Screening of Lentinula edodes Strains for High Polysaccharide Production and In Vitro Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Determination of Mycelial Biomass
2.3. Polysaccharide Extractions and Determinations
2.4. In Vitro Antioxidant Activities of Polysaccharides
2.5. Enzyme Activities and Expression Profiles of Key Genes in Polysaccharide Synthesis
2.6. Determination of Polysaccharide Monosaccharide Components and Molecular Weight
2.7. Data Analysis
3. Results
3.1. Polysaccharide Content of Mycelium from Different Strains Under Shaking and Static Culture Conditions
3.2. In Vitro Antioxidant Activity of Mycelium Polysaccharides from Different Strains Under Shaking and Static Culture Conditions
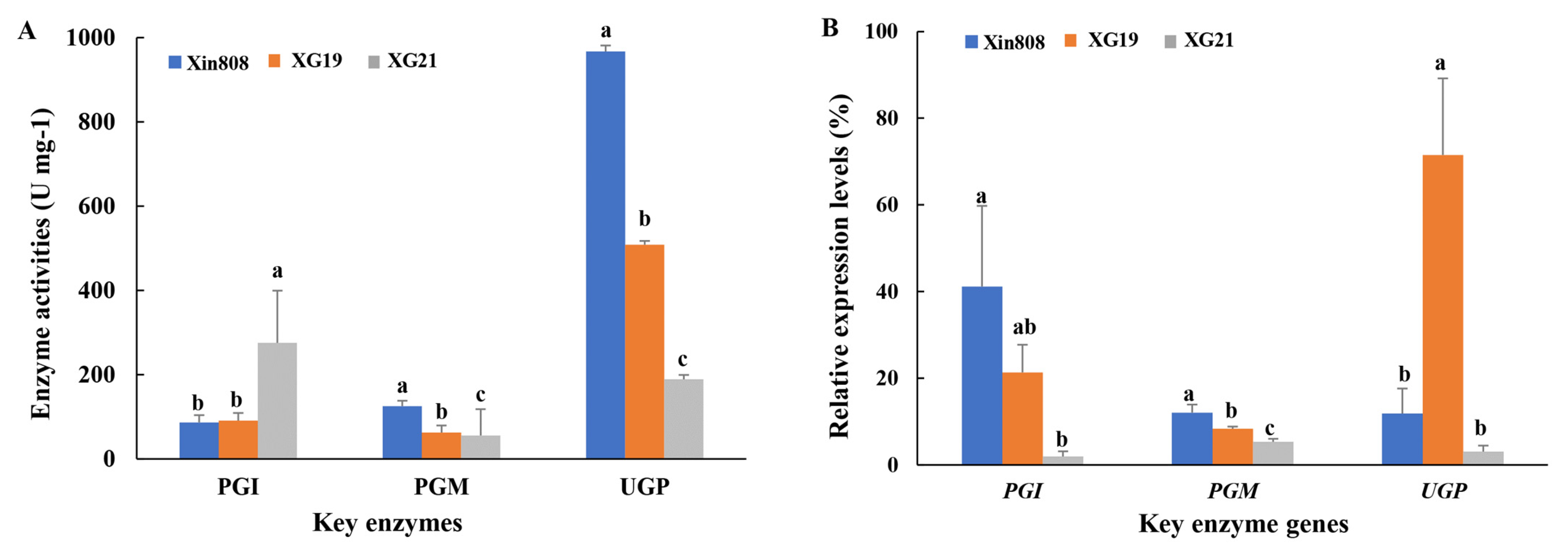
3.3. Transcriptional Expression Levels and Enzyme Activities of Key Enzymes in Polysaccharide Biosynthesis
3.4. Monosaccharide Composition of Mycelial Polysaccharides
3.5. Analysis of Molecular Weight of Mycelial Polysaccharides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPS | Intracellular polysaccharide |
| EPS | Extracellular polysaccharide |
| TPS | Total polysaccharide |
| PGI | Phosphoglucose isomerase |
| PGM | Phosphoglucomutase |
| UGP | UDPG-pyrophosphorylase |
References
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A Review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 5, 1095–1105. [Google Scholar]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B.K.S. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef]
- Duan, Y.Q.; Xing, Z.C.; Xu, J.W. Screening of a high yield polysaccharide strain from ten edible and medicinal fungi and optimization of its culture conditions. Res. J. Biotechnol. 2013, 8, 11–15. [Google Scholar]
- Wei, Z.H.; Duan, Y.Y.; Qian, Y.Q.; Guo, X.F.; Li, Y.J.; Jin, S.H.; Zhou, Z.X.; Shan, S.Y.; Wang, C.R.; Chen, X.J. Screening of Ganoderma strains with high polysaccharides and ganoderic acid contents and optimization of the fermentation medium by statistical methods. Bioproc. Biosyst. Eng. 2014, 37, 1789–1797. [Google Scholar] [CrossRef]
- Adil, B.; Xiang, Q.J.; He, M.L.; Wu, Y.T.; Muhammad Ahsan, A.; Muhammad, A.; Qin, P.; Gu, Y.F.; Yu, X.M.; Zhao, K.; et al. Effect of sodium and calcium on polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Lentinus edodes. Amb Express 2020, 10, 47. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Zhang, H.J.; Chen, X.Q.; Hou, S.Y.; Gu, Y.F.; Yu, X.M.; Zhao, K.; Zhang, X.P.; Ma, M.G.; Chen, Q. Enhanced effects of iron on mycelial growth, metabolism and in vitro antioxidant activity of polysaccharides from Lentinula edodes. Bioengineering 2022, 9, 581. [Google Scholar] [CrossRef]
- Hu, G.; Zhai, M.; Niu, R.; Xu, X.; Liu, Q.; Jia, J. Optimization of culture condition for ganoderic acid production in Ganoderma lucidum liquid static culture and design of a suitable bioreactor. Molecules 2018, 23, 2563. [Google Scholar] [CrossRef]
- Hou, Z.; Cao, J. Comparative study of the P2X gene family in animals and plants. Purinergic Signal 2016, 12, 269–281. [Google Scholar] [CrossRef]
- Luo, J.X.; Zhang, J.S.; Jia, W.; Feng, N.; Yang, Y.; Tang, Q.J.; Liu, Y.F.; Zhang, H. Influences of culture methods on the yield and antineoplastic activity of intracellular triterpene of Ganoderma lucidum mycelia. Acta Agric. Shanghai 2014, 2, 33–37. (In Chinese) [Google Scholar]
- Jin, Y.; Lina Zhang, L.N.; Zhang, M.; Chen, L.; Peter, C.K.C.; Oi, V.E.C.; Lin, Y.Y. Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media. Carbohydr. Res. 2003, 338, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Ju, Y.; Li, J.J.; Yu, M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int. J. Biol. Macromol. 2012, 50, 214–218. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef]
- Yin, C.M.; Li, C.; Ma, K.; Fan, X.Z.; Yao, F.; Shi, D.F.; Wu, W.J.; Qiu, J.H.; Hu, G.Y.; Gao, H. The physicochemical, antioxidant, hypoglycemic and prebiotic properties of γ-irradiated polysaccharides extracted from Lentinula edodes. Food Sci. Biotechnol. 2023, 32, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.L.; Wang, L.; Wang, X.Q.; Li, Z.T.; Gao, M.J.; Yang, Y.H.; Wang, Q.; et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhang, W.; Zhong, J.J. Performance analyses of a pH-shift and DOT-shift integrated fed-batch fermentation process for the production of ganoderic acid and ganoderma polysaccharides by medicinal mushroom Ganoderma lucidum. Bioresour. Technol. 2009, 100, 1852–1859. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, Y.X.; Wu, L.X.; Wu, X.Q.; Huang, Y.F.; Liu, B. Optimization of polysaccharides extraction from Dictyophora indusiata and determination of its antioxidant activity. Int. J. Biol. Macromol. 2017, 103, 175–181. [Google Scholar] [CrossRef]
- Salvador, L.D.; Suganuma, T.; Kitahara, K.; Tanoue, H.Y.; Ichiki, M. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. J. Agric. Food Chem. 2000, 48, 3448–3454. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, R.M.; Wen, P.; Song, Y.; He, B.L.; Tan, J.L.; Hao, H.L.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohyd. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Hu, T.; Huang, Q.L.; Wong, K.H.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2017, 194, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; You, Q.; Li, X.; Chang, Q.; Zhang, Y.; Zheng, B.D.; Hu, X.K.; Zeng, H.L. Polysaccharide fractions from Fortunella margarita affect proliferation of Bifidobacterium adolescentis ATCC 15703 and undergo structural changes following fermentation. Int. J. Biol. Macromol. 2019, 123, 1070. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Q.; Nan, T.G.; Yuan, Y.; Jin, Y.; Hu, K.Y. Study on polysaccharide content and monosaccharide composition of Polyporus umbellatus from different production areas. China J. Chin. Mater. Medica 2019, 17, 3608–3614. [Google Scholar]
- Zhang, H.; Yan, Y.; Nie, S.P.; Chen, L.; Xie, M.Y. Monosaccharide composition and antioxidant activity in vitro of polysaccharides from different parts of Ganoderma atrum. Food Sci. 2011, 32, 56–61. [Google Scholar]
- Zheng, C.C.; Li, T.; Tang, Y.Y.; Lu, T.; Wu, M.K.; Sun, J.; Man, R.J.; He, X.M.; Zhou, Z.G. Structural and functional investigation on stem and peel polysaccharides from different varieties of pitaya. Int. J. Biol. Macromol. 2024, 259 Pt 1, 129172. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments. Food Chem. 2016, 197, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Juliet, I.C.; Wen, C.; Duan, Y.; Zhou, J.; He, Y.; Zhang, H.; Ma, H. Effects of multi-mode divergent ultrasound pretreatment on the physicochemical and functional properties of polysaccharides from Sagittaria sagittifolia L. Food Biosci. 2021, 42, 101145. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, P.; Qu, Z.; Bai, D.; Gao, X.; Zhao, C.; Chen, J.; Gao, W. Physicochemical characterizations of polysaccharides from Angelica Sinensis Radix under different drying methods for various applications—ScienceDirect. Int. J. Biol. Macromol. 2019, 121, 381–389. [Google Scholar] [CrossRef]
- Li, Z.; Nie, K.; Wang, Z.; Luo, D. Quantitative structure activity relationship models for the antioxidant activity of polysaccharides. PLoS ONE 2016, 11, e0163536. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohyd Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohyd Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
| Strain No. | TPS (mg) a | ||
|---|---|---|---|
| Shaking b | Static c | Shaking and Static d | |
| Xin808 | 23.40 ± 2.80 ef | 19.90 ± 1.13 i | 43.30 ± 2.24 h |
| XG2 | 14.77 ± 0.81 g | 19.36 ± 0.29 i | 34.14 ± 0.66 i |
| XG3 | 29.86 ± 2.23 cd | 33.36 ± 0.91 c | 63.23 ± 2.35 bcd |
| XG4 | 19.97 ± 2.36 f | 27.95 ± 0.24 ef | 47.92 ± 1.98 fg |
| XG5 | 12.28 ± 0.99 g | 19.03 ± 1.15 i | 31.31 ± 0.98 i |
| XG6 | 17.40 ± 0.56 fg | 24.47 ± 0.5 g | 41.86 ± 0.85 h |
| XG8 | 8.36 ± 0.43 h | 9.23 ± 0.2 k | 17.59 ± 0.34 j |
| XG9 | 27.29 ± 2.66 de | 32.64 ± 0.41 c | 59.93 ± 2.20 de |
| XG12 | 20.27 ± 0.29 f | 11.01 ± 0.18 j | 31.28 ± 0.36 i |
| XG13 | 25.65 ± 1.43 e | 35.28 ± 0.75 b | 60.93 ± 1.45 cde |
| XG14 | 14.90 ± 0.17 g | 28.92 ± 0.93 e | 43.82 ± 0.89 gh |
| XG19 | 25.70 ± 1.43 e | 24.14 ± 0.24 g | 49.85 ± 1.30 f |
| XG20 | 29.15 ± 1.84 d | 46.06 ± 0.41 a | 75.21 ± 1.83 a |
| XG21 | 44.06 ± 2.69 a | 34.74 ± 0.47 b | 78.80 ± 2.58 a |
| XG22 | 18.18 ± 2.24 f | 21.22 ± 0.58 h | 39.40 ± 1.36 h |
| XG24 | 37.54 ± 4.15 b | 27.53 ± 0.21 f | 65.07 ± 3.54 bc |
| XG26 | 27.44 ± 2.04 de | 30.07 ± 0.4 d | 57.51 ± 1.65 e |
| XG30 | 32.39 ± 1.58 c | 33.34 ± 0.27 c | 65.72 ± 1.51 b |
| Strain No. | Total IC50 (mg mL−1) a | ||
|---|---|---|---|
| Shaking b | Static c | Shaking and Static d | |
| Xin808 | 27.79 ± 2.64 c | 15.06 ± 2.60 h | 42.85 |
| XG2 | 18.26 ± 1.38 f | 8.63 ± 1.08 j | 26.89 |
| XG3 | 53.99 ± 3.83 a | 23.22 ± 2.71 d | 77.21 |
| XG4 | 5.83 ± 0.31 j | 3.48 ± 0.31 no | 9.31 |
| XG5 | 3.21 ± 0.19 m | 19.13 ± 0.83 f | 22.34 |
| XG6 | 33.23 ± 3.55 b | 4.16 ± 0.23 l | 37.39 |
| XG8 | 4.77 ± 0.34 k | 37.54 ± 2.34 b | 42.31 |
| XG9 | 6.18 ± 0.97 j | 4.28 ± 1.11 l | 10.46 |
| XG12 | 3.21 ± 0.25 m | 15.76 ± 0.48 g | 18.97 |
| XG13 | 23.45 ± 2.61 e | 65.29 ± 5.82 a | 88.74 |
| XG14 | 4.05 ± 0.30 l | 3.82 ± 0.79 m | 7.87 |
| XG19 | 3.11 ± 0.21 m | 3.38 ± 0.14 o | 6.49 |
| XG20 | 3.11 ± 0.39 m | 4.11 ± 0.46 l | 7.22 |
| XG21 | 7.10 ± 1.40 i | 8.99 ± 0.12 i | 16.09 |
| XG22 | 26.90 ± 2.42 d | 37.36 ± 3.27 c | 64.26 |
| XG24 | 7.99 ± 0.26 h | 3.57 ± 0.11 n | 11.56 |
| XG26 | 4.01 ± 0.36 l | 22.24 ± 3.37 e | 26.25 |
| XG30 | 15.86 ± 1.74 g | 5.86 ± 1.17 k | 21.72 |
| Enzyme Activity | IPS | EPS | TPS |
|---|---|---|---|
| PGI | 0.426 | 0.981 ** | 0.987 ** |
| PGM | 0.125 | −0.851 ** | −0.782 * |
| UGP | 0.455 | −0.603 | −0.533 |
| Monosaccharide Composition | Monosaccharide Percentage (%) | |||||
|---|---|---|---|---|---|---|
| Xin 808 | XG19 | XG21 | ||||
| IPS | EPS | IPS | EPS | IPS | EPS | |
| Fucose | 6.00 | 3.50 | 4.04 | 1.93 | 2.66 | 1.07 |
| Galactose | 30.32 | 11.16 | 30.90 | 6.74 | 31.43 | 6.25 |
| Glucose | 20.62 | 23.06 | 24.19 | 29.77 | 28.09 | 33.08 |
| Xylose | 5.55 | 2.79 | 2.22 | 1.38 | 3.24 | - |
| Mannose | 35.11 | 51.41 | 34.59 | 51.91 | 30.65 | 51.65 |
| Ribose | 1.06 | - | 1.11 | - | 1.33 | - |
| Galacturonic acid | 0.56 | 2.11 | 1.22 | 2.43 | 0.88 | 2.22 |
| Glucuronic acid | 0.77 | 5.97 | 1.74 | 5.83 | 1.72 | 5.74 |
| Molecular Weight | Xin 808 | XG19 | XG21 | |||
|---|---|---|---|---|---|---|
| IPS | EPS | IPS | EPS | IPS | EPS | |
| Mw (kDa) | 269.783 | 79.875 | 702.924 | 83.894 | 547.913 | 53.824 |
| Mn (kDa) | 69.401 | 8.865 | 29.752 | 4.750 | 29.229 | 3.168 |
| Mz(kDa) | 1780.179 | 2375.146 | 6448.187 | 5629.469 | 3551.611 | 1500.835 |
| PDI (Mw/Mn) | 3.887 | 9.010 | 23.626 | 17.663 | 18.746 | 16.992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Rida, K.; Wen, J.; Yu, X.; Gu, Y.; He, M.; Chen, Q.; Xiang, Q. Screening of Lentinula edodes Strains for High Polysaccharide Production and In Vitro Antioxidant Activities. J. Fungi 2025, 11, 347. https://doi.org/10.3390/jof11050347
Zhang J, Rida K, Wen J, Yu X, Gu Y, He M, Chen Q, Xiang Q. Screening of Lentinula edodes Strains for High Polysaccharide Production and In Vitro Antioxidant Activities. Journal of Fungi. 2025; 11(5):347. https://doi.org/10.3390/jof11050347
Chicago/Turabian StyleZhang, Jie, Kanwal Rida, Jiahao Wen, Xiumei Yu, Yunfu Gu, Maoqiang He, Qiang Chen, and Quanju Xiang. 2025. "Screening of Lentinula edodes Strains for High Polysaccharide Production and In Vitro Antioxidant Activities" Journal of Fungi 11, no. 5: 347. https://doi.org/10.3390/jof11050347
APA StyleZhang, J., Rida, K., Wen, J., Yu, X., Gu, Y., He, M., Chen, Q., & Xiang, Q. (2025). Screening of Lentinula edodes Strains for High Polysaccharide Production and In Vitro Antioxidant Activities. Journal of Fungi, 11(5), 347. https://doi.org/10.3390/jof11050347








