Histopathological Study of Host–Pathogen Interactions Between Cordyceps javanica PSUC002 and Hypothenemus hampei
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Coffee Berry Borer Samples
2.2. Preparation of Insect Pathogenic Fungi
2.3. Infestation Mechanism of C. javanica on the Coffee Berry Borer
2.4. Histology and Histopathology
2.5. Production and Measurement of Cuticle-Degrading Enzymes
2.6. TUNEL Analysis of Cell Death
2.7. Ultrastructural Investigations
2.8. Statistical Analysis
3. Results
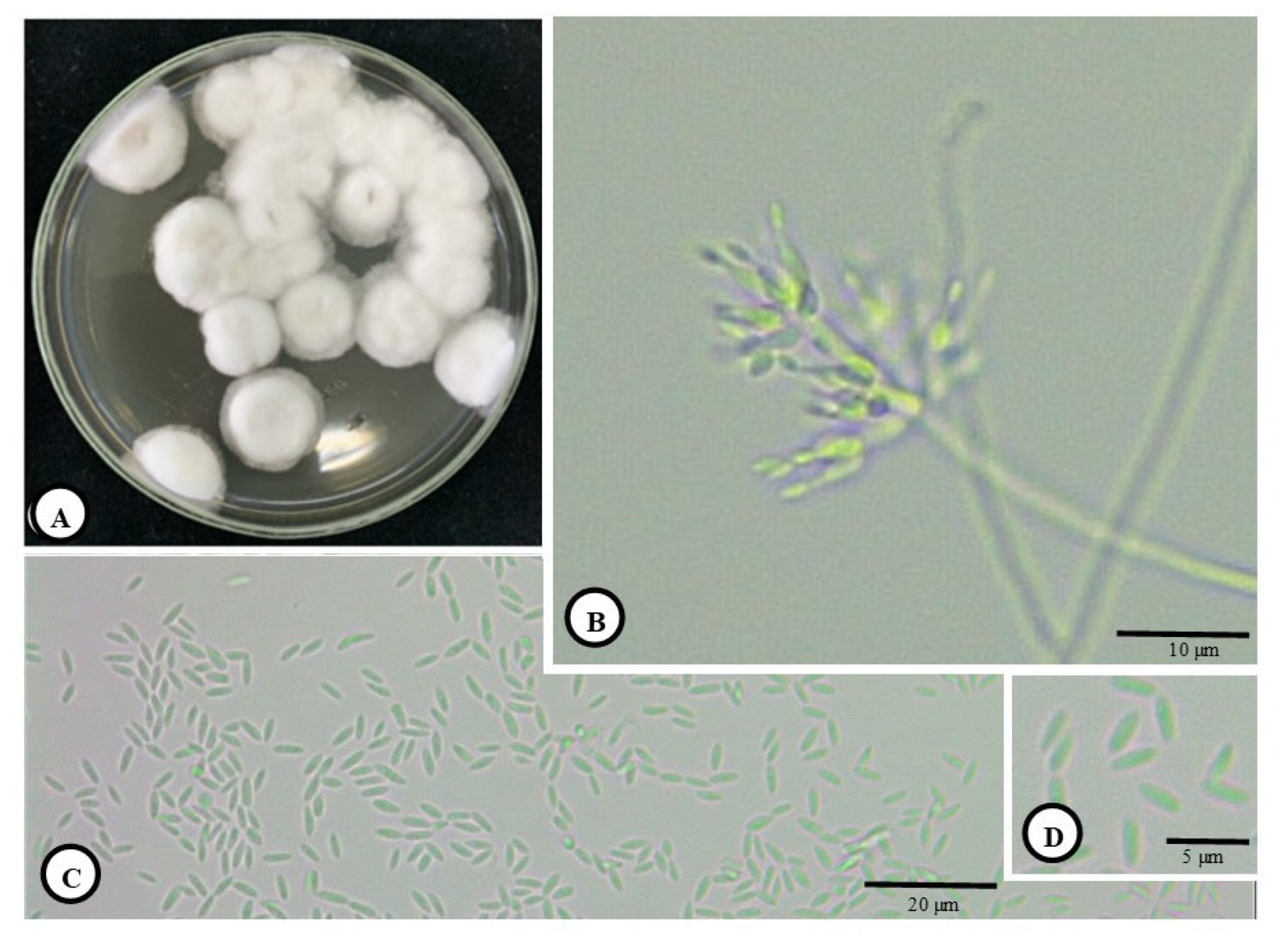
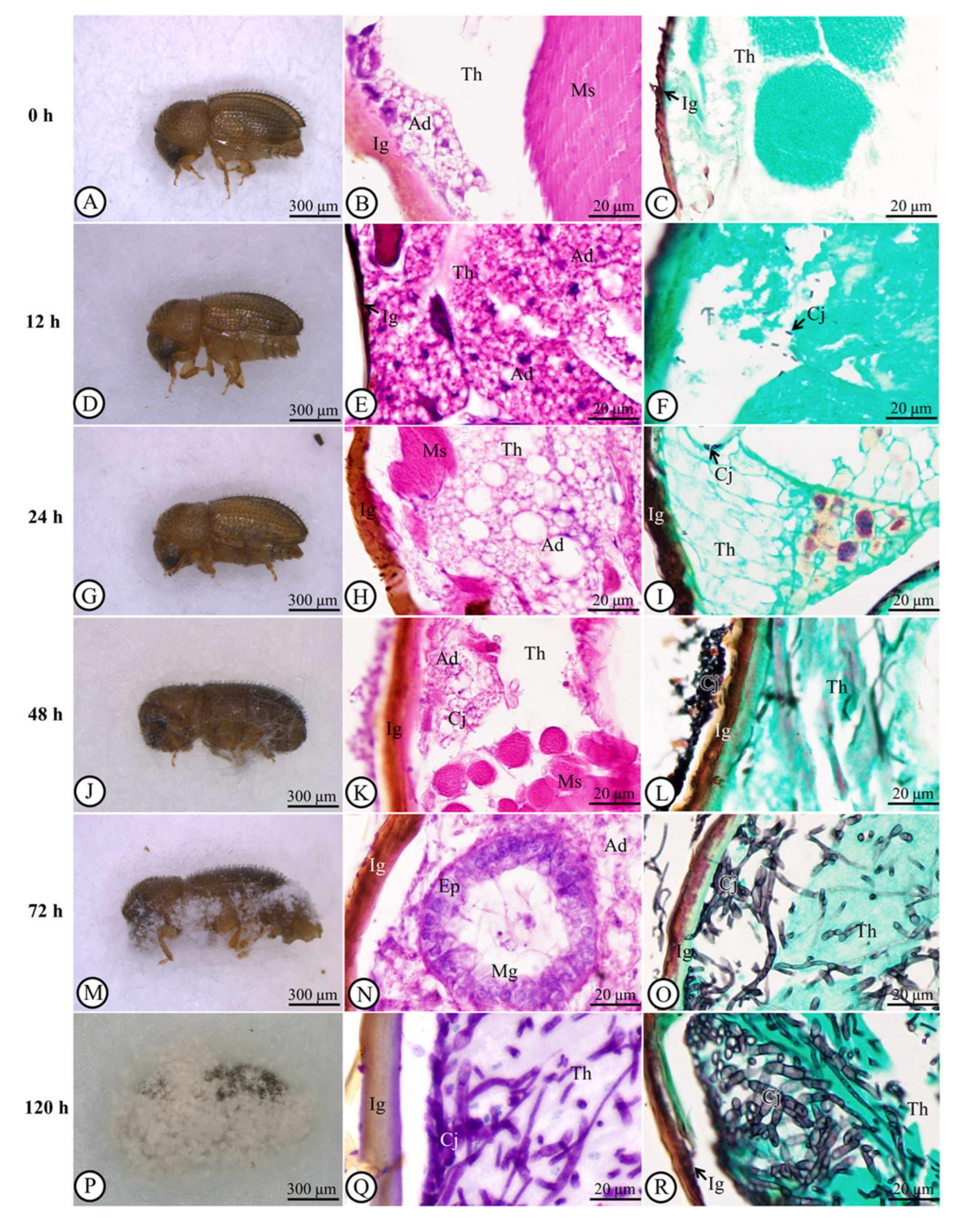
3.1. Morphology and Development of C. javanica and Penetration of Host Cuticle
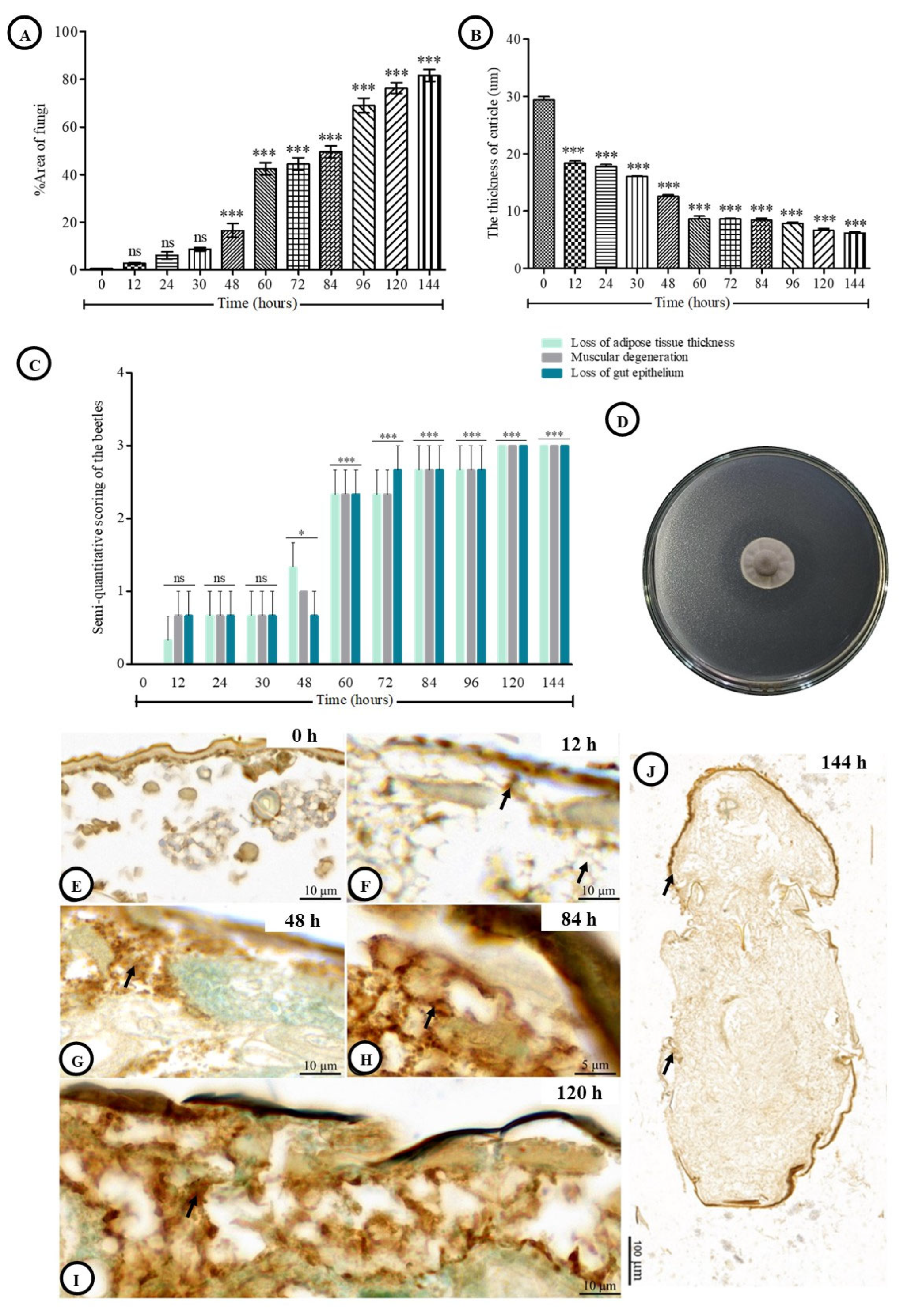
3.2. Fungal Spread in the Cuticle and Post-Inoculation Cuticle Thickness of Host
3.3. Histological Alteration Indexes (HAI)
3.4. The Determination of Lipase Activity
3.5. Semiquantitative Analytical Score of TUNEL-Positive Cells and PAS Reaction
3.6. Ultrastructural Observation
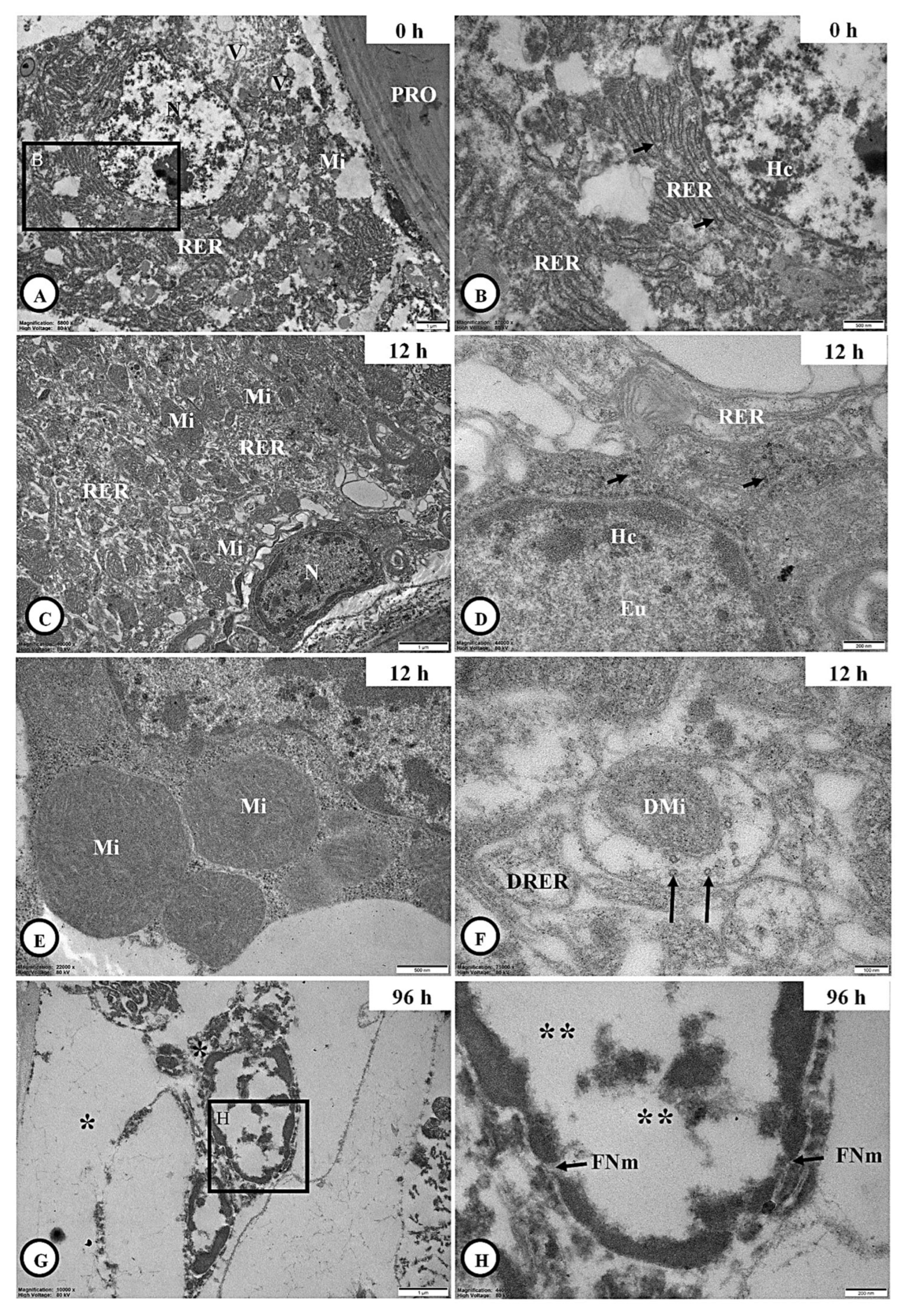
3.6.1. Integumental Ultrastructure
3.6.2. Adipocyte Ultrastructure
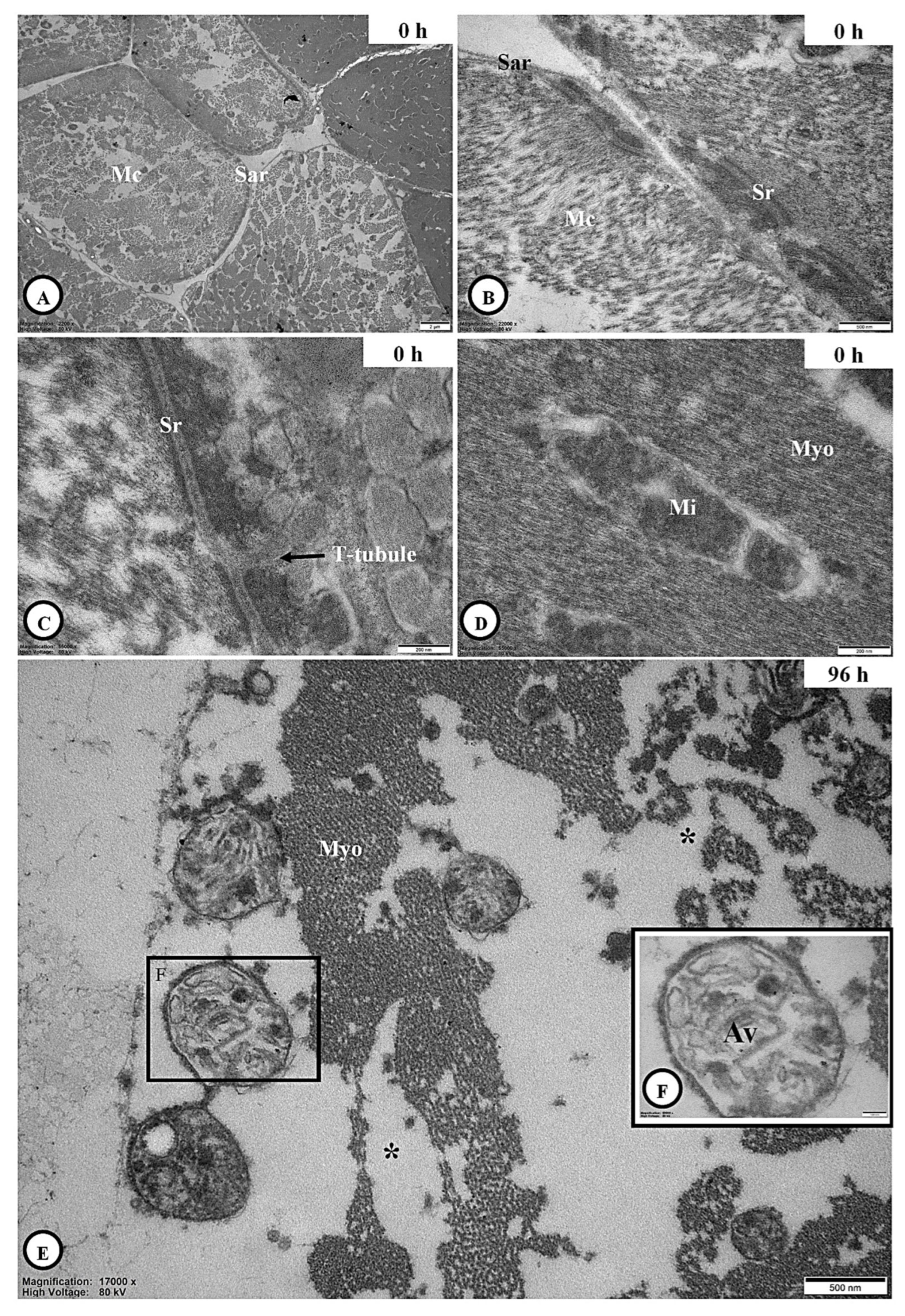
3.6.3. Skeletal Muscle Ultrastructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avelino, J.; Ten Hoopen, G.M.; DeClerck, F. Ecological mechanisms for pest and disease control in coffee and cacao agroecosystems of the neotropics. In Ecosystem Services from Agriculture and Agroforestry: Measurement and Payment, 4th ed.; Rapidel, B., DeClerck, F., Le Coq, J.F., Beer, J., Eds.; Earthscan Publications: London, UK, 2011; pp. 91–117. [Google Scholar]
- Harelimana, A.; Rukazambuga, D.; Hance, T. Pests and diseases regulation in coffee agroecosystems by management systems and resistance in changing climate conditions: A review. J. Plant Dis. Prot. 2022, 129, 1041–1052. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Arendse, W.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Roman, J.C.; van Bijsterveldt-Gels, J.E.M.; Wick, M.; Messéan, A. Challenges and opportunities for integrated pest management in Europe: A telling example of minor uses. Crop Prot. 2015, 74, 42–47. [Google Scholar] [CrossRef]
- Groenen, D. The effects of climate change on the pests and diseases of coffee crops in mesoamerica. J. Climatol. Weather Forecast. 2018, 6, 239. [Google Scholar] [CrossRef]
- Rice, R.A. Coffee in the crosshairs of climate change: Agroforestry as abatis. Agroecol. Sustain. Food Syst. 2018, 42, 1058–1076. [Google Scholar] [CrossRef]
- Vega, S.P.; Williams, C.J.; Brooks, E.S.; Pierson, F.B.; Strand, E.K.; Robichaud, P.R.; Brown, R.E.; Seyfried, M.S.; Lohse, K.A.; Glossner, K.; et al. Interaction of wind and cold-season hydrologic processes on erosion from complex topography following wildfire in sagebrush steppe. Earth Surf. Process. Landf. 2020, 45, 841–861. [Google Scholar] [CrossRef]
- Jaramillo, J.; Muchugu, E.; Vega, F.E.; Davis, A.; Borgemeister, C.; Chabi-Olaye, A. Some like it hot: The influence and implications of climate change on coffee berry borer (Hypothenemus hampei) and coffee production in East Africa. PLoS ONE 2011, 6, e24528. [Google Scholar] [CrossRef]
- Jonsson, M.; Ijala, A.R.; Ekbom, B.; Kyamanywa, S.; Karungi, J. Contrasting effect of shade levels and altitude on two important coffee pests. J. Pest Sci. 2015, 88, 281–287. [Google Scholar] [CrossRef]
- Barrera, J.F. Coffee Pests and their Management. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 961–998. [Google Scholar]
- Johnson, M.A.; Fortna, S.; Manoukis, N.C. Evaluation of Exclusion Netting for Coffee Berry Borer (Hypothenemus hampei) Management. Insects 2020, 11, 364. [Google Scholar] [CrossRef]
- Escobar-Ramírez, S.; Grass, I.; Armbrecht, I.; Tscharntke, T. Biological control of the coffee berry borer: Main natural enemies, control success, and landscape influence. Biol. Control 2019, 136, 103992. [Google Scholar] [CrossRef]
- Warit, S.; Stateva, L.I.; Palittapongarnpim, P. Cloning and heterologous expression of Cryptococcus neoformans CnSRB1 cDNA in Saccharomyces cerevisiae. Southeast Asian J. Trop. Med. Public Health 2008, 39, 484–491. [Google Scholar]
- Benavides, P.; Gongora, C.; Bustillo, A. IPM Program to control coffee berry borer Hypothenemus hampei, with emphasis on highly pathogenic mixed strains of Beauveria bassiana, to overcome insecticide resistance in Colombia. In Insecticides—Advances in Integrated Pest Management; InTech: London, UK, 2012; pp. 511–540. [Google Scholar]
- Sánchez-Pérez, R.; Del Cueto, J.; Dicenta, F.; Martínez-Gómez, P. Recent advancements to study flowering time in almond and other Prunus species. Front. Plant Sci. 2014, 5, 334. [Google Scholar]
- Thaochan, N.; Ngampongsai, A. Effects of autodisseminated Metarhizium guizhouense PSUM02 on mating propensity and mating competitiveness of Bactrocera cucurbitae (Diptera: Tephritidae). Biocontrol Sci. Technol. 2015, 25, 629–644. [Google Scholar] [CrossRef]
- Lacey, C.M.; Lacey, L.A.; Roberts, D.R. Route of invasion and histopathology of Metarhizium anisopliae in Culex quinquefasciatus. J. Invertebr. Pathol. 1988, 52, 108–118. [Google Scholar] [CrossRef]
- Toledo, A.V.; de Remes Lenicov, A.M.; López Lastra, C.C. Histopathology caused by the entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae, in the adult planthopper, Peregrinus maidis, a maize virus vector. J. Insect Sci. 2010, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, Í.J.; Pereira, R.; Freire, I.V.; de Oliveira, B.G.; Casotti, C.A.; Boery, E.N. Stress and quality of life among university students: A systematic literature review. Health Prof. Educ. 2018, 4, 70–77. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Musolino, V.; Lupia, C.; Palma, E.; Britti, D.; Musella, V. Entomopathogenic Fungi for Pests and Predators Control in Beekeeping. Vet. Sci. 2022, 9, 95. [Google Scholar] [CrossRef]
- Ibrahim, R.; Alahmadi, S.; Binnaser, Y.S.; Shawer, D. Seasonal prevalence and histopathology of Beauveria bassiana infecting larvae of the leopard moth, Zeuzera pyrina L. (Lepidoptera: Cossidae). Egypt. J. Biol. Pest. Control 2019, 29, 65. [Google Scholar] [CrossRef]
- Mondal, M.; Kumar, S.; Haris, A.A.; Dwivedi, S.K.; Bhatt, B.P.; Mishra, J.S. Effect of different rice establishment methods on soil physical properties in drought- prone, rainfed lowlands of Bihar. India Soil Res. 2016, 54, 997–1006. [Google Scholar] [CrossRef]
- Ali, S.; Wu, J.H.; Huang, Z.; Ren, S.X. Production and regulation of extracellular chitinase from the entomopathogenic fungus Isaria fumosorosea. Biocontrol Sci. Technol. 2010, 20, 723–738. [Google Scholar] [CrossRef]
- Gandarilla-Pacheco, F.L.; Arévalo-Niño, K.; Galán-Wong, L.J.; Coronado, C.F.; Quintero-Zapata, I. Evaluation of conidia production and mycelial growth in solid culture media from native strains of entomopathogenic fungi isolated from citrus-growing areas of México. Afr. J. Biotechnol. 2012, 11, 14453–14460. [Google Scholar] [CrossRef]
- Rojas, V.M.A.; Iwanicki, N.S.A.; D’Alessandro, C.P.; Fatoretto, M.B.; Demétrio, C.G.B.; Delalibera, I., Jr. Characterization of Brazilian Cordyceps fumosorosea isolates: Conidial production, tolerance to ultraviolet-B radiation, and elevated temperature. J. Invertebr. Pathol. 2023, 197, 107888. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Holmes, M.; Reynolds, P.N. Apoptosis in COPD. Curr. Respir. Med. Rev. 2005, 1, 33–41. [Google Scholar] [CrossRef]
- Luangsa-ard, J.J.; Hywel-Jones, N.L.; Manoch, L.; Samson, R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005, 109, 581–589. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Quintela, E.D.; Silva, E.G.; Arthurs, S.P. Precision micro-spray tower for application of entomopathogens. BioAssay 2013, 8, 1–4. [Google Scholar]
- Tian, W.; Zhang, Z.H.; Hu, X.F.; Tian, R.; Zhang, J.B.; Xiao, X.J.; Xi, Y.G. Short-term changes in total heavy metal concentration and bacterial community composition after replicated and heavy application of pig manure-based compost in an organic vegetable production system. Biol. Fertil. Soils. 2015, 51, 593–603. [Google Scholar] [CrossRef]
- Meyer, J.M.; Hoy, M.A. Removal of fungal contaminants and their DNA from the surface of Diaphorina citri (Hemiptera: Psyllidae) prior to a molecular survey of endosymbionts. Fla. Entomol. 2008, 91, 702–705. [Google Scholar]
- Kushiyev, R.; Tuncer, C.; Erper, I.; Ozdemir, I.O.; Saruhan, I. Efficacy of native entomopathogenic fungus, Isaria fumosorosea, against bark and ambrosia beetles, Anisandrus dispar Fabricius and Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). Egypt. J. Biol. Pest Control 2018, 28, 55. [Google Scholar] [CrossRef]
- Mier, T.; Reyes-Montes, M.d.R.; Barranco, H.N.; Rodríguez, A.Z.; Pérez-Torres, A.; Toriello, C.; Castellanos-Moguel, J. Fungal growth development index and ultrastructural study of whiteflies infected by three Isaria fumosorosea isolates of different pathogenicity. Rev. Mex. Mic 2013, 38, 23–33. [Google Scholar]
- Chen, Q.L.; Ding, J.; Zhu, Y.G.; He, J.Z.; Hu, H.W. Soil bacterial taxonomic diversity is critical to maintaining the plant productivity. Environ. Int. 2020, 140, 105766. [Google Scholar] [CrossRef]
- Johnson, A.; LeMay, G.; Hulcr, J. Identification of Coffee Berry Borer from Similar Bark Beetles in Southeast Asia and Oceania (Publication No. FR447). 2024. Available online: https://edis.ifas.ufl.edu/publication/FR447 (accessed on 1 March 2025).
- Rännbäck, L.M.; Cotes, B.; Anderson, P.; Rämert, B.; Meyling, N.V. Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J. Invertebr. Pathol. 2015, 124, 78–86. [Google Scholar] [CrossRef]
- Wilson, R.W.; Millero, F.J.; Taylor, J.R.; Walsh, P.J.; Christensen, V.; Jennings, S.; Grosell, M. Contribution of fish to the marine inorganic carbon cycle. Science 2009, 323, 359–362. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone Pub.: Edinburgh, UK, 2002; pp. 172–175, 593–620. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone Elsevier: Oxford, UK, 2013; p. 654. [Google Scholar]
- Dietrich, D.; Krieger, H.O. Histological Analysis of Endocrine Disruptive Effects in Small Laboratory Fish; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bunsap, P.; Senarat, S.; Niyomdecha, S.; Pornsuriya, C.; Kaneko, G.; Thaochan, N. Histopathological alterations in Nilaparvata lugens (Hemiptera: Delphacidae) after exposure to Cordyceps javanica. Insects 2024, 15, 565. [Google Scholar] [CrossRef]
- Ahmed, E.H.; Raghavendra, T.; Madamwar, D. An alkaline lipase from organic solvent tolerant Acinetobacter sp. EH28: Application for ethyl caprylate synthesis. Bioresour. Technol. 2010, 101, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.; Gillings, M.; Nevalainen, K. Qualitative assessment of hydrolytic activities in Antarctic microfungi grown at different temperatures on solid media. World J. Microbiol. Biotechnol. 1999, 15, 131–132. [Google Scholar] [CrossRef]
- Rojo, M.C.; Gonzalez, M.E. In situ detection of apoptotic cells by TUNEL in the gill epithelium of the developing brown trout (Salmo trutta). J. Anat. 1998, 193, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Samuels, R.I.; Pereira, R.C.; Gava, C.A. Infection of the coffee berry borer Hypothenemus hampei (Coleoptera: Scolytidae) by Brazilian isolates of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae (Deuteromycotina: Hyphomycetes). Biocontrol Sci. Technol. 2002, 12, 631–635. [Google Scholar] [CrossRef]
- Cure, J.R.; Rodríguez, D.; Gutierrez, A.P.; Ponti, L. The coffee agroecosystem: Bio-economic analysis of coffee berry borer control (Hypothenemus hampei). Sci. Rep. 2020, 10, 12262. [Google Scholar] [CrossRef]
- Bayman, P.; Mariño, Y.A.; García-Rodríguez, N.M.; Oduardo-Sierra, O.F.; Rehner, S.A. Local isolates of Beauveria bassiana for control of the coffee berry borer Hypothenemus hampei in Puerto Rico: Virulence, efficacy, and persistence. Biol. Control 2021, 155, 104533. [Google Scholar] [CrossRef]
- Chang, F.M.; Lu, H.L.; Nai, Y.S. Evaluation of potential entomopathogenic fungus, Beauveria bassiana, for controlling the coffee berry borer Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae) in Taiwa. J. Asia-Pac. Entomol. 2023, 26, 102118. [Google Scholar] [CrossRef]
- Boucias, D.G.; Pendland, J.C.; Latge, J.P. Nonspecific factors involved in attachment of entomopathogenic deuteromycetes to host insect cuticle. Appl. Environ. Microbiol. 1988, 54, 1795–1805. [Google Scholar] [CrossRef]
- Bitencourt, R.D.O.B.; Santos-Mallet, J.R.D.; Lowenberger, C.; Ventura, A.; Gôlo, P.S.; Bittencourt, V.R.E.P.; Angelo, I.D.C. A novel model of pathogenesis of Metarhizium anisopliae propagules through the midguts of Aedes aegypti larvae. Insects 2023, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Intodia, A.; Prasad, A.; Veerwal, B. Histopathology of Beauveria bassiana (balsamo) vuillemin, an entomopathogenic fungus, infection in the midgut of termite, Odontotermes obesus (r.) worker. Int. J. Recent Sci. Res. 2019, 10, 34326–34330. [Google Scholar]
- Soliman, A.; Nada, M.; Gad, A. Evaluation the Effects of the Entomopathogenic Fungus Beauveria bassiana (Ascomycota: Hypocrales) on some Histological and Physiological Parameters for the Green Bug Nezara viridula (L.) (Hemiptera: Pentatomidae). Alex. Sci. Exch. J. 2022, 43, 229–238. [Google Scholar] [CrossRef]
- Lei, Y.; Hussain, A.; Guan, Z.; Wang, D.; Jaleel, W.; Lyu, L.; He, Y. Unraveling the Mode of Action of Cordyceps fumosorosea: Potential Biocontrol Agent against Plutella xylostella (Lepidoptera: Plutellidae). Insects 2021, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Boguś, M.I.; Włóka, E.; Wrońska, A.K.; Krawiel, A.; Kazek, M.; Zalewska, K.; Kłocińska-Biały, K.; Sobocińska, M.; Gliniewicz, A.; et al. The interaction between cuticle free fatty acids (FFAs) of the cockroaches Blattella germanica and Blatta orientalis and hydrolases produced by the entomopathogenic fungus Conidiobolus coronatus. PLoS ONE 2020, 15, e0235785. [Google Scholar] [CrossRef]
- Arias-Aravena, M.; Altimira, F.; Gutiérrez, D.; Ling, J.; Tapia, E. Identification of exoenzymes secreted by entomopathogenic fungus Beauveria pseudobassiana RGM 2184 and their effect on the degradation of cocoons and pupae of Quarantine Pest Lobesia botrana. J. Fungi. 2022, 8, 1083. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Extracellular enzyme activity of entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae and their pathogenicity potential as a bio-control agent against whitefly pests, Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). BMC. Res. Notes 2022, 15, 117. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Kitsiou, F.; Natsiopoulos, D.; Eliopoulos, P.A. Entomopathogenic Fungi: Interactions and applications. Encyclopedia 2022, 2, 646–656. [Google Scholar] [CrossRef]
- Bihal, R.; Al-Khayri, J.M.; Banu, A.N.; Kudesia, N.; Ahmed, F.K.; Sarkar, R.; Arora, A.; Abd-Elsalam, K.A. Entomopathogenic fungi: An eco-friendly synthesis of sustainable nanoparticles and their nanopesticide properties. Microorganisms 2023, 11, 1617. [Google Scholar] [CrossRef]
- Liu, D.; Smagghe, G.; Liu, T.X. Interactions between entomopathogenic fungi and insects and prospects with glycans. J. Fungi. 2023, 9, 575. [Google Scholar] [CrossRef]

| Parameter | Times | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 12 | 48 | 84 | 96 | 120 | 144 | |
| TUNEL-positive cells | − | + | ++ | +++ | +++ | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senarat, S.; Bunsap, P.; Poolprasert, P.; Inchan, A.; Charoenphon, N.; Sornying, P.; Thaochan, N. Histopathological Study of Host–Pathogen Interactions Between Cordyceps javanica PSUC002 and Hypothenemus hampei. J. Fungi 2025, 11, 423. https://doi.org/10.3390/jof11060423
Senarat S, Bunsap P, Poolprasert P, Inchan A, Charoenphon N, Sornying P, Thaochan N. Histopathological Study of Host–Pathogen Interactions Between Cordyceps javanica PSUC002 and Hypothenemus hampei. Journal of Fungi. 2025; 11(6):423. https://doi.org/10.3390/jof11060423
Chicago/Turabian StyleSenarat, Sinlapachai, Peerasak Bunsap, Pisit Poolprasert, Anjaree Inchan, Natthawut Charoenphon, Peerapon Sornying, and Narit Thaochan. 2025. "Histopathological Study of Host–Pathogen Interactions Between Cordyceps javanica PSUC002 and Hypothenemus hampei" Journal of Fungi 11, no. 6: 423. https://doi.org/10.3390/jof11060423
APA StyleSenarat, S., Bunsap, P., Poolprasert, P., Inchan, A., Charoenphon, N., Sornying, P., & Thaochan, N. (2025). Histopathological Study of Host–Pathogen Interactions Between Cordyceps javanica PSUC002 and Hypothenemus hampei. Journal of Fungi, 11(6), 423. https://doi.org/10.3390/jof11060423








