis NKG-2, as reported by Myo et al. [56], is capable of producing siderophores, chitinase, cellulase, amylase, and β-glucosidase. Variations in strains and experimental conditions may be the cause of these disparities. Numerous related publications have linked Bacillus’s capacity to release extracellular hydrolases to their inhibitory effects on plant fungal infections [57,58]. Furthermore, siderophores produced by plant-beneficial bacteria are crucial for defending plants against fungal diseases [59]. These small molecular organic compounds, which have a strong affinity for Fe3+, can inhibit the growth of harmful fungi by maintaining iron levels below what is required for their regular metabolism [60]. Additionally, in iron-limited environments, Bacillus can release abundant iron chelators and siderophores to facilitate the rapid acquisition of iron ions by plants, thereby promoting healthy growth and reducing the risk of disease. In line with the findings of Feng et al. [61], who reported that four endophytic bacteria strains isolated in their study could synthesize IAA by utilizing intermediary metabolites, such as tryptamine and indole ethyl amide, strain B5 was also capable of producing IAA. Most bacteria adapt to complex ecological environments by forming biofilms. Sun et al. [39] investigated the ability of Pseudomonas putida A1 to colonize tomato root surfaces and utilized a colorimetric technique to assess the bacterium’s biofilm-forming capacity. Their results indicated that the concentration of the fermentation fluid increased as well, and this strain’s capacity to produce biofilms. Furthermore, P. putida A1 could colonize tomato roots in large numbers, with a preference for colonization near wounds. Similarly, strain B5 exhibits a high capacity for biofilm formation; as the concentration of the initial fermentation broth increases, the amount of biofilm formed by this strain first increases notably before rapidly decreasing. Preliminary speculation suggests that strain B5 may possess exceptional colonization abilities.
These secondary metabolites can stimulate plant growth, induce systemic disease resistance in plants, and directly inhibit fungal pathogens. According to Moon et al. [62], B. velezensis CE 100 was investigated for its ability to reduce the suppression of mycelial growth in Phytophthora root rot, resulting in mycelial enlargement and distortion. The sterile fermentation broth of strain B5 demonstrated potent antifungal effects against A alternata in this investigation. Additionally, treated samples displayed significant structural deformations, such as collapse, depression, folding, and creasing, when observed under a scanning electron microscope. The active components of strain B5’s supernatant were extracted using ethyl acetate as the organic solvent, and the bioactive compounds present in the B5 crude extract were identified through LC-MS analysis. The identified bioactive compounds, including surfactin and iturin, are well-documented for their antifungal properties and their ability to enhance plant defense mechanisms. Similar to our findings, B. methylotrophicus DR-08 has been reported to produce antimicrobial compounds, such as difficidin, which significantly reduces tomato bacterial wilt and inhibits the growth of a broad range of plant pathogens [63]. Specifically, fengycin is known to inhibit mycelial growth by inducing deformation, oxidative damage, and mitochondrial dysfunction in fungal cells, while surfactin enhances the activity of plant defense-related genes and enzymes, thereby contributing to improved disease resistance [64].
Plant defense-related enzymes can neutralize reactive oxygen species in plants and activate the salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) signaling pathways to enhance plant resistance to pathogen infection [65,66,67]. Plants activate defense enzymes, including POD, PPO, PAL, CAT, and SOD, in response to exposure to a variety of pathogenic organisms. These enzymes are essential for the metabolism of ROS [68,69], the synthesis of secondary metabolites involved in disease resistance, including lignin, phenolic compounds, and plant defense hormones [70,71], and directly inhibit and kill pathogenic bacteria, thereby enhancing plant resistance [72]. Several studies have demonstrated a positive correlation between the disease resistance index and the activity of enzymes such as POD, PPO, PAL, CAT, and SOD, suggesting a specific defense mechanism against infections. Strain B5 substantially increased the activity of several defense-related enzymes in tobacco plants in this study, and this effect became more apparent as the concentration increased. These results are in agreement with the findings reported by Qiu et al. [66]. Similar biocontrol activity has been reported for B. velezensis SDTB022, a tomato rhizosphere isolate, while our study demonstrates comparable efficacy with strain B5.
The aforementioned findings imply that B5’s biocontrol activities against tobacco brown spot disease might be linked to a number of mechanisms and their combined effects. Future research is required to determine the components of B5’s antifungal chemicals and learn more about the biocontrol mechanisms behind its antimicrobial activity.
5. Conclusions
It is of the utmost importance to manage plant diseases in a safe, effective, and alternative manner. Biological control, which employs antagonistic microorganisms, is a sustainable and long-term method of inhibiting plant pathogens in contrast to chemical control methods. In this study, a B5 strain was isolated from M. haplocalyx Briq, which exhibited potent inhibitory effects against the tobacco red star disease pathogen, A. alternata. This strain was identified as Bacillus velezensis through sequencing. It exhibits substantial inhibitory effects under greenhouse conditions and possesses broad-spectrum antifungal activity against various fungal pathogens. Furthermore, it was found to enhance the expression of defense enzymes and extracellular enzymes in the host plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11060446/s1, Figure S1. Detection of cell wall-degrading enzymes, siderophores, and indole-3-acetic acid (IAA): (a) Amylase activity, (b) Siderophore production, (c) Glucanase activity, (d) Protease activity, (e) Cellulase activity, (f) IAA production. Figure S2. Inhibitory activity of strain B5 crude extracts against the growth of A. alternata. The ethylacetate fraction and aqueous fraction of the crude extracts (10%, v/v) were added to PDA medium and incubated at 25°C for 7 days. Pathogen-inoculated PDA medium (without extracts) served as the control. Figure S3. Mass spectrometry analysis of lipopeptide bioactive compounds produced by strain B5.Identified compounds: (a) Uridine; (b) Toxenutin; (c) Cyclo(Proline-Leucine) dipeptide; (d) Surfactin A; (e) Cyclo(D-Phenylalanine-L-Proline); (f) 4’,5-Trimethoxy-trans-stilbene; (g) Iturin A-7; (h) Betaine; (i) Bacillaene; (j) Surfactin C.
Author Contributions
Q.Q.: writing—review and editing, pot experiments, LC-MS analysis. B.L.: assistance with relevant experiments. B.M.: assistance with relevant experiments. X.W.: writing—review and editing. Y.Z.: supervision, formal analysis, conceptualization. Z.S.: manuscript revision, supervision, project administration, funding acquisition, conceptual design. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Pests and Diseases Green Prevention and Control Major Special Project (110202201023 (LS-07)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Wuhan Majorbio Bio-pharm Technology Co., Ltd.’s technical personnel for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiang, L.G.; Wang, H.C.; Cai, L.T.; Guo, T.; Luo, F.; Hsiang, T.; Yu, Z.H. Variations in leaf phyllosphere microbial communities and development of tobacco brown spot before and after fungicide application. Front. Microbiol. 2022, 13, 1068158. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Mei, G.; Long, Z.; Luo, D. Effects of Radix Curcuma fungicides on tobacco brown spot and yield of flue-cured tobacco leaves. Agric. Sci. Technol. 2016, 17, 1736. [Google Scholar]
- Hu, D.; Fan, Y.; Tan, Y.; Tian, Y.; Liu, N.; Wang, L.; Wu, A. Metabolic profiling on Alternaria toxins and components of Xinjiang jujubes incubated with pathogenic Alternaria alternata and Alternaria tenuissima via orbitrap high-resolution mass spectrometry. J. Agric. Food Chem. 2017, 65, 8466–8474. [Google Scholar] [CrossRef]
- Báez-Flores, M.E.; Troncoso-Rojas, R.; Osuna, M.A.I.; Domínguez, M.R.; Pryor, B.; Tiznado-Hernández, M.E. Differentially expressed cDNAs in Alternaria alternata treated with 2-propenyl isothiocyanate. Microbiol. Res. 2011, 166, 566–577. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Llorens, E.; Fernández-Crespo, E.; Vicedo, B.; Lapeña, L.; García-Agustín, P. Enhancement of the citrus immune system provides effective resistance against Alternaria brown spot disease. J. Plant Physiol. 2013, 170, 146–154. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, L.; Li, H.; Dong, Y.; Xu, H.; Guan, Y.; Rong, S.; Gao, X.; Chen, R.; Li, L.; et al. The isolation of the antagonistic strain Bacillus australimaris CQ07 and the exploration of the pathogenic inhibition mechanism of Magnaporthe oryzae. PLoS ONE 2019, 14, e0220410. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases–review and future prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Kiesewalter, H.T.; Lozano-Andrade, C.N.; Wibowo, M.; Strube, M.L.; Maróti, G.; Snyder, D.; Jørgensen, T.S.; Larsen, T.O.; Cooper, V.S.; Weber, T.; et al. Genomic and chemical diversity of Bacillus subtilis secondary metabolites against plant pathogenic fungi. Msystems 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Naik, S.; Shaanker, R.U.; Ravikanth, G.; Dayanandan, S. How and why do endophytes produce plant secondary metabolites? Symbiosis 2019, 78, 193–201. [Google Scholar] [CrossRef]
- Bacon, C.W.; Whit, J.F. Physiological adaptations in the evolution of endophytism in the Clavicipitaceae. In Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000; pp. 251–276. [Google Scholar]
- Venieraki, A.; Dimou, M.; Katinakis, P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hell. Plant Prot. J. 2017, 2, 51–56. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, S.; Gupta, A.R.; Gupta, A.; Singh, U.B.; Manzar, N.; Bhowmik, A.; Singh, H.V.; Saxena, A.K. Endophytic bacilli from medicinal-aromatic perennial Holy basil (Ocimum tenuiflorum L.) modulate plant growth promotion and induced systemic resistance against Rhizoctonia solani in rice (Oryza sativa L.). Biol. Control 2020, 150, 104353. [Google Scholar] [CrossRef]
- Iantas, J.; Savi, D.C.; Schibelbein, R.D.; Noriler, S.A.; Assad, B.M.; Dilarri, G.; Ferreira, H.; Rohr, J.; Thorson, J.S.; Shaaban, K.A.; et al. Endophytes of Brazilian medicinal plants with activity against phytopathogens. Front. Microbiol. 2021, 12, 714750. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. Int. Sch. Res. Not. 2012, 2012, 718645. [Google Scholar]
- Gul, P. Seasonal variation of oil and menthol content in Mentha arvensis Linn. Pak. J. For. 1994, 44, 16–20. [Google Scholar]
- AFarco, J.; Grundmann, O. Menthol-pharmacology of an important naturally medicinal “cool”. Mini Rev. Med. Chem. 2013, 13, 124–131. [Google Scholar] [CrossRef]
- Silva, F.C.D.; Chalfoun, S.M.; Siqueira, V.M.D.; Botelho, D.M.D.S.; Lima, N.; Batista, L.R. Evaluation of antifungal activity of essential oils against potentially mycotoxigenic Aspergillus flavus and Aspergillus parasiticus. Rev. Bras. De Farmacogn. 2012, 22, 1002–1010. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Tarighi, S. Antifungal activity of various essential oils against Rhizoctonia solani and Macrophomina phaseolina as major bean pathogens. J. Appl. Microbiol. 2015, 118, 704–717. [Google Scholar] [CrossRef]
- Lopes, R.; Tsui, S.; Gonçalves, P.J.; de Queiroz, M.V. A look into a multifunctional toolbox: Endophytic Bacillus species provide broad and underexploited benefits for plants. World J. Microbiol. Biotechnol. 2018, 34, 94. [Google Scholar] [CrossRef]
- Alamri, S.A. Enhancing the efficiency of the bioagent Bacillus subtilis JF419701 against soil-borne phytopathogens by increasing the productivity of fungal cell wall degrading enzymes. Arch. Phytopathol. Plant Prot. 2015, 48, 159–170. [Google Scholar] [CrossRef]
- Jangir, M.; Pathak, R.; Sharma, S.; Sharma, S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol. Control 2018, 123, 60–70. [Google Scholar] [CrossRef]
- Xu, S.J.; Park, D.H.; Kim, J.Y.; Kim, B.S. Biological control of gray mold and growth promotion of tomato using Bacillus spp. isolated from soil. Trop. Plant Pathol. 2016, 41, 169–176. [Google Scholar] [CrossRef]
- Thines, M. Phylogeny and evolution of plant pathogenic oomycetes—A global overview. Eur. J. Plant Pathol. 2014, 138, 431–447. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.; Xiao, Y.; Wass, T.J.; Schenk, P.M. Identification of soil bacterial isolates suppressing different Phytophthora spp. and promoting plant growth. Front. Plant Sci. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Harwood, C.R.; Mouillon, J.M.; Pohl, S.; Arnau, J. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. Rev. 2018, 42, 721–738. [Google Scholar] [CrossRef]
- Zheng, T.W.; Liu, L.; Nie, Q.W.; Hsiang, T.; Sun, Z.X.; Zhou, Y. Isolation, identification, and biocontrol mechanisms of endophytic bacterium D61-A from Fraxinus hupehensis against Rhizoctonia solani. Biol. Control 2021, 158, 104621. [Google Scholar] [CrossRef]
- AN, I.; Grebenshchikova, A.V.; Yatsenko, E.S.; Speranskaya, N.Y.; Matsyura, A.V. Morphological diversity of Bacillus subtilis. Ukr. J. Ecol. 2018, 8, 365–370. [Google Scholar]
- Krieg, N.R.; Holt, J.G. Bergey’s Manual of Systematic Bacteriology; Yi Hsien Publishing Co.: Taipei, Taiwan, 1984. [Google Scholar]
- Patel, J.B. 16S rRNA gene sequencing for bacterial pathogen identification in the clinical laboratory. Mol. Diagn. 2001, 6, 313–321. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Noormohammadi, Z.; Behzadi, P.; Ranjbar, R. Molecular detection of gyrA mutation in clinical strains of Klebsiella pneumoniae. Iran. J. Public Health 2022, 51, 2334. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Fravel, D.R.; Spurr, H.W.; Harvey, J. Biocontrol of tobacco brown-spot disease by Bacillus cereus subsp. mycoides in a controlled environment. Phytopathology 1977, 67, 930–932. [Google Scholar] [CrossRef]
- Meng, X.J.; Wang, L.Q.; Ma, B.G.; Wei, X.H.; Zhou, Y.; Sun, Z.X.; Li, Y.Y. Screening, identification, and evaluation of an acidophilic strain of Bacillus velezensis B4-7 for the biocontrol of tobacco bacterial wilt. Front. Plant Sci. 2024, 15, 1360173. [Google Scholar] [CrossRef] [PubMed]
- GB/T 23222-2008; Grade and Investigation Method of Tobacco Diseases and Insect Pests. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Ad-ministration of the People’s Republic of China: Beijing, China, 2008.
- Murthy, N.; Bleakley, B. Simplified method of preparing colloidal chitin used for screening of chitinase-producing microorganisms. Internet J Microbiol 2012, 10, e2bc3. [Google Scholar]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Methods 2001, 44, 89–95. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-Protocol 2019, 9, e3230. [Google Scholar] [CrossRef]
- Sun, D.; Zhuo, T.; Hu, X.; Fan, X.; Zou, H. Identification of a Pseudomonas putida as a biocontrol agent for tomato bacterial wilt disease. Biol. Control 2017, 114, 45–50. [Google Scholar] [CrossRef]
- Guardado-Valdivia, L.; Tovar-Pérez, E.; Chacón-López, A.; López-García, U.; Gutiérrez-Martínez, P.; Stoll, A.; Aguilera, S. Identification and characterization of a new Bacillus atrophaeus strain B5 as biocontrol agent of postharvest anthracnose disease in soursop (Annona muricata) and avocado (Persea americana). Microbiol. Res. 2018, 210, 26–32. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Chen, B. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS, TEM–EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef]
- Mandal, S.M.; Sharma, S.; Pinnaka, A.K.; Kumari, A.; Korpole, S. Isolation and characterization of diverse antimicrobial lipopeptides produced by Citrobacter and Enterobacter. BMC Microbiol. 2013, 13, 152. [Google Scholar] [CrossRef]
- Jin, P.; Wang, H.; Tan, Z.; Xuan, Z.; Dahar, G.Y.; Li, Q.X.; Miao, W.; Liu, W. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic. Biochem. Physiol. 2020, 163, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tian, Z.; Luo, Y.; Cheng, Y.; Long, C.A. Antagonistic activity and the mechanism of Bacillus amyloliquefaciens DH-4 against citrus green mold. Phytopathology 2018, 108, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, Z.; Li, Y.; Zhang, X.; Duan, Y.; Wang, Q.I. Biocontrol of bacterial fruit blotch by Bacillus subtilis 9407 via surfactin-mediated antibacterial activity and colonization. Front. Microbiol. 2017, 8, 1973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.F.; Wang, S.Q.; Xu, Z.L.; Liao, M.D. Tobacco-acquired resistance induced by an exopolysaccharide of Paenibacillus kribbensis PS04 against bacterial wilt. Biocontrol Sci. Technol. 2020, 30, 370–383. [Google Scholar] [CrossRef]
- Qiu, J.E.; Zhao, L.; Jiang, S.; Godana, E.A.; Zhang, X.; Zhang, H. Efficacy of Meyerozyma caribbica in the biocontrol of blue mold in kiwifruit and mechanisms involved. Biol. Control 2022, 173, 105000. [Google Scholar] [CrossRef]
- Jiang, C.H.; Yao, X.F.; Mi, D.D.; Li, Z.J.; Yang, B.Y.; Zheng, Y.; Qi, Y.; Guo, J.H. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis F21 against Fusarium wilt on watermelon. Front. Microbiol. 2019, 10, 652. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Kim, J.W.; Choi, H.G.; Song, J.H.; Kang, K.S.; Shim, S.H. Bioactive secondary metabolites from an endophytic fungus, Phoma sp. PF2 derived from Artemisia princeps Pamp. J. Antibiot. 2019, 72, 174–177. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, H.; Hao, J.J.J.B.C. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Tan, T.; Zhu, J.; Shen, A.; Li, J.; Yu, Y.; Zhang, M.; Zhao, M.; Li, Z.; Chen, J.; Gao, C.; et al. Isolation and identification of a Bacillus subtilis HZ-72 exhibiting biocontrol activity against flax seedling blight. Eur. J. Plant Pathol. 2019, 153, 825–836. [Google Scholar] [CrossRef]
- Chen, L.; Shi, H.; Heng, J.; Wang, D.; Bian, K. Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol. Res. 2019, 218, 41–48. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Wang, D.; Wang, F.; Shen, H.; Sun, G.; Feng, C.; Wang, X.; Chen, D.; Sun, X. Biocontrol efficacy of Bacillus siamensis LZ88 against brown spot disease of tobacco caused by Alternaria alternata. Biol. Control 2021, 154, 104508. [Google Scholar] [CrossRef]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Chang, W.T.; Chen, Y.C.; Jao, C.L. Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour. Technol. 2007, 98, 1224–1230. [Google Scholar] [CrossRef]
- Shanmugaiah, V.; Mathivanan, N.; Balasubramanian, N.; Manoharan, P.T. Optimization of cultural conditions for the production of chitinase by Bacillus heterosporous MML2270 isolated from rice rhizosphere soil. Afr. J. Biotechnol. 2008, 7, 2562–2568. [Google Scholar]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Feng, Y.; Tian, B.; Xiong, J.; Lin, G.; Cheng, L.; Zhang, T.; Li, X. Exploring IAA biosynthesis and plant growth promotion mechanism for tomato root endophytes with incomplete IAA synthesis pathways. Chem. Biol. Technol. Agric. 2024, 11, 187. [Google Scholar] [CrossRef]
- Moon, J.H.; Won, S.J.; Maung, C.E.H.; Choi, J.H.; Choi, S.I.; Ajuna, H.B.; Ahn, Y.S. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms 2021, 9, 821. [Google Scholar] [CrossRef]
- Im, S.M.; Yu, N.H.; Joen, H.W.; Kim, S.O.; Park, H.W.; Park, A.R.; Kim, J.C. Biological control of tomato bacterial wilt by oxydifficidin and difficidin-producing Bacillus methylotrophicus DR-08. Pestic. Biochem. Physiol. 2020, 163, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, J.; Peng, X.; Tang, X.; Zhou, P.; Xiang, J.; Deng, X. Rice WRKY4 acts as a transcriptional activator mediating defense responses toward Rhizoctonia solani, the causing agent of rice sheath blight. Plant Mol. Biol. 2015, 89, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, H.; Jang, J.C.; Xiao, T.; He, H.; Jiang, D.; Tang, X. OsWRKY80-OsWRKY4 module as a positive regulatory circuit in rice resistance against Rhizoctonia solani. Rice 2016, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yan, H.H.; Sun, S.M.; Wang, Y.Q.; Zhao, X.R.; Wang, H.Y. Use of Bacillus velezensis SDTB022 against tobacco black shank (TBS) and the biochemical mechanism involved. Biol. Control 2022, 165, 104785. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liang, J.; Wang, L.; Gao, W.; Jiang, J.; Chang, R. Surfactin and fengycin B extracted from Bacillus pumilus W-7 provide protection against potato late blight via distinct and synergistic mechanisms. Appl. Microbiol. Biotechnol. 2020, 104, 7467–7481. [Google Scholar] [CrossRef]
- Nakata, M.; Shiono, T.; Watanabe, Y.; Satoh, T. Salt stress-induced dissociation from cells of a germin-like protein with Mn-SOD activity and an increase in its mRNA in a moss, Barbula unguiculata. Plant Cell Physiol. 2002, 43, 1568–1574. [Google Scholar] [CrossRef][Green Version]
- Shine, M.B.; Yang, J.W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.Q.; Navarre, D.; Kachroo, A. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. New Phytol. 2016, 212, 627–636. [Google Scholar] [CrossRef]
- Richter, H.; Lieberei, R.; Strnad, M.; Novák, O.; Gruz, J.; Rensing, S.A.; von Schwartzenberg, K. Polyphenol oxidases in Physcomitrella: Functional PPO1 knockout modulates cytokinin-dependent development in the moss Physcomitrella patens. J. Exp. Bot. 2012, 63, 5121–5135. [Google Scholar] [CrossRef]
- Singh, R.; Sunder, S.; Kumar, P. Sheath blight of rice: Current status and perspectives. Indian Phytopathol 2016, 69, 340–351. [Google Scholar]
- Wei, Z.M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Collmer, A.; Beer, S.V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).