Arsenic Stress Resistance in the Endophytic Fungus Cladosporium cladosporioides: Physiological and Transcriptomic Insights into Heavy Metal Detoxification
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Morphological and Molecular Identification of the Target Endophytic Fungus
2.3. Tolerance of C. cladosporioides to As(V)
2.4. Growth Curve of C. cladosporioides
2.5. Concentration and Distribution of Total As and Different Valence States of As in Mycelium
2.6. Antioxidant System of C. cladosporioides
2.7. Transcriptome Analysis and Availability of Supporting Data
2.8. Statistical Analysis
3. Results
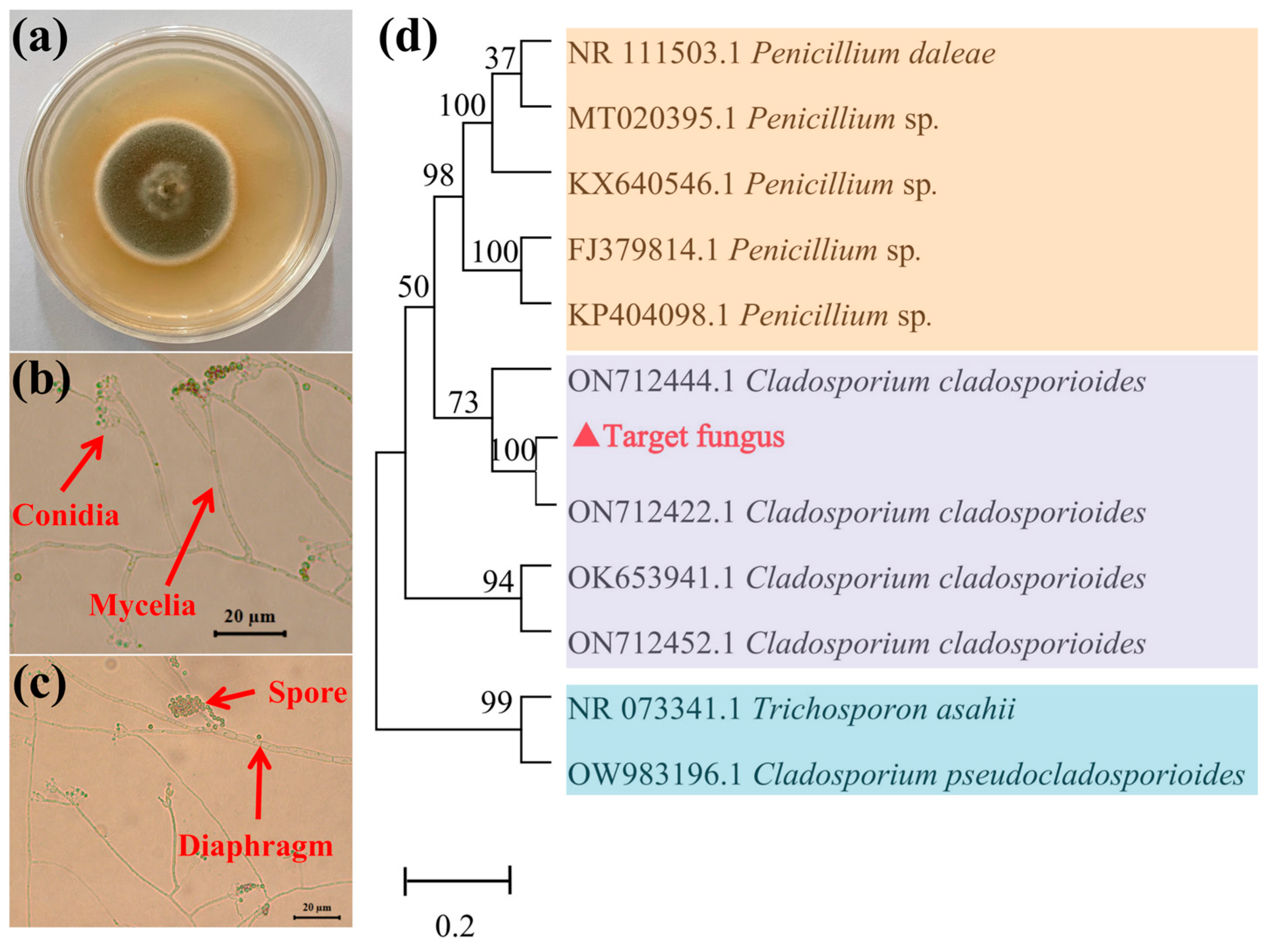
3.1. Taxonomic Identification of Target Fungus
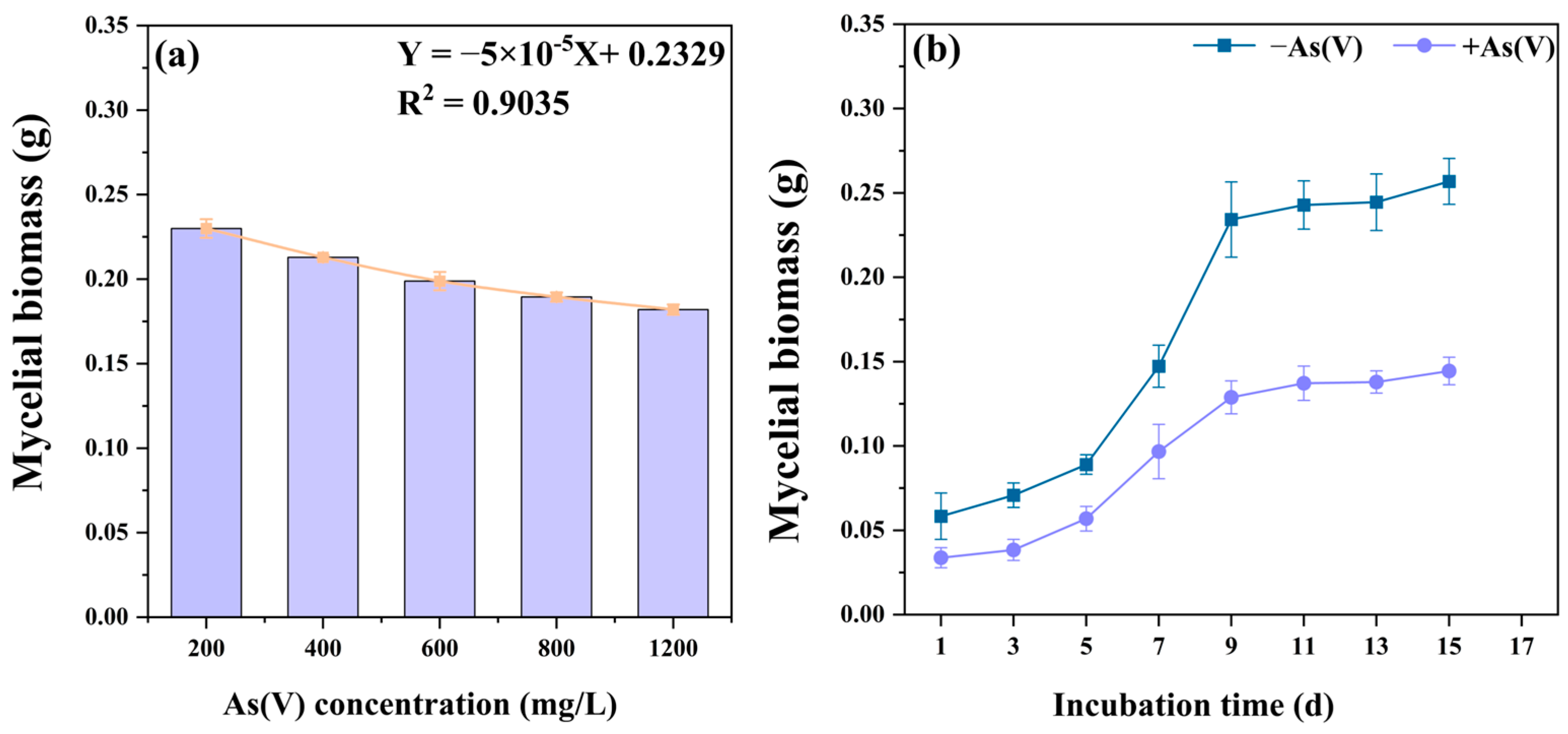
3.2. Tolerance of C. cladosporioides to As(V)
3.3. Accumulation of Total and Different Valence States of As in Mycelium
3.4. Changes in the Antioxidant System of C. cladosporioides Under As(V)
3.5. Transcriptome Sequencing and Assembly
3.6. Screening and Validation of DEGs and Functional Annotation
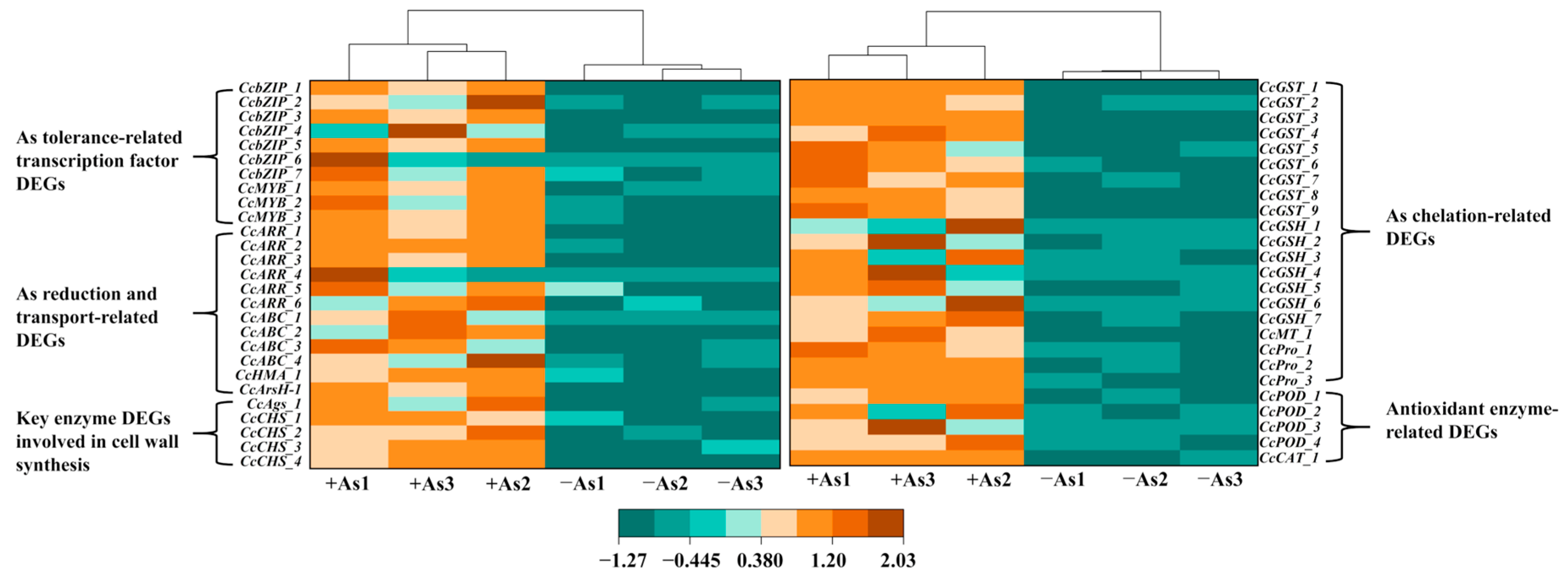
3.7. Identification of DEGs Involved in the As Resistance of C. cladosporioides
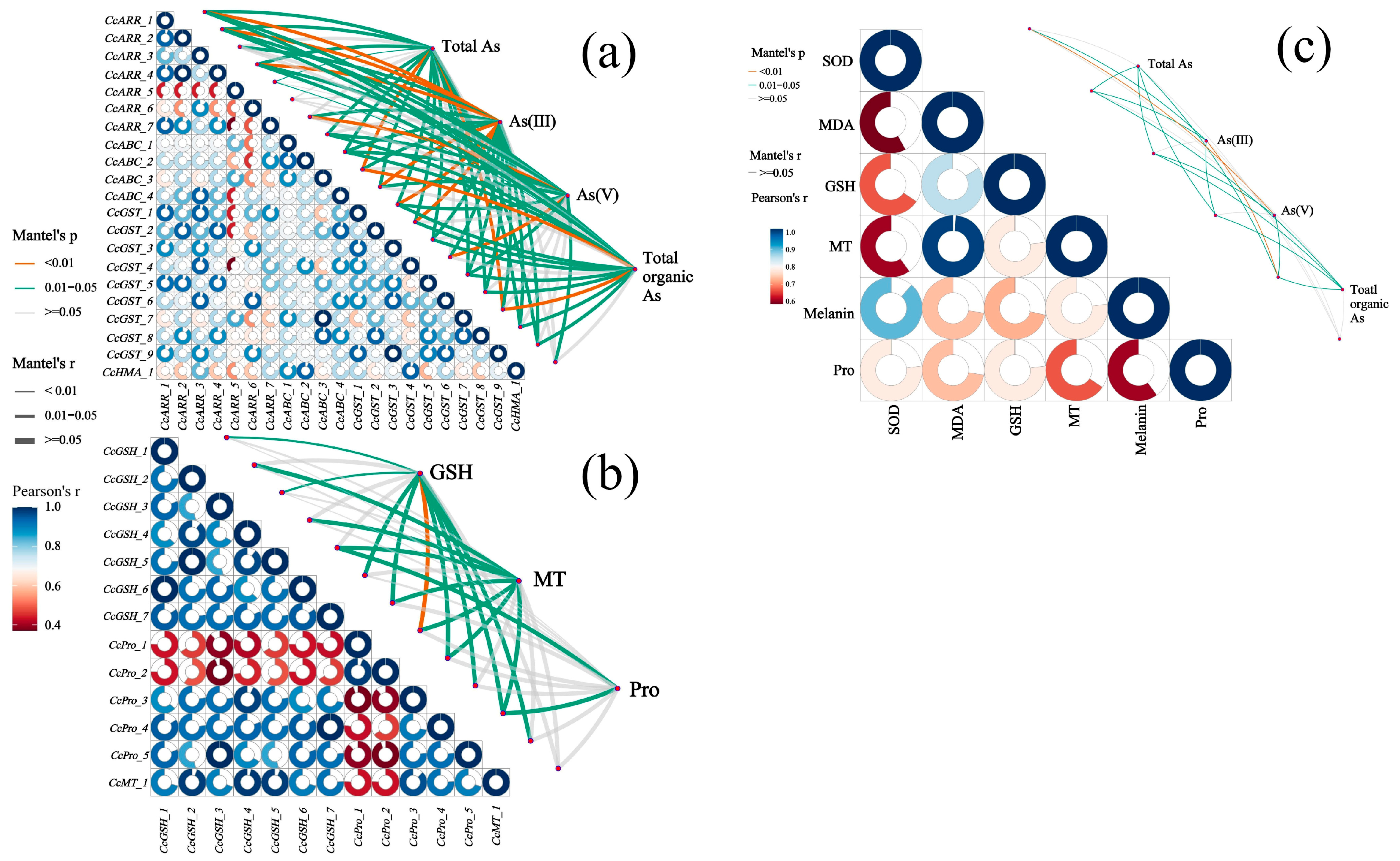
3.8. Multiple Correlation Analysis Among As Speciation, Antioxidant System, and Related DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustafa, A.M.; Caprioli, G.; Dikmen, M.; Kaya, E.; Maggi, F.; Sagratini, G.; Vittori, S.; Öztürk, Y. Evaluation of neuritogenic activity of cultivated, wild and commercial roots of Gentiana lutea L. J. Funct. Foods 2015, 15, 164–173. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Fang, Y.; Ma, C.; Zhang, Z.; Zhu, X.; Wang, J.; Liu, D. Investigation and analysis on resources of chinese material Gentiana rigescens Franch. Southwest China J. Agric. Sci. 2017, 30, 267–272. (In Chinese) [Google Scholar]
- Pan, Y.; Zhao, Y.L.; Zhang, J.; Li, W.Y.; Wang, Y.Z. Phytochemistry and pharmacological activities of the genus Gentiana (Gentianaceae). Chem. Biodivers. 2016, 13, 107–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Zou, J.Y.; Sun, H.; Qin, J.H.; Yang, J.Y. Metals in Traditional Chinese medicinal materials (TCMM): A systematic review. Ecotoxicol. Environ. Saf. 2021, 207, 111311. [Google Scholar] [CrossRef]
- Chen, J.; Qin, L.P. Microbial interactions within Chinese traditional medicinal plants. Chin. Herb. Med. 2024, 16, 169–171. [Google Scholar] [CrossRef]
- Domka, A.M.; Rozpaądek, P.; Turnau, K. Are fungal endophytes merely mycorrhizal copycats? The role of fungal endophytes in the adaptation of plants to metal toxicity. Front. Microbiol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Andrade, G.V.S.; Rodrigues, F.A.; Nadal, M.C.; da Silva Dambroz, C.M.; Martins, A.D.; Rodrigues, V.A.; dos Reis Ferreira, G.M.; Pasqual, M.; Buttros, V.H.; Dória, J. Plant-endophytic bacteria interactions associated with root and leaf microbiomes of Cattleya walkeriana and their effect on plant growth. Sci. Hort. 2023, 309, 111656. [Google Scholar] [CrossRef]
- Lindblom, S.D.; Wangeline, A.L.; Barillas, J.R.V.; Devilbiss, B.; Fakra, S.C.; Pilon-Smits, E.A.H. Fungal endophyte Alternaria tenuissima can affect growth and selenium accumulation in its hyperaccumulator host Astragalus bisulcatus. Front. Plant Sci. 2018, 9, 1213. [Google Scholar] [CrossRef]
- Lu, W.; Huang, S.; Zhang, N.; Li, F.; Wang, J.; Han, D.; Bao, L. Occurrence, Origin and Risk Assessment of Trace Elements in High Geological Background Impacted Soil-crop Systems in Yunnan, China. Soil Sediment Contam. 2021, 31, 515–532. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, M.F.; Ahmad, I.; Ashfaq, F.; Wahab, S.; Alsayegh, A.A.; Kumar, S.; Hakeem, K.R. Arsenic Exposure through Dietary Intake and Associated Health Hazards in the Middle East. Nutrients 2022, 14, 2136. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathogen. 2015, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the Role of Dark Septate Endophytes in the Plant Resistance to Abiotic and Biotic Stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.; Mohd, S.; Kushwaha, A.S.; Narayan, S.; Saxena, P.N.; Bahadur, L.; Mishra, A.; Shirke, P.A.; Kumar, M. Endophytic fungus Serendipita indica reduces arsenic mobilization from root to fruit in colonized tomato plant. Environ. Pollut. 2021, 298, 118830. [Google Scholar] [CrossRef]
- Babu, A.G.; Shim, J.; Bang, K.S.; Shea, P.J.; Oh, B.T. Trichoderma virens PDR-28: A heavy metal-tolerant and plant growth-promoting fungus for remediation and bioenergy crop production on mine tailing soil. J. Environ. Manag. 2014, 132, 129–134. [Google Scholar] [CrossRef]
- El-Mahdy, O.M.; Mohamed, H.I.; Mogazy, A.M. Biosorption effect of Aspergillus niger and Penicillium chrysosporium for Cd- and Pb-contaminated soil and their physiological effects on Vicia fab L. Environ. Sci. Pollut. Res. 2021, 28, 67608–67631. [Google Scholar] [CrossRef]
- Meier, S.; Borie, F.; Bolan, N.; Cornejo, P. Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit. Rev. Environ. Sci. Technol. 2012, 42, 741–775. [Google Scholar] [CrossRef]
- Bibi, S.; Hussain, A.; Hamayun, M.; Rahman, H.; Iqbal, A.; Shah, M.; Irshad, M.; Qasim, M.; Islam, B. Bioremediation of hexavalent chro-mium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 2018, 211, 653–663. [Google Scholar] [CrossRef]
- Vasak, M.; Hasler, D. Metallothioneins: New functional and structural insights. Curr. Opin. Chem. Biol. 2000, 4, 177–183. [Google Scholar] [CrossRef]
- Chen, C.; Yang, B.Y.; Gao, A.X.; Yu, Y.; Zhao, F.J. Transformation of arsenic species by diverse endophytic bacteria of rice roots. Environ. Pollut. 2022, 309, 119825. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, D.K.; Qiao, Q.; Liu, L.; Wang, J.L.; Cao, G.H.; Li, T.; Zhao, Z.W. Identification of glutathione S-transferase (GST) genes from a dark septate endophytic fungus (Exophiala pisciphila) and their expression patterns under varied metals stress. PLoS ONE 2015, 10, e0123418. [Google Scholar] [CrossRef]
- Reddy, M.S.; Prasanna, L.; Marmeisse, R.; Fraissinet-Tachet, L. Differential expression of metallothioneins in response to heavy metals and their involvement in metal tolerance in the symbiotic basidiomycete Laccaria bicolor. Microbiology 2014, 160, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, Y.; Dong, Z.; Guo, Y.; Luo, J.; Wang, F.; Yan, L.; Zou, X. Endophytic Fungal Diversity and Its Interaction Mechanism with Medicinal Plants. Molecules 2025, 30, 1028. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Shrestha, R.; Maharjan, S.; Selosse, M.-A.; Pant, B. Isolation and Characterization of Plant Growth-Promoting Endophytic Fungi from the Roots of Dendrobium moniliforme. Plants 2019, 8, 5. [Google Scholar] [CrossRef]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef]
- Wei, J.C. Fungal Identification Handbook; Shanghai Scientific and Technical Publishers: Shanghai, China, 1979. (In Chinese) [Google Scholar]
- Crouch, J.A.; Clarke, B.B.; Hillman, B.I. Phylogenetic relationships and fungicide sensitivities of Colletotrichum graminicola isolates from turfgrass in North America. Int. Turfgrass Soc. Res. 2005, 10, 186–195. [Google Scholar]
- Sieber, T.N.; Grünig, C.R. Fungal Root Endophytes; Marcel Dekker: New York, NY, USA, 2013. [Google Scholar]
- Gharsallah, H.; Ksentini, I.; Naayma, S.; Taieb, K.H.; Abdelhedi, N.; Schuster, C.; Triki, M.A.; Ksantini, M.; Leclerque, A. Identification of fungi in Tunisian olive orchards: Characterization and biological control potential. BMC Microbiol. 2020, 20, 307. [Google Scholar] [CrossRef]
- Li, X.C. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food. Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef]
- Diao, Y.H.; Li, T.; Zhao, Z.W. Zinc accumulation characteristics of two Exophiala strains and their antioxidant response to Zn2+ stress. J. Environ. Prot. Ecol. 2013, 4, 12–19. [Google Scholar] [CrossRef]
- Lankinen, P.; Kähkönen, M.A.; Rajasärkkä, J.; Virta, M.P.; Hatakka, A.I. The effect of nickel contamination on the growth of litter decomposing fungi, extracellular enzyme activities and toxicity in soil. Boreal Environ. Res. 2011, 16, 229–239. [Google Scholar]
- Teng, Y.; Du, X.Z.; Wang, T.; Mi, C.Y.; Yu, H.Y.; Zou, L.Y. Isolation of a fungus Pencicillium sp. with zinc tolerance and its mechanism of resistance. Arch. Microbiol. 2018, 200, 159–169. [Google Scholar]
- Sun, C.L.; Lu, L.L.; Liu, L.J.; Liu, W.J.; Yu, Y.; Liu, X.X.; Hu, Y.; Jin, C.W.; Lin, Y.X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.H.; Li, X.G.; Zhang, C.R.; Xiong, Y.R.; Li, X.; Li, T.; He, S.; Cui, Z.G.; Yu, J. Physiological response mechanism of heavy metal-resistant endophytic fungi isolated from the roots of Polygonatum kingianum. Environ. Microbiol. Rep. 2023, 15, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Zhang, G.J.; Gao, W.J.; Zhao, X.P.; Deng, C.L.; Lu, L.D. Plant growth, development and change in GSH level in safflower (Carthamus tinctorius L.) exposed to copper and lead. Arch. Biol. Sci. 2015, 67, 385–396. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.B.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Jamil, M.; Malook, I.; Rehman, S.U.; Aslam, M.M.; Fayyaz, M.; Shah, G.; Kaplan, A.; Khan, M.N.; Ali, B.; Roy, R.; et al. Inoculation of heavy metal resistant bacteria alleviated heavy metal-induced oxidative stress biomarkers in spinach (Spinacia oleracea L.). BMC Plant Bio. 2024, 24, 221. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.Y.; Yu, X.L.; Kiprotich, F.; Han, H.; Guan, R.Z.; Wang, R.; Shen, W.B. Nitric Oxide is Required for Melatonin-Enhanced Tolerance against Salinity Stress in Rapeseed (Brassica napus L.) Seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Gene Ontol. Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Huang, H. linkET: Everything is Linkable. R Package Version 0.0.3. Available online: https://github.com/Hy4m/linKET (accessed on 10 June 2024).
- Wang, H.R.; Du, X.R.; Zhang, Z.Y.; Feng, F.J.; Zhang, J.M. Rhizosphere interface microbiome reassembly by arbuscular mycorrhizal fungi weakens cadmium migration dynamics. Imeta 2023, 2, 133. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress. Sci. Rep. 2016, 6, 22028. [Google Scholar]
- Parmar, S.; Sharma, V.K.; Li, T.; Tang, W.; Li, H. Fungal Seed Endophyte FZT214 Improves Dysphania ambrosioides Cd Tolerance Throughout Different Developmental Stages. Front. Microbiol. 2022, 12, 783475. [Google Scholar] [CrossRef]
- Yang, X.F.; Yan, C.X.; Zhang, R.; Sun, Y.P.; Li, Z.G.; Liu, Y.; Yang, S.C.; Shen, L.; Wen, X.D. Determination of trace metal ions in Gentiana rigescens by inductively coupled plasma-optical emission spectrometry after deep eutectic solvent-based digestion and related pharmacodynamic evaluation. Anal. Chim. Acta. 2022, 1221, 340109. [Google Scholar] [CrossRef]
- Văcar, C.L.; Covaci, E.; Chakraborty, S.; Li, B.; Weindorf, D.C.; Frentiu, T.; Pârvu, M.; Podar, D. Heavy Metal-Resistant Filamentous Fungi as Potential Mercury Bioremediators. J. Fungi 2021, 7, 386. [Google Scholar] [CrossRef]
- Wymer, L.; Vesper, S.; Struewing, I.; Allen, J.; Lu, J.R. Possible Antagonism between Cladosporium cladosporioides and Microcystis aeruginosa in a Freshwater Lake during Bloom Seasons. Life 2022, 12, 742. [Google Scholar] [CrossRef]
- Shadmani, L.; Jamali, S.; Fatemi, A. Isolation, identification, and characterization of cadmium-tolerant endophytic fungi isolated from barley (Hordeum vulgare L.) roots and their role in enhancing phytoremediation. Braz. J. Microbiol. 2021, 52, 1097–1106. [Google Scholar] [CrossRef]
- Lin, L.C. Zinc resistance of six dark septate endophytic fungi and the growth response of tomato. Appl. Ecol. Environ. Res. 2019, 18, 389–400. [Google Scholar] [CrossRef]
- Ban, Y.H.; Tang, M.; Chen, H.; Xu, Z.Y.; Zhang, H.H.; Yang, Y.R. The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS ONE 2012, 7, e47968. [Google Scholar] [CrossRef]
- Hou, L.F.; Yu, J.; Zhao, L.L.; He, X.L. Dark Septate Endophytes Improve the Growth and the Tolerance of Medicago sativa and Ammopiptanthus mongolicus Under Cadmium Stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.D.; He, Y.M.; Li, T.; Yang, Y.Y.; Toor, G.S.; Zhao, Z.W. Tolerance and Antioxidant Response of a Dark Septate Endophyte (DSE), Exophiala pisciphila, to Cadmium Stress. Bull. Environ. Contam. Toxicol. 2015, 94, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mohd, S.; Shukla, J.; Kushwaha, A.S.; Mandrah, K.; Shankar, J.; Arjaria, N.; Saxena, P.N.; Narayan, R.; Roy, S.K.; Kumar, M. Endophytic Fungi Piriformospora indica Mediated Protection of Host from Arsenic Toxicity. Front. Microbiol. 2017, 8, 754. [Google Scholar] [CrossRef]
- Shakya, M.; Sharma, P.; Meryem, S.S.; Mahmood, Q.; Kumar, A. Heavymetal removal from industrial wastewater using fungi: Uptake mechanism and biochemical aspects. J. Environ. Eng. 2016, 142, 6015001. [Google Scholar] [CrossRef]
- Berthelot, C.; Chalot, M.; Leyval, C.; Blaudez, D. From Darkness to Light: Emergence of the Mysterious Dark Septate Endophytes in Plant Growth Promotion and Stress Alleviation. In Endophytes for a Growing World; Hodkinson, T.R., Doohan, F.M., Saunders, M.J., Murphy, B.R., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 143–164. [Google Scholar]
- Gupta, A.; Dubey, P.; Kumar, M.; Roy, A.; Sharma, D.; Khan, M.M.; Bajpai, A.B.; Shukla, R.P.; Pathak, N.; Hasanuzzaman, M. Consequences of Arsenic Contamination on Plants and Mycoremediation-Mediated Arsenic Stress Tolerance for Sustainable Agriculture. Plants 2022, 11, 3220. [Google Scholar] [CrossRef]
- Mandal, D.; Aghababaei, M.; Das, S.K.; Majumder, S.; Chatterjee, D.; Basu, A. Isolation and Identification of Arsenic Hyper-Tolerant Bacterium with Potential Plant Growth Promoting Properties from Soil. Minerals 2022, 12, 1452. [Google Scholar] [CrossRef]
- Thomas, D.J. Arsenic methylation-Lessons from three decades of research. Toxicology 2021, 457, 152800. [Google Scholar] [CrossRef]
- Dong, O.; Powers, M.; Liu, Z.; Yoshinaga, M. Arsenic Metabolism, Toxicity and Accumulation in the White Button Mushroom Agaricus bisporus. Toxics 2022, 10, 554. [Google Scholar] [CrossRef]
- Sun, H.Q.; Meng, M.; Wu, L.R.; Zheng, X.M.; Zhu, Z.Y.; Dai, S.H. Function and mechanism of polysaccharide on enhancing tolerance of Trichoderma asperellum under Pb2+ stress. Int. J. Biol. Macromol. 2020, 151, 509–518. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Cui, P.; Shen, E.H.; Farooq, M.A.; Islam, F.; Ali, S.; Mao, B.Z.; Zhou, W.J. Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genom. 2016, 17, 885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Lu, J.M.; Zhu, Y.C.; Shen, X.X.; Zhu, B.; Qin, L.P. Roles of endophytic fungi in medicinal plant abiotic stress response and TCM quality development. Chin. Herb. Med. 2024, 16, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Das, D.; Mondal, S.K.; Biswas, R.; Das, T.K.; Boujedaini, N.; Khuda-Bukhsh, A.R. Tolerance of arsenate-induced stress in Aspergillus niger, a possible candidate for bioremediation. Ecotoxicol. Environ. Saf. 2010, 73, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Bellion, M.; Courbot, M.; Jacob, C.; Guinet, F.; Blaudez, D.; Chalot, M. Metal induction of a Paxillus involutus metal-lothionein and its heterologous expression in Hebeloma cylin-drosporum. New Phytol. 2007, 174, 151–158. [Google Scholar] [CrossRef]
- Khullar, S.; Sudhakara Reddy, M.S. Cadmium and arsenic responses in the ectomycorrhizal fungus Laccaria bicolor: Gluta-thione metabolism and its role in metal (loid) homeostasis. Environ. Microbiol. Rep. 2019, 11, 53–61. [Google Scholar] [CrossRef]
- Zhao, D.K.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.W.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, A.; Banerjee, A.; Das, N. Role of melatonin in fungi, with special emphasis to morphogenesis and stress tolerance. S. Afr. J. Bot. 2024, 166, 413–422. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Zeng, G.M.; Chen, A.W.; Chen, G.Q.; Hu, X.J.; Guan, S.; Shang, C.; Lu, L.H.; Zou, Z.J. Responses of Phan-erochaete chrysosporium to Toxic Pollutants: Physio- logical Flux, Oxidative Stress, and Detoxification. Environ. Sci. Ecotechnol. 2012, 46, 7818–7825. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, F.; Chen, J.; Sun, G.X. Arsenic removal from contaminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. J. Environ. Sci. 2011, 23, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.H.; He, S.; Chen, D.; Li, T.; Zhao, Z.W. EpABC Genes in the Adaptive Responses of Exophiala pisciphila to Metal Stress: Functional Importance and Relation to Metal Tolerance. Appl. Environ. Microbiol. 2019, 85, e01844-19. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Park, M.; Park, J.I.; Kim, J.K.; Yum, S.; Kim, Y.J. Transcriptomic Changes Induced by Low and High Concentrations of Heavy Metal Exposure in Ulva pertusa. Toxics 2023, 1, 549. [Google Scholar] [CrossRef]
- Flores-Alvarez, L.J.; Corrales-Escobosa, A.R.; Cortés-Penagos, C.; Martínez-Pacheco, M.; Wrobel-Zasada, K.; Wrobel-Kaczmarczyk, K.; Cervantes, C.; Gutiérrez-Corona, F. The Neurospora crassa chr-1 gene is up-regulated by chromate and its encoded CHR-1 protein causes chromate sensitivity and chromium accumulation. Curr. Genet. 2012, 58, 281–290. [Google Scholar] [CrossRef]
- Ma, J.; Chen, D.W.; Xu, Y.F.; Liu, Y.; Liu, L.L.; Huang, J.; Gao, R.C.; Bai, J.; Hou, Q.Z. Effects of Different Heavy Metal Stressors on the Endophytic Community Composition and Diversity of Symphytum officinale. Microorganisms 2024, 12, 477. [Google Scholar] [CrossRef]
- Khan, I.; Asaf, S.; Kang, S.M.; Lee, I.J. Physiological mechanisms of heavy metal detoxification in tomato plants mediated by endophytic fungi under nickel and cadmium stress. Plant Physiol. Biochem. 2025, 221, 109589. [Google Scholar] [CrossRef]
- Rahman, S.U.; Khalid, M.; Kayani, S.I.; Tang, K. The ameliorative effects of exogenous inoculation of Piriformospora indica on molecular, biochemical and physiological parameters of Artemisia annua L. under arsenic stress condition. Ecotoxicol. Environ. Saf. 2020, 206, 111202. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, X.-X.; Li, X.-G.; Zhang, X.-K.; Gu, W.; Chen, D.; He, S.; Cao, G.-H. Arsenic Stress Resistance in the Endophytic Fungus Cladosporium cladosporioides: Physiological and Transcriptomic Insights into Heavy Metal Detoxification. J. Fungi 2025, 11, 374. https://doi.org/10.3390/jof11050374
You X-X, Li X-G, Zhang X-K, Gu W, Chen D, He S, Cao G-H. Arsenic Stress Resistance in the Endophytic Fungus Cladosporium cladosporioides: Physiological and Transcriptomic Insights into Heavy Metal Detoxification. Journal of Fungi. 2025; 11(5):374. https://doi.org/10.3390/jof11050374
Chicago/Turabian StyleYou, Xiao-Xu, Xiao-Gang Li, Xing-Kai Zhang, Wen Gu, Di Chen, Sen He, and Guan-Hua Cao. 2025. "Arsenic Stress Resistance in the Endophytic Fungus Cladosporium cladosporioides: Physiological and Transcriptomic Insights into Heavy Metal Detoxification" Journal of Fungi 11, no. 5: 374. https://doi.org/10.3390/jof11050374
APA StyleYou, X.-X., Li, X.-G., Zhang, X.-K., Gu, W., Chen, D., He, S., & Cao, G.-H. (2025). Arsenic Stress Resistance in the Endophytic Fungus Cladosporium cladosporioides: Physiological and Transcriptomic Insights into Heavy Metal Detoxification. Journal of Fungi, 11(5), 374. https://doi.org/10.3390/jof11050374





