Phenotypic and Molecular Characterization of Candida albicans Isolates from Mexican Women with Vulvovaginitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Sampling
2.6. Yeast Isolation
2.7. Phenotypic Identification of C. albicans
Germ Tube Test
2.8. DNA Extraction
2.9. Molecular Identification of C. albicans
2.10. Genotypic Characterization
ABC Genotyping
2.11. Phenotypic Characterization
Hydrolytic Enzyme Activity
2.12. Statistical Analysis
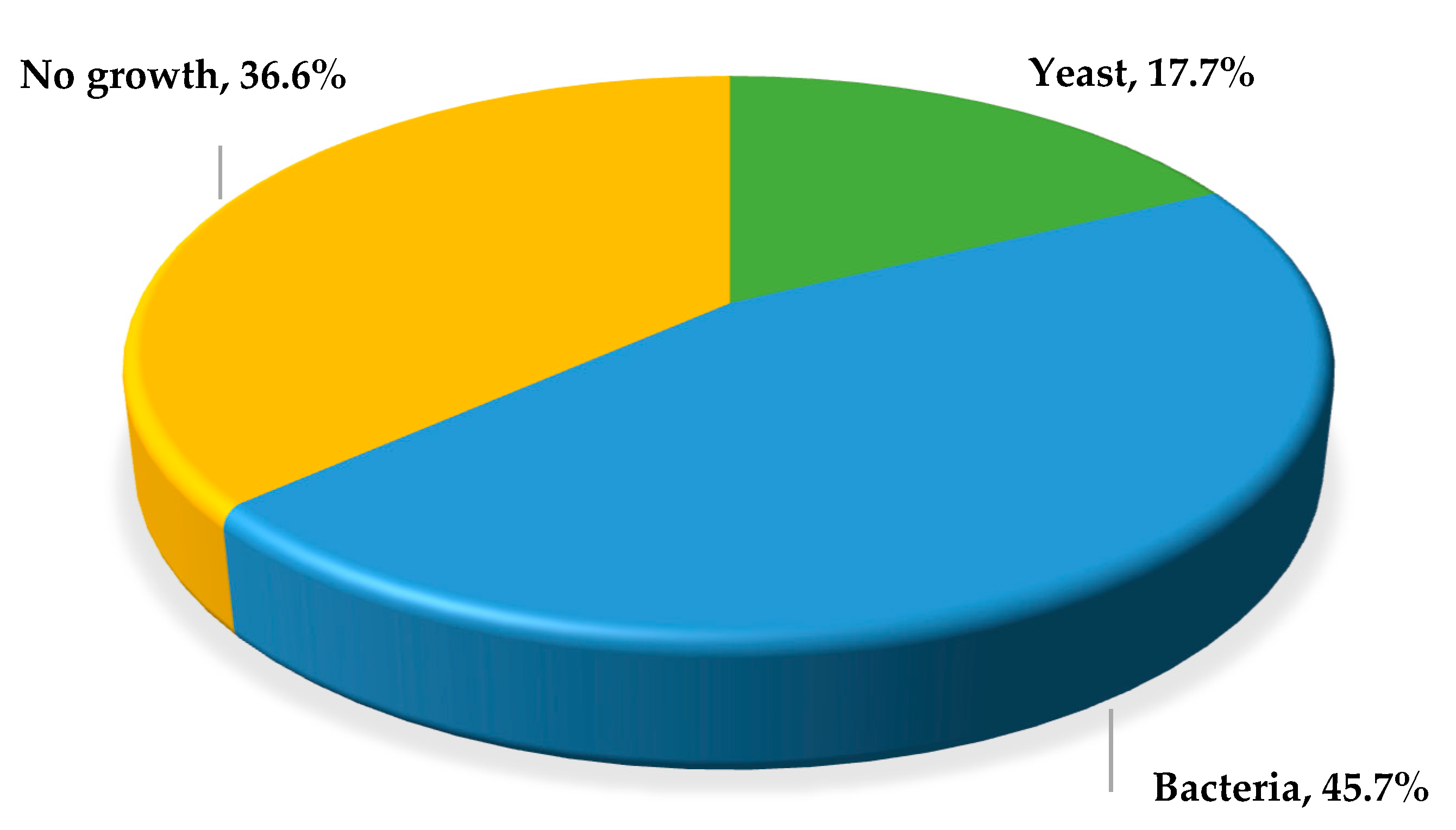
3. Results
3.1. Identification of C. albicans
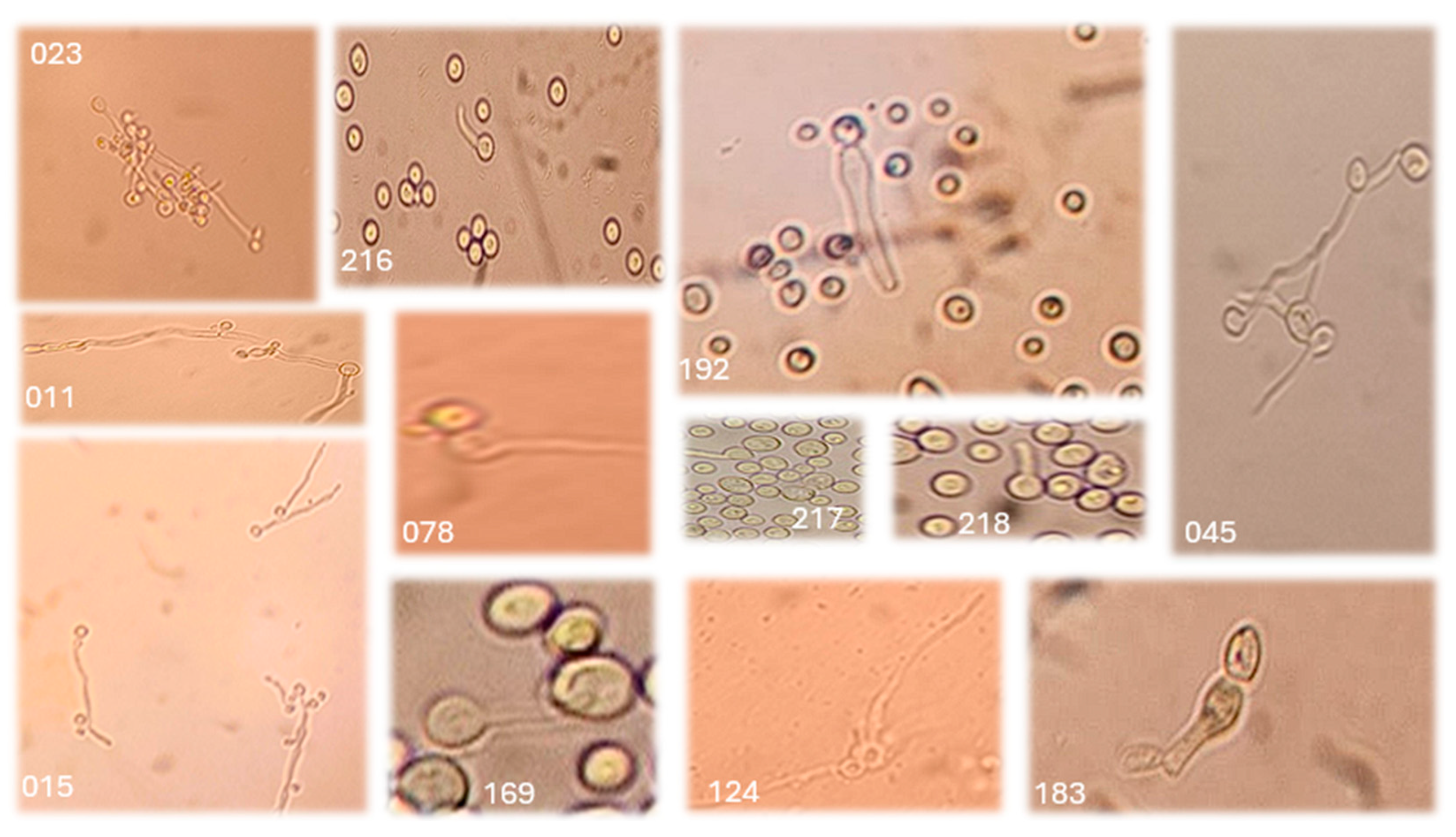
Germ Tube Test
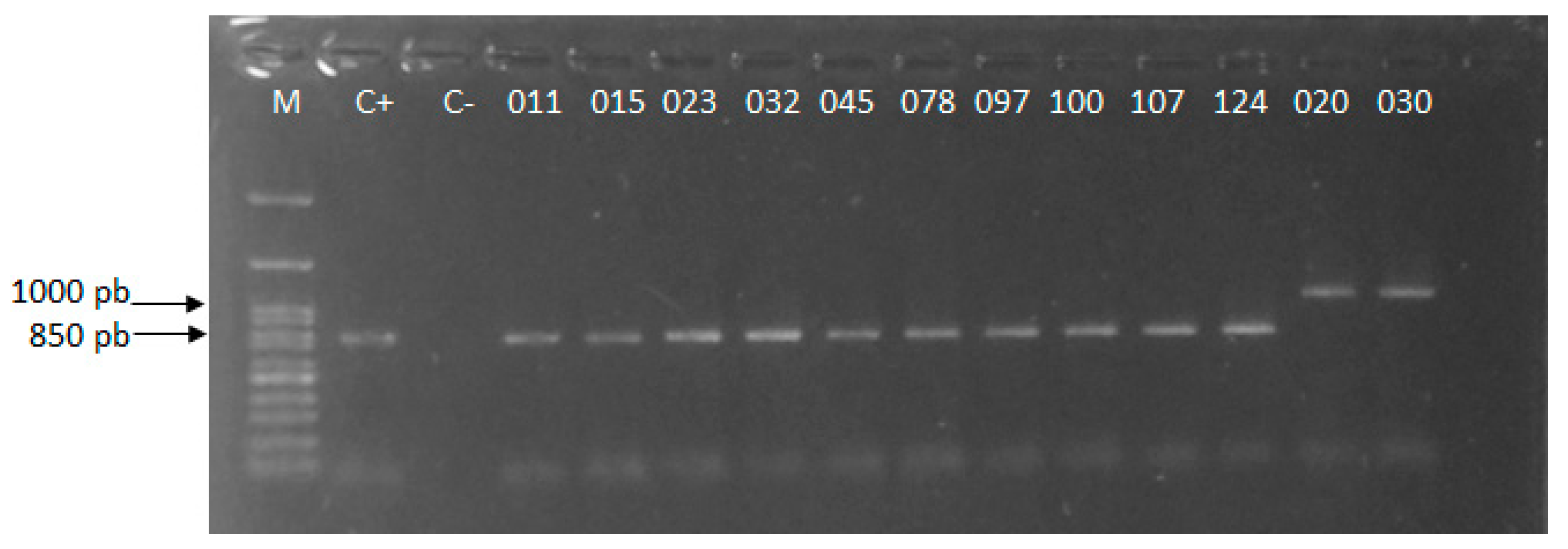
3.2. Molecular Identification
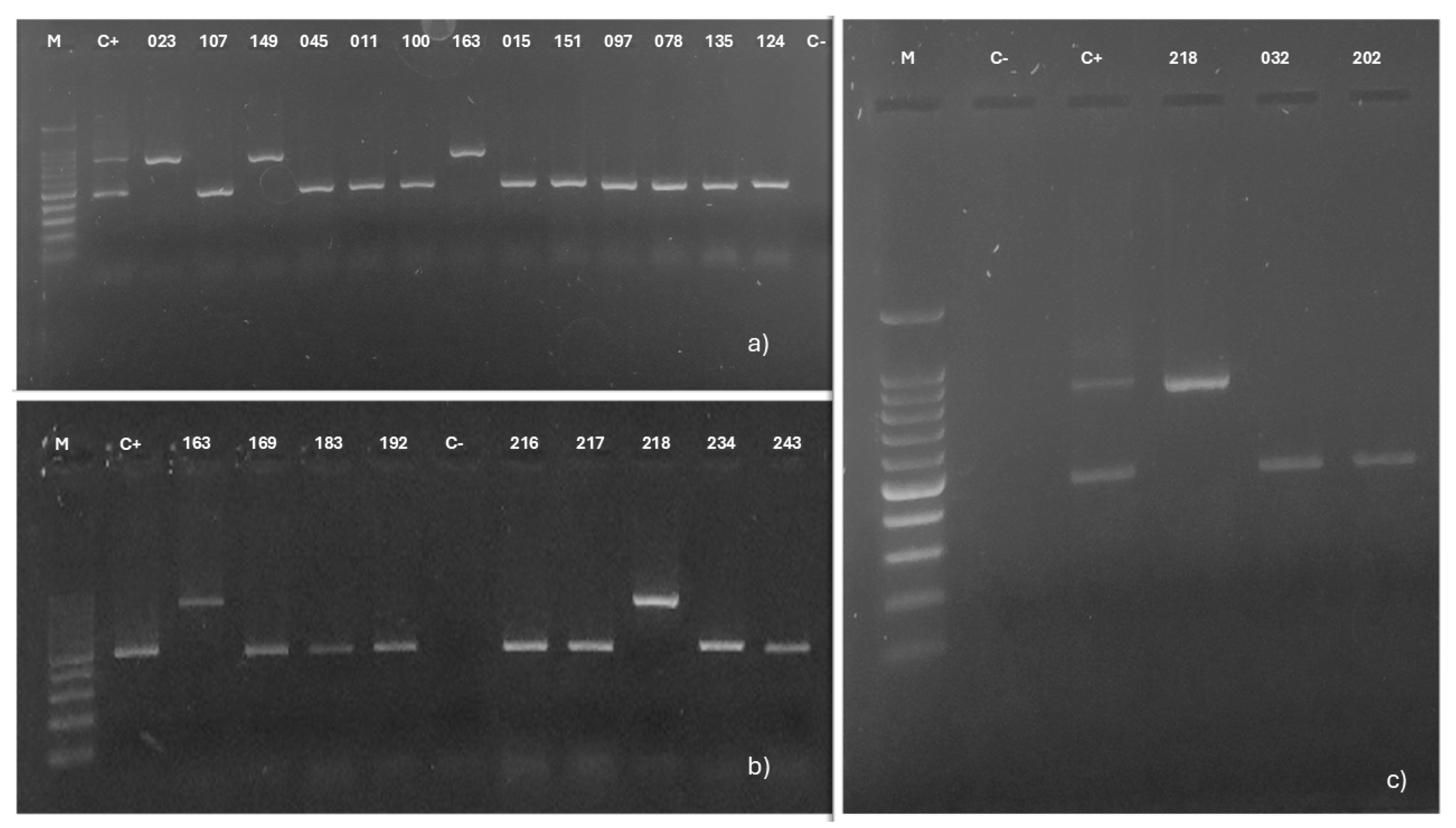
3.3. Genotypic Characterization
ABC Genotyping
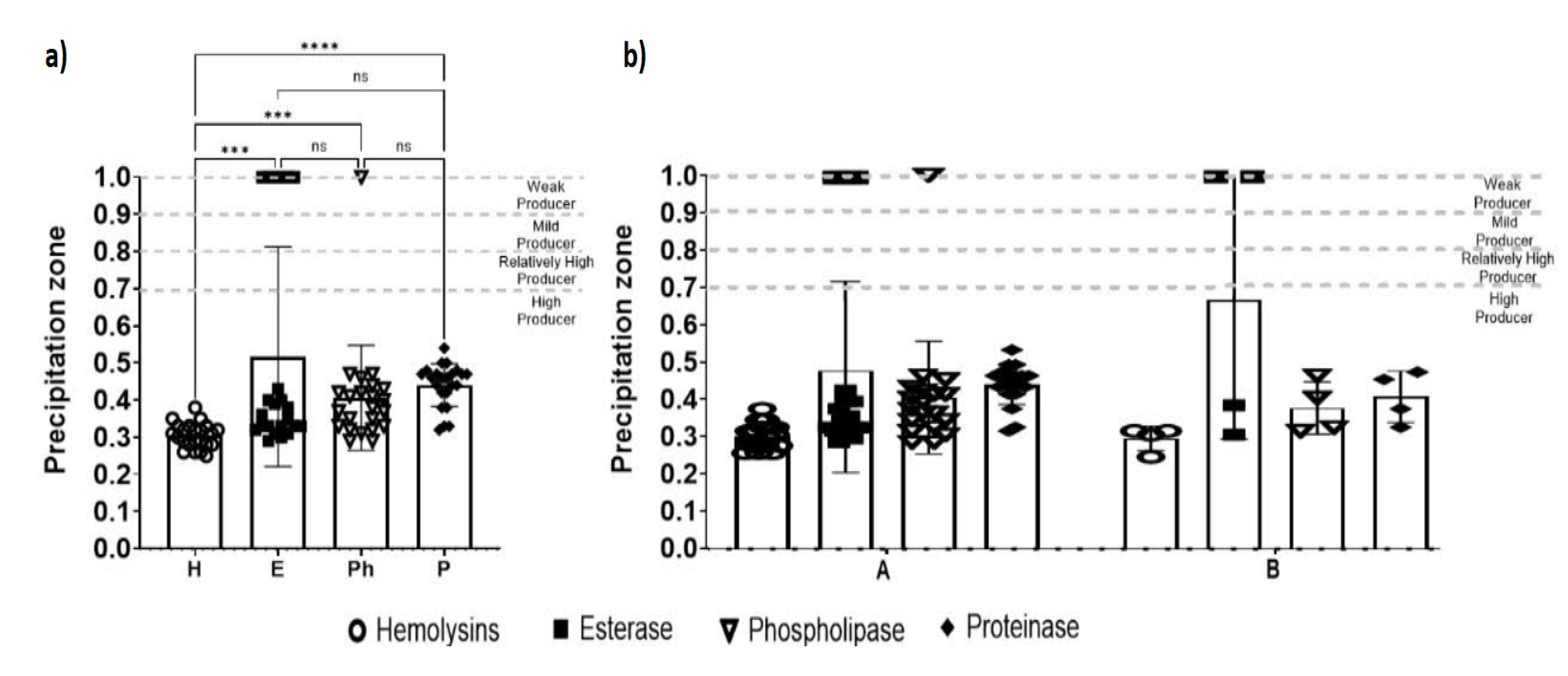
3.4. Phenotypic Characterization
Hydrolytic Enzyme Activity
3.5. Statistical Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Martin Lopez, J.E. Candidiasis (vulvovaginal). BMJ Clin. Evid. 2015, 2015, 0815. [Google Scholar]
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L., Jr. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Women’s Health 2019, 19, 48. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Caetano, C.F.; Gaspar, C.; Oliveira, A.S.; Palmeira-de-Oliveira, R.; Rodrigues, L.; Gonçalves, T.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A.; Rolo, J. Study of ecological relationship of yeast species with Candida albicans in the context of vulvovaginal infections. Microorganisms 2023, 11, 2398. [Google Scholar] [CrossRef]
- Czechowicz, P.; Nowicka, J.; Gościniak, G. Virulence factors of Candida spp. and host immune response important in the pathogenesis of vulvovaginal candidiasis. Int. J. Mol. Sci. 2022, 23, 5895. [Google Scholar] [CrossRef]
- Fornari, G.; Vicente, V.A.; Gomes, R.R.; Muro, M.D.; Pinheiro, R.L.; Ferrari, C.; Herkert, P.F.; Takimura, M.; Carvalho, N.S.; Queiroz-Telles, F. Susceptibility and molecular characterization of Candida species from patients with vulvovaginitis. Braz. J. Microbiol. 2016, 47, 373–380. [Google Scholar] [CrossRef]
- Saghrouni, F.; Ben Abdeljelil, J.; Boukadida, J.; Ben Said, M. Molecular methods for strain typing of Candida albicans: A review. J. Appl. Microbiol. 2013, 114, 1559–1574. [Google Scholar] [CrossRef]
- Ngouana, T.K.; Drakulovski, P.; Krasteva, D.; Toghueo, R.K.; Kouanfack, C.; Reynes, J.; Delaporte, E.; Boyom, F.F.; Mallié, M.; Bertout, S. Genetic diversity of the Hwp1 gene and HIS3, EF3, CDC3 microsatellites and antifungal susceptibility profiles of Candida albicans isolates from Yaoundé HIV-infected patients. Med. Mycol. 2017, 55, 546–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Liu, H.; Yan, J.; Liu, N.; Zhang, H.; Zhao, C.; Liu, Y. Molecular typing and genetic relatedness of 72 clinical Candida albicans isolates from poultry. Vet. Microbiol. 2018, 214, 36–43. [Google Scholar] [CrossRef]
- Jafarian, H.; Gharaghani, M.; Seyedian, S.S.; Mahmoudabadi, A.Z. Genotyping, antifungal susceptibility, enzymatic activity, and phenotypic variation in Candida albicans from esophageal candidiasis. J. Clin. Lab. Anal. 2021, 35, e23826. [Google Scholar] [CrossRef] [PubMed]
- Amanloo, S.; Zanjani, M.; Serajian, S.; Ahmadi, F.; Kakavand, F. Genotyping of Candida albicans isolates obtained from vulvovaginal candidiasis patients in Zanjan, Iran, based on ABC and RPS typing systems. Curr. Med. Mycol. 2022, 8, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Gharaghani, M.; Shabanzadeh, M.; Jafarian, H.; Zarei Mahmoudabadi, A. ABC typing and extracellular enzyme production of Candida albicans isolated from Candida vulvovaginitis. J. Clin. Lab. Anal. 2022, 36, e24117. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, G.E.; Zeid, M.S. Candida albicans genotyping and relationship of virulence factors with fluconazole tolerance in infected pediatric patients. Infect. Drug Resist. 2022, 15, 2035–2043. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Wang, D.; Li, C.; Wang, Y.; Diao, Y.; Tang, Y. Isolation, identification and genotyping of Candida albicans from Landes geese. Transbound. Emerg. Dis. 2022, 69, 349–359. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Nouraei, H.; Nasr, R.; Zomorodian, K.; Khodadadi, H.; Pakshir, K. Phenotypes characterization and ABC genotypes distribution of clinical Candida albicans isolates. J. Clin. Lab. Anal. 2023, 37, e24888. [Google Scholar] [CrossRef]
- Owotade, F.J.; Gulube, Z.; Patel, M. Oral Candida albicans strain diversity and maintenance in HIV positive women in South Africa. Arch. Oral. Biol. 2024, 164, 106007. [Google Scholar] [CrossRef]
- Dalvand, A.; Katiraee, F.; Jafari Joozani, R.; Shokri, H. Genotyping of Candida albicans isolated from animals using 25S ribosomal DNA and ALT repeats polymorphism in repetitive sequence. Curr. Med. Mycol. 2018, 4, 12–19. [Google Scholar] [CrossRef]
- Wang, M.; Cao, Y.; Xia, M.; Al-Hatmi, A.M.S.; Ou, W.; Wang, Y.; Sibirny, A.A.; Zhao, L.; Zou, C.; Liao, W.; et al. Virulence and antifungal susceptibility of microsatellite genotypes of Candida albicans from superficial and deep locations. Yeast 2019, 36, 363–373. [Google Scholar] [CrossRef]
- Marot-Leblond, A.; Nail-Billaud, S.; Pilon, F.; Beucher, B.; Poulain, D.; Robert, R. Efficient diagnosis of vulvovaginal candidiasis by use of a new rapid immunochromatography test. J. Clin. Microbiol. 2009, 47, 3821–3825. [Google Scholar] [CrossRef]
- Taschdjian, C.L.; Burchall, J.J.; Kozinn, P.J. Rapid identification of Candida albicans by filamentation on serum and serum substitutes. Am. J. Clin. Pathol. 1960, 99, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Lõoke, M.; Kristjuhan, K.; Kristjuhan, A. Extraction of genomic DNA from yeasts for PCR-based applications. BioTechniques 2011, 50, 325–328. [Google Scholar] [CrossRef] [PubMed]
- García-Salazar, E.; Acosta-Altamirano, G.; Betancourt-Cisneros, P.; Reyes-Montes, M.R.; Rosas-De-Paz, E.; Duarte-Escalante, E.; Sánchez-Conejo, A.R.; Ocharan Hernández, E.; Frías-De-León, M.G. Detection and molecular identification of eight Candida species in clinical samples by simplex PCR. Microorganisms 2022, 10, 374. [Google Scholar] [CrossRef]
- Maheronnaghsh, M.; Fatahinia, M.; Dehghan, P.; Zarei Mahmoudabadi, A.; Kheirkhah, M. Comparison of virulence factors of different Candida Species isolated from the oral cavity of cancer patients and normal individuals. Jundishapur J. Microbiol. 2019, 12, e91556. [Google Scholar] [CrossRef]
- Erum, R.; Samad, F.; Khan, A.; Kazmi, S.U. A comparative study on production of extracellular hydrolytic enzymes of Candida species isolated from patients with surgical site infection and from healthy individuals and their co-relation with antifungal drug resistance. BMC Microbiol. 2020, 20, 368. [Google Scholar] [CrossRef]
- Rezk, S.; Alqabbasi, O. Bacterial vaginosis, vulvovaginal candidiasis, trichomonal vaginitis and aerobic vaginitis in women from Egypt. Germs 2023, 13, 130–136. [Google Scholar] [CrossRef]
- Satora, M.; Grunwald, A.; Zaremba, B.; Frankowska, K.; Żak, K.; Tarkowski, R.; Kułak, K. Treatment of vulvovaginal candidiasis-an overview of guidelines and the latest treatment methods. J. Clin. Med. 2023, 12, 5376. [Google Scholar] [CrossRef]
- Lipsky, M.S.; Waters, T.; Sharp, L.K. Impact of vaginal antifungal products on utilization of health care services: Evidence from physician visits. J. Am. Board. Fam. Pract. 2000, 13, 178–182. [Google Scholar] [CrossRef]
- Ward, A.; Alvarez, P.; Vo, L.; Martin, S. Direct medical costs of complications of diabetes in the United States: Estimates for event-year and annual state costs (USD 2012). J. Med. Econ. 2014, 17, 176–183. [Google Scholar] [CrossRef]
- Deorukhkar, S.C. Virulence traits contributing to pathogenicity of Candida species. J. Microbiol. Exp. 2017, 5, 00140. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M. Virulence factors in Candida species. Curr. Protein Pept. Sci. 2020, 21, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Silva-Rocha, W.P.; de Brito Lemos, V.L.; Ferreira, M.R.; Soares, L.A.; Svidzisnki, T.I.; Milan, E.P.; Chaves, G.M. Effect of the crude extract of Eugenia uniflora in morphogenesis and secretion of hydrolytic enzymes in Candida albicans from the oral cavity of kidney transplant recipients. BMC Complement. Altern. Med. 2015, 15, 6. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef]
- Makanjuola, O.; Bongomin, F.; Fayemiwo, S.A. An update on the roles of non-albicans Candida species in vulvovaginitis. J. Fungi 2018, 4, 121. [Google Scholar] [CrossRef] [PubMed]
- Arafa, S.H.; Elbanna, K.; Osman, G.E.H.; Abulreesh, H.H. Candida diagnostic techniques: A review. J. Umm. Al-Qura Univ. Appll. Sci. 2023, 9, 360–377. [Google Scholar] [CrossRef]
- Ameen, F.; Moslem, M.; Al Tami, M.; Al-Ajlan, H.; Al-Qahtani, N. Identification of Candida species in vaginal flora using conventional and molecular methods. J. Mycol. Med. 2017, 27, 364–368. [Google Scholar] [CrossRef]
- Kumar, K.P.P.; Tejashree, A. Genotyping of Candida albicans and comparison of its antifungal resistance pattern in the South Indian region. J. Pure Appl. Microbiol. 2022, 16, 2123–2130. [Google Scholar] [CrossRef]
- Karahan, Z.C.; Guriz, H.; Agirbasli, H.; Balaban, N.; Gocmen, J.S.; Aysev, D.; Akar, N. Genotype distribution of Candida albicans isolates by 25S intron analysis with regard to invasiveness. Mycoses 2004, 47, 465–469. [Google Scholar] [CrossRef]
- Tamai, I.A.; Salehi, T.Z.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R. Repetitive sequences based on genotyping of Candida albicans isolates obtained from Iranian patients with human immunodeficiency virus. Iran. J. Basic. Med. Sci. 2014, 17, 831–835. [Google Scholar]
- Kianifar, S.; Rezaei-Matehkolaei, A.; Zarei-Mahmoudabadi, A. Genotypes analysis of Candida albicans species complex from healthy individual saliva in Ahvaz, Iran. Lett. Appl. Microbiol. 2022, 75, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Lee, C.H.; Cheng, Y.C.; Lu, J.J.; Wang, S.H. Association between CAI microsatellite, multilocus sequence typing, and clinical significance within Candida albicans isolates. Med. Mycol. 2021, 59, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.S.P.; Chu, F.C.S.; Leung, W.K.; Jin, L.J.; Samaranayake, L.P.; Siu, S.C. Phospholipase, proteinase and haemolytic activities of Candida albicans isolated from oral cavities of patients with type 2 diabetes mellitus. J. Med. Microbiol. 2007, 56 Pt 10, 1393–1398. [Google Scholar] [CrossRef]
- Tanaka, W.T.; Nakao, N.; Mikami, T.; Matsumoto, T. Hemoglobin is utilized by Candida albicans in the hyphal form but not yeast form. Biochem. Biophys. Res. Commun. 1997, 232, 350–353. [Google Scholar] [CrossRef]
- Fatahinia, M.; Halvaeezadeh, M.; Rezaei-Matehkolaei, A. Comparison of enzymatic activities in different Candida species isolated from women with vulvovaginitis. J. Mycol. Med. 2017, 27, 188–194. [Google Scholar] [CrossRef]
- Tsuboi, R.; Komatsuzaki, H.; Ogawa, H. Induction of an extracellular esterase from Candida albicans and some of its properties. Infect. Immun. 1996, 64, 2936–2940. [Google Scholar] [CrossRef]
- Bras, G.; Satala, D.; Juszczak, M.; Kulig, K.; Wronowska, E.; Bednarek, A.; Zawrotniak, M.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Proteinasas aspárticas secretadas: Factores clave en las infecciones por Candida y las interacciones huésped-patógeno. Int. J. Molecular Sci. 2024, 25, 4775. [Google Scholar] [CrossRef]
- Chauhan, V.; Kumar, A.; Tripathi, S.; Jha, M.; Kumar, N.; Poluri, K.M.; Gupta, P. An update on the pathogenesis and ethnopharmacological therapeutic approaches of vulvovaginal candidiasis. Discov. Public Health 2024, 21, 195. [Google Scholar] [CrossRef]
- Zhang, N.; Wheeler, D.; Truglio, M.; Lazzarini, C.; Upritchard, J.; McKinney, W.; Rogers, K.; Prigitano, A.; Tortorano, A.M.; Cannon, R.D.; et al. Multi-locus next-generation sequence typing of DNA extracted from pooled colonies detects multiple unrelated Candida albicans strains in a significant proportion of patient samples. Front. Microbiol. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Colabella, C.; Casagrande Pierantoni, D.; Corte, L.; Roscini, L.; Conti, A.; Bassetti, M.; Tascini, C.; Robert, V.; Cardinali, G. Single strain high-depth NGS reveals high rDNA (ITS-LSU) variability in the four prevalent pathogenic species of the genus Candida. Microorganisms 2021, 9, 302. [Google Scholar] [CrossRef]
| Isolate | Genotype | Enzyme Activity Index (Pz) | |||
|---|---|---|---|---|---|
| Hemolysin | Esterase | Phospholipase | Proteinase | ||
| 011 | A | 0.28 b | 0.31 b | 0.47 b | 0.42 b |
| 015 | A | 0.38 b | 0.30 b | 0.43 b | 0.38 b |
| 032 | A | 0.29 b | 0.29 b | 0.29 b | 0.47 b |
| 045 | A | 0.29 b | 0.36 b | 0.44 b | 0.46 b |
| 078 | A | 0.29 b | 1.00 a | 0.46 b | 0.44 b |
| 097 | A | 0.31 b | 0.32 b | 0.40 b | 0.44 b |
| 100 | A | 0.33 b | 0.35 b | 0.42 b | 0.47 b |
| 107 | A | 0.28 b | 0.38 b | 0.35 b | 0.46 b |
| 124 | A | 0.32 b | 0.33 b | 0.33 b | 0.33 b |
| 135 | A | 0.35 b | 0.33 b | 0.41 b | 0.32 b |
| 151 | A | 0.30 b | 1.00 a | 0.32 b | 0.42 b |
| 169 | A | 0.32 b | 0.40 b | 1.00 a | 0.48 b |
| 183 | A | 0.33 b | 0.43 b | 0.31 b | 0.50 b |
| 192 | A | 0.35 b | 0.33 b | 0.29 b | 0.47 b |
| 202 | A | 0.26 b | 1.00 a | 0.35 b | 0.47 b |
| 216 | A | 0.26 b | 0.33 b | 0.37 b | 0.44 b |
| 217 | A | 0.33 b | 0.33 b | 0.37 b | 0.47 b |
| 234 | A | 0.28 b | 1.00 a | 0.42 b | 0.50 b |
| 243 | A | 0.26 b | 0.40 b | 0.38 b | 0.54 b |
| 023 | B | 0.31 b | 0.31 b | 0.33 b | 0.38 b |
| 149 | B | 0.32 b | 0.39 b | 0.32 b | 0.33 b |
| 163 | B | 0.32 b | 1.00 a | 0.41 b | 0.46 b |
| 218 | B | 0.25 b | 1.00 a | 0.47 b | 0.48 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Huerta, H.; García-Salazar, E.; Ramírez-Magaña, X.; Martínez-Herrera, E.; Pinto-Almazán, R.; Betancourt-Cisneros, P.; Duarte-Escalante, E.; Reyes-Montes, M.d.R.; Hernández-Castro, R.; Frías-De-León, M.G. Phenotypic and Molecular Characterization of Candida albicans Isolates from Mexican Women with Vulvovaginitis. J. Fungi 2025, 11, 354. https://doi.org/10.3390/jof11050354
Díaz-Huerta H, García-Salazar E, Ramírez-Magaña X, Martínez-Herrera E, Pinto-Almazán R, Betancourt-Cisneros P, Duarte-Escalante E, Reyes-Montes MdR, Hernández-Castro R, Frías-De-León MG. Phenotypic and Molecular Characterization of Candida albicans Isolates from Mexican Women with Vulvovaginitis. Journal of Fungi. 2025; 11(5):354. https://doi.org/10.3390/jof11050354
Chicago/Turabian StyleDíaz-Huerta, Hugo, Eduardo García-Salazar, Xóchitl Ramírez-Magaña, Erick Martínez-Herrera, Rodolfo Pinto-Almazán, Paola Betancourt-Cisneros, Esperanza Duarte-Escalante, María del Rocío Reyes-Montes, Rigberto Hernández-Castro, and María Guadalupe Frías-De-León. 2025. "Phenotypic and Molecular Characterization of Candida albicans Isolates from Mexican Women with Vulvovaginitis" Journal of Fungi 11, no. 5: 354. https://doi.org/10.3390/jof11050354
APA StyleDíaz-Huerta, H., García-Salazar, E., Ramírez-Magaña, X., Martínez-Herrera, E., Pinto-Almazán, R., Betancourt-Cisneros, P., Duarte-Escalante, E., Reyes-Montes, M. d. R., Hernández-Castro, R., & Frías-De-León, M. G. (2025). Phenotypic and Molecular Characterization of Candida albicans Isolates from Mexican Women with Vulvovaginitis. Journal of Fungi, 11(5), 354. https://doi.org/10.3390/jof11050354










