The Resistance Mechanisms of Anilinopyrimidine Fungicide Pyrimethanil in Sclerotinia sclerotiorum
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates and Fungicides
2.2. Biological Characteristics of Pyrimethanil-Resistant S. sclerotiorum Mutants
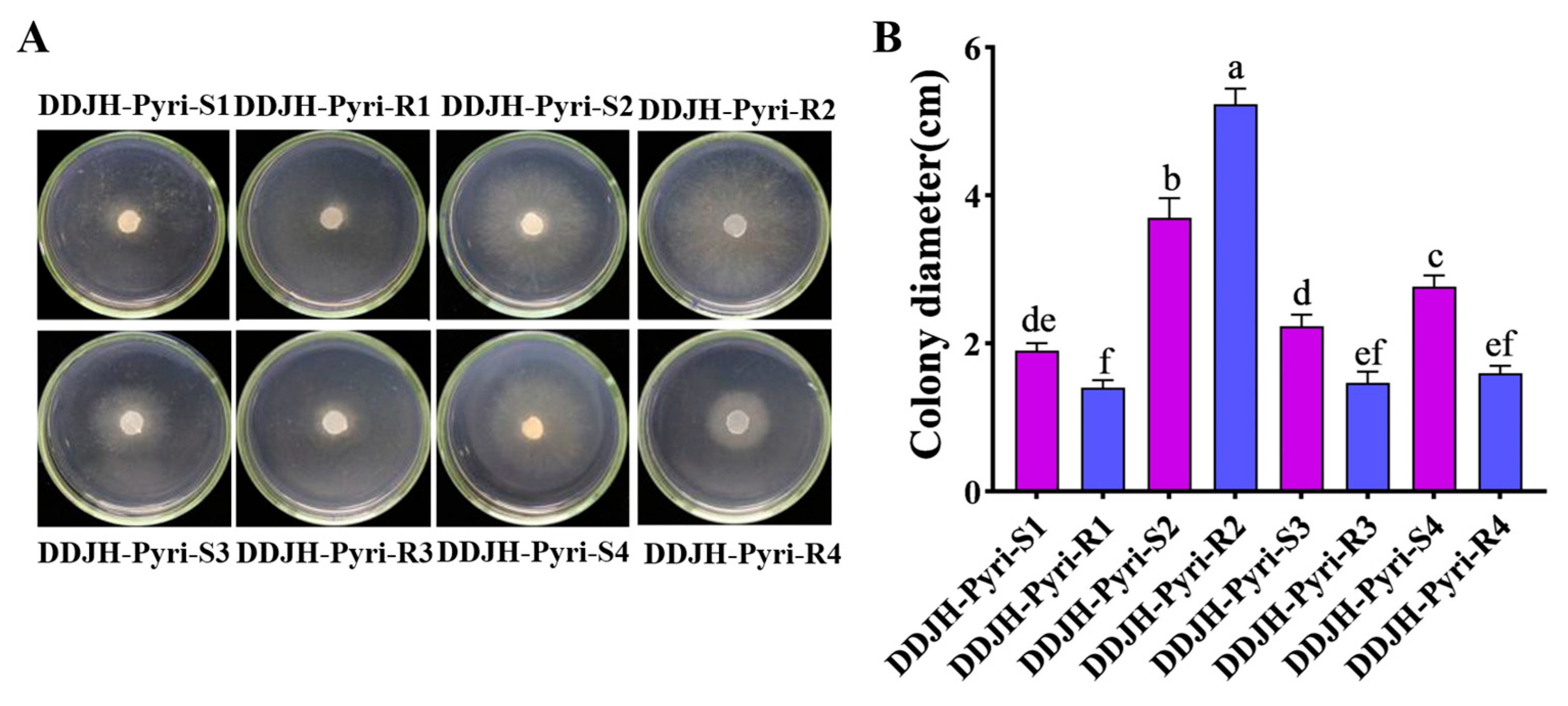
2.2.1. Mycelial Growth
2.2.2. Sclerotia Production on PDA Medium
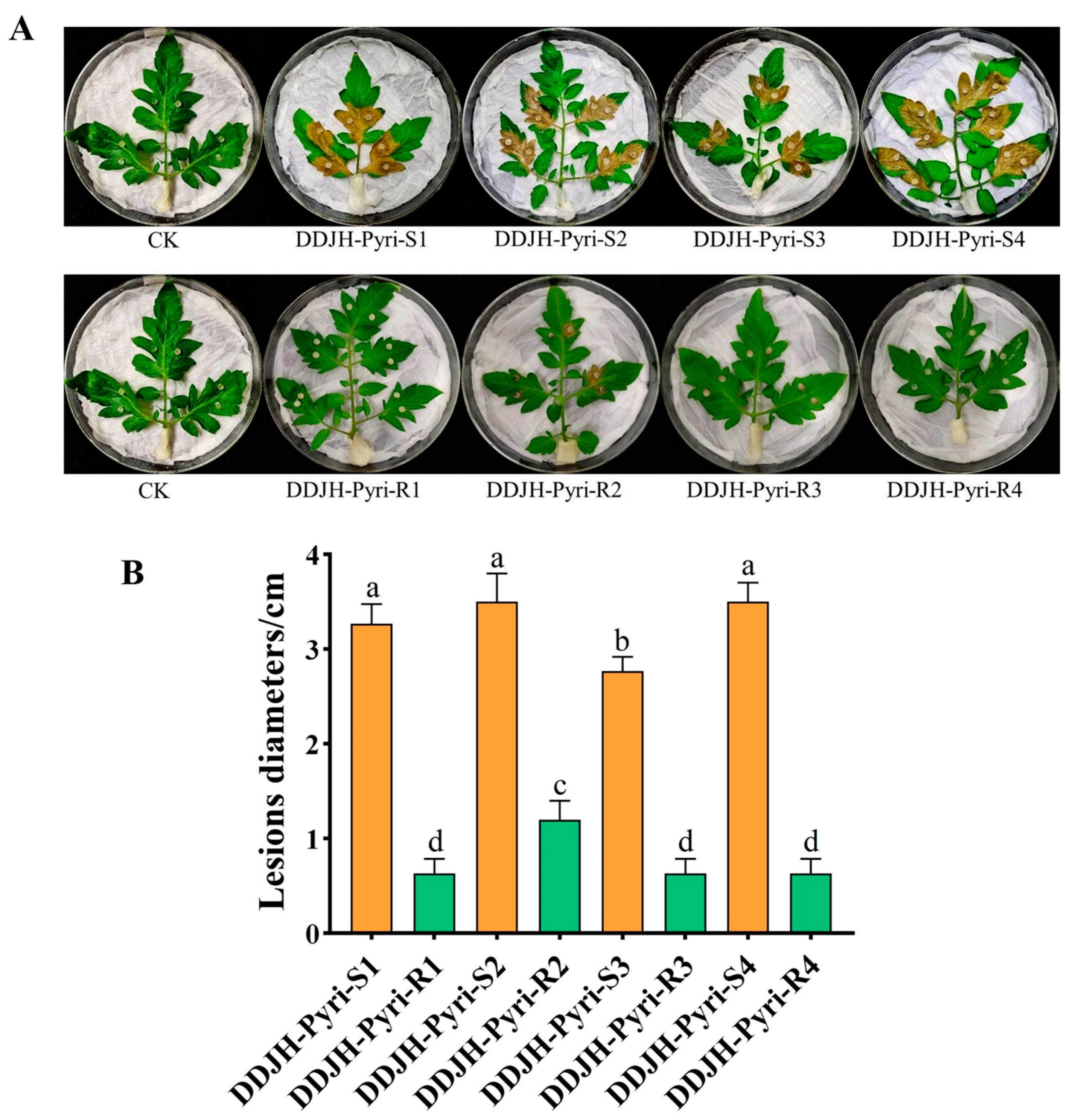
2.2.3. Pathogenicity on Detached Tomato Leaves
2.3. Cloning and Sequence of SsCGS1 and SsCGS2 Genes
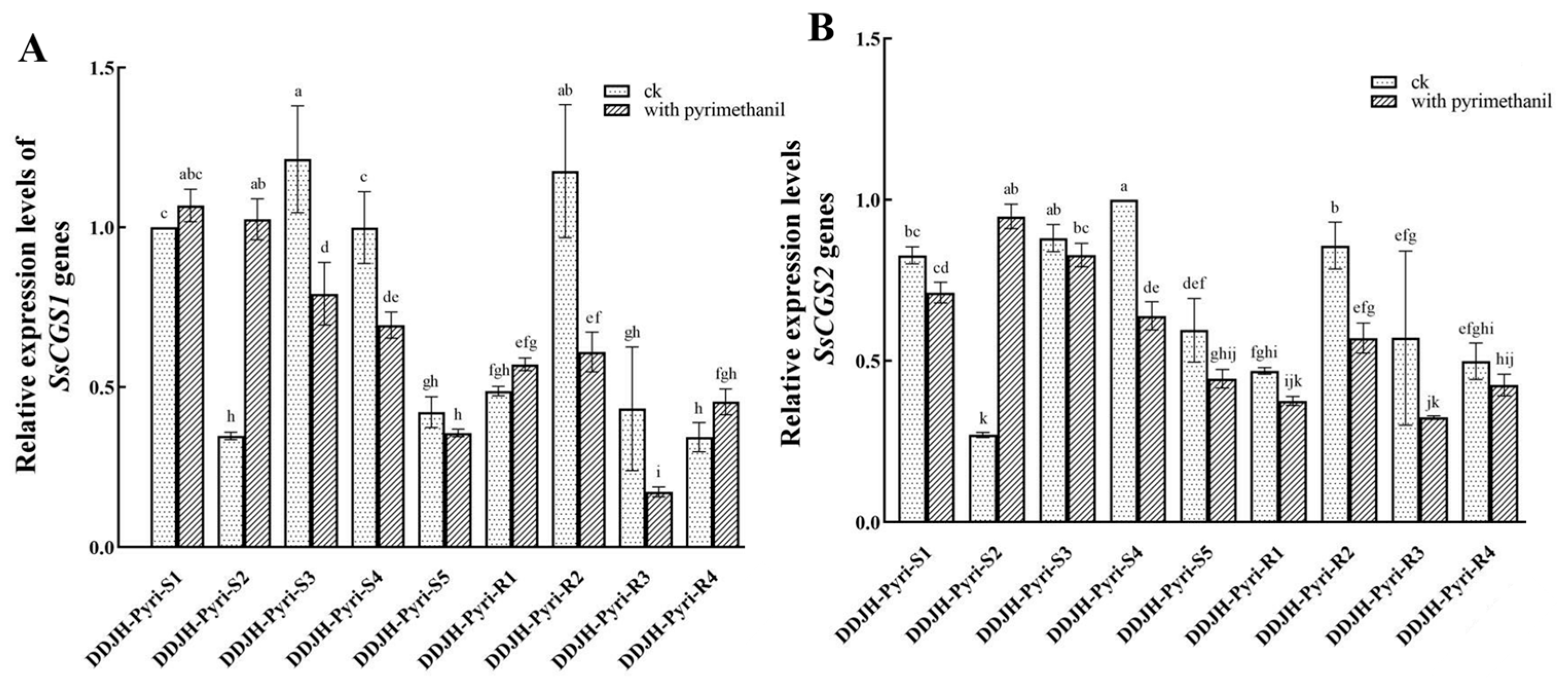
2.4. Comparison of SsCGS1 and SsCGS2 Expression Levels in Pyrimethanil-Resistant Mutants of S. sclerotiorum
2.5. Molecular Docking
2.6. Cross-Resistance of Pyrimethanil with Other Fungicides
2.7. Data Analysis
3. Results
3.1. Biological Characteristics of Four Pyrimethanil-Resistant Mutants of S. sclerotiorum
3.2. Sequence Analysis of the SsCGS1 and SsCGS2 Protein in Pyrimethanil-Resistant Mutants of S. sclerotiorum
3.3. Relative Expression of the SsCGS1 and SsCGS2 Genes in Pyrimethanil-Resistant Mutants of S. sclerotiorum
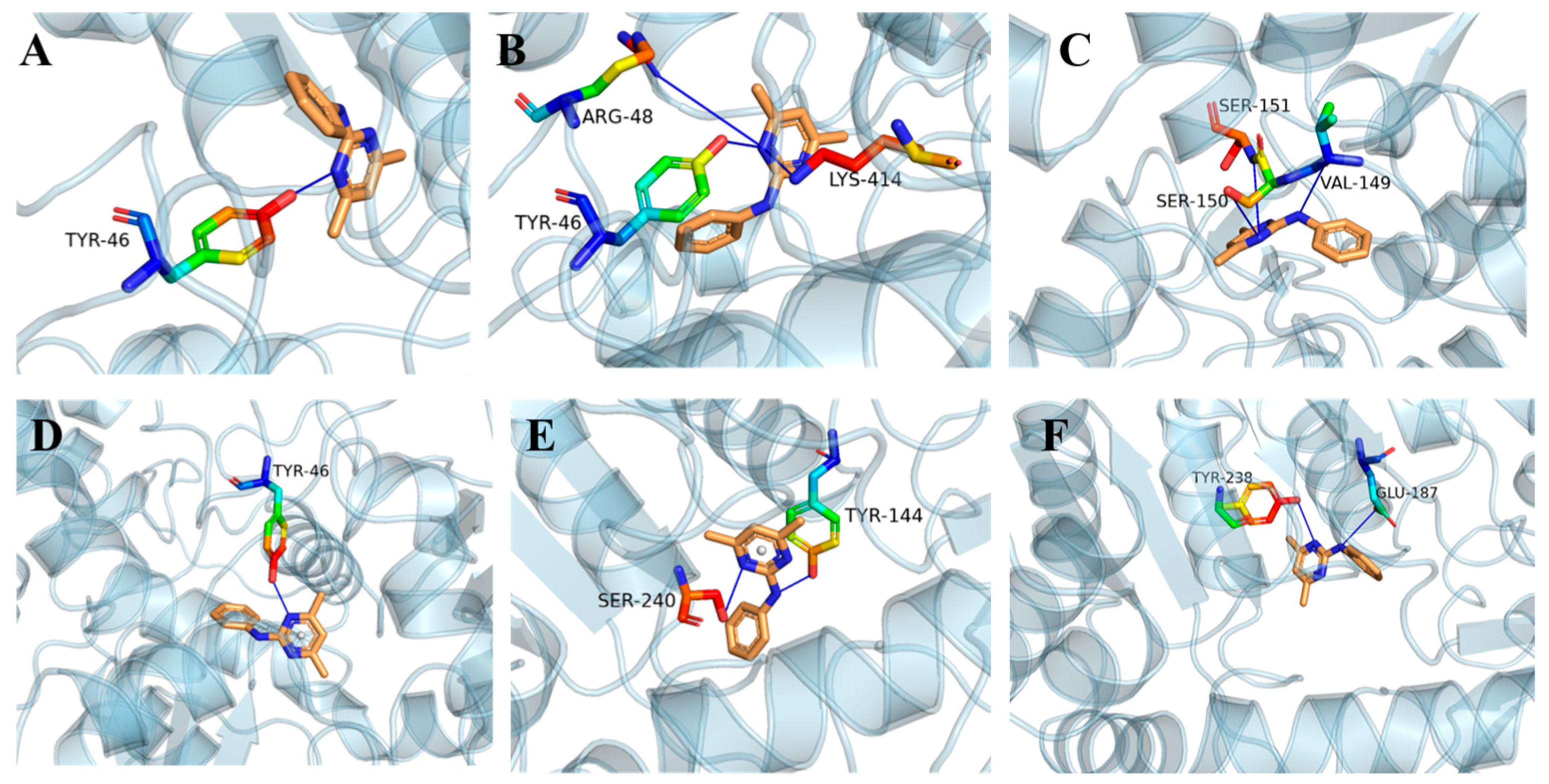
3.4. Molecular Docking Analysis
3.5. Cross-Resistance Between Pyrimethanil and Other Fungicides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Liu, S.L.; Wang, Z.; Yuan, Y.Q.; Zhang, Z.F.; Liang, Q.J.; Yang, X.; Duan, Z.B.; Liu, Y.C.; Kong, F.J.; et al. Progress in soybean functional genomics over the past decade. Plant Biotechnol. J. 2022, 20, 256–282. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, M.M.; Guan, Y.J.; Guo, W.; Zhang, C.; Wei, Y.C.; Zhao, Z.W.; Ma, H.Y.; Wang, L.F.; Jiang, X.Y.; et al. Transcriptional regulatory network reveals key transcription factors for regulating agronomic traits in soybean. Genome Biol. 2024, 25, 313. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zuo, X.; Zuo, D.; Lin, H.; Huang, X.; Zang, C. Study on soybean potential productivity and food security in China under the influence of COVID-19 outbreak. Geogr. Sustain. 2020, 1, 163–171. [Google Scholar] [CrossRef]
- Xiao, K.; Qiao, K.; Cui, W.; Xu, X.; Pan, H.Y.; Wang, F.T.; Wang, S.D.; Yang, F.; Xuan, Y.H.; Li, A.; et al. Comparative transcriptome profiling reveals the importance of GmSWEET15 in soybean susceptibility to Sclerotinia sclerotiorum. Front. Microbiol. 2023, 14, 1119016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, L.Y.; Cao, J.; Li, Y.L.; Ding, L.N.; Zhu, K.M.; Yang, Y.H.; Tan, X.L. Recent advances in mechanisms of plant defense to Sclerotinia sclerotiorum. Front. Plant Sci. 2019, 10, 1314. [Google Scholar] [CrossRef]
- Wang, Y.F.; Lu, N.H.; Wang, K.K.; Li, Y.; Zhang, M.L.; Liu, S.; Li, Y.L.; Zhou, F. Fluxapyroxad resistance mechanisms in Sclerotinia sclerotiorum. Plant Dis. 2023, 107, 1035–1043. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.S.; Mao, H.X.; Zhang, C.Q.; Wu, J.Y. Genetic structure and pyrimethanil resistance of Botrytis spp. causing gray mold on strawberry from greenhouses in Zhejiang, China. Pestic. Biochem. Physiol. 2024, 205, 106128. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Wang, C.; Liu, S.Q. Synergistic antifungal effect and potential mechanism of dimethomorph combined with pyrimethanil against Phytophthora capsici. Food Chem. 2024, 457, 140158. [Google Scholar] [CrossRef]
- Amiri, A.; Ali, M.E.; De Angelis, D.R.; Mulvaney, K.A.; Pandit, L.K. Prevalence and distribution of Penicillium expansum and Botrytis cinerea in apple packinghouses across Washington State and their sensitivity to the postharvest fungicide pyrimethanil. Acta Horticulturae 2021, 167–172. [Google Scholar] [CrossRef]
- Caiazzo, R.; Kim, Y.K.; Xiao, C.L. Occurrence and phenotypes of pyrimethanil resistance in Penicillium expansum from apple in Washington State. Plant Dis. 2014, 98, 924–928. [Google Scholar] [CrossRef]
- Madan, P.; Achour, A. High resistance levels to pyrimethanil and fludioxonil among fourteen Penicillium spp. from pome fruits in the U.S. Pacific Northwest. Pestic. Biochem. Physiol. 2024, 206, 106206. [Google Scholar]
- Zhang, Y.C.; Fu, Y.P.; Luo, C.X.; Zhu, F.X. Pyrimethanil sensitivity and resistance mechanisms in Penicillium digitatum. Plant Dis. 2021, 105, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, B.; Li, J.; Zhang, K.; Ran, L.; Ge, B. Effect of combining wuyiencin and pyrimethanil on Controlling grape gray mold and delaying resistance development in Botrytis cinerea. Microorganisms 2024, 12, 1383. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.P.; Mao, X.W.; Qu, X.P.; Wang, J.X.; Chen, C.J.; Zhou, M.G. Molecular and biological characterization of Sclerotinia sclerotiorum resistant to the anilinopyrimidine fungicide cyprodinil. Pestic. Biochem. Physiol. 2018, 146, 80–89. [Google Scholar] [CrossRef]
- Wang, Y.F.; Lu, N.H.; Wang, K.K.; Li, Y.N.; Zhang, M.L.; Liu, S.; Li, Y.L.; Zhou, F. Baseline sensitivity and potential resistance mechanisms for Fusarium pseudograminearum to fludioxonil. Plant Dis. 2021, 106, 2138–2144. [Google Scholar]
- Zhou, F.; Cui, Y.X.; Ma, Y.H.; Wang, J.Y.; Hu, H.Y.; Li, S.W.; Zhang, F.L.; Li, C.W. Investigating the potential mechanism of pydiflumetofen resistance in Sclerotinia sclerotiorum. Plant Dis. 2022, 105, 3580–3585. [Google Scholar] [CrossRef]
- Qiu, J.B.; Yu, M.Z.; Yin, Q.; Xu, J.H.; Shi, J.R. Molecular and characterization, fitness and mycotoxin production of Fusarium asiaticum strains resistant to fludioxonil. Plant Dis. 2018, 102, 1759–1765. [Google Scholar] [CrossRef]
- Nicolau, J.M.; Giovana, B.; Brocco, I.B.; Aparecida, P.N.; May De, M.L.; Duarte, H.S. Sensitivity of brazilian Botrytis cinerea isolates from strawberry to pyrimethanil and its control efficacy. Tropical Plant Pathol. 2023, 49, 141–146. [Google Scholar]
- FracList FRAC Code List©* 2024, Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). Available online: https://www.frac.info/media/kufnaceb/frac-code-list-2024.pdf (accessed on 1 January 2025).
- Avenot, H.F.; Michailides, T.J. Detection of isolates of Alternaria alternata with multiple-resistance to fludioxonil, cyprodinil, boscalid and pyraclostrobin in California pistachio orchards. Crop Prot. 2015, 78, 214–221. [Google Scholar] [CrossRef]
- Yan, H.J.; Gaskins, V.L.; Vico, I.; Luo, Y.G.; Jurick, W.M. First report of Penicillium expansum isolates resistant to pyrimethanil from stored apple fruit in Pennsylvania. Plant Dis. 2014, 98, 1004. [Google Scholar] [CrossRef]
- Liu, S.M.; Che, Z.P.; Chen, G.Q. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop Prot. 2016, 84, 56–61. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, G.X.; Huang, J.B.; Wang, Z.Y.; Li, X.H. Evaluation on resistance of grape gray mold pathogen Botrytis cinerea to pyrimethanil in China. Sci. Agric. Sin. 2013, 46, 1208–1212. [Google Scholar]
- Liu, Y.; Liu, Z.Y.; Hamada, M.S.; Yin, Y.N.; Ma, Z.H. Characterization of laboratory pyrimethanil-resistant mutants of Aspergillus flavus from groundnut in China. Crop Prot. 2014, 60, 5–8. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, B.; Gao, X.; Cui, Y.; Qian, L.; Xu, J.; Liu, S. In vitro determination of the sensitivity of Fusarium graminearum to fungicide fluopyram and investigation of the resistance mechanism. J. Agric. Food Chem. 2025, 73, 4001–4011. [Google Scholar] [CrossRef]

| Fungicide | Sensitive Parental Isolates | Pyrimethanil-Resistant Mutants | ||||||
|---|---|---|---|---|---|---|---|---|
| DDJH-Pyri-S1 | DDJH-Pyri-S2 | DDJH-Pyri-S3 | DDJH-Pyri-S4 | DDJH-Pyri-R1 | DDJH-Pyri-R2 | DDJH-Pyri-R3 | DDJH-Pyri-R4 | |
| Pyrimethanil | 0.441 Z | 0.539 | 0.610 | 0.513 | 7.247 | 14.526 | 24.718 | 13.84 |
| Cyprodinil | 0.009 | 0.045 | 0.022 | 0.002 | 0.091 | 0.014 | 0.058 | 0.026 |
| Fludioxonil | 0.001 | 0.002 | 0.003 | 0.002 | 0.001 | 0.004 | 0.001 | 0.006 |
| Prochloraz | 0.001 | 0.001 | 0.001 | 0.002 | 0.004 | 0.003 | 0.002 | 0.011 |
| Fluazinam | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.002 |
| Tebuconazole | 0.074 | 0.091 | 0.089 | 0.090 | 0.066 | 0.095 | 0.001 | 0.234 |
| Pyraclostrobin | 0.017 | 0.052 | 0.074 | 0.051 | 0.225 | 0.022 | 0.089 | 0.132 |
| Carbendazim | 0.131 | 0.399 | 0.117 | 0.069 | 0.061 | 0.679 | 0.293 | 0.534 |
| Boscalid | 0.185 | 0.167 | 0.214 | 0.169 | 0.032 | 0.002 | 0.007 | 0.039 |
| Genes | Mutants | Amino Acid Changes | Species/Fungicides/References |
|---|---|---|---|
| SsCGS1 | DDJH-Pyri-R1 | V117G, Y285H, N421H, V490A | S. sclerotiorum/Pyrimethanil/Current study |
| DDJH-Pyri-R2 | / N | S. sclerotiorum/Pyrimethanil/Current study | |
| DDJH-Pyri-R3 | M457T | S. sclerotiorum/Pyrimethanil/Current study | |
| DDJH-Pyri-R4 | H487R | S. sclerotiorum/Pyrimethanil/Current study | |
| SsCGS2 | DDJH-Pyri-R1 | G289R | S. sclerotiorum/Pyrimethanil/Current study |
| DDJH-Pyri-R2 | / | S. sclerotiorum/Pyrimethanil/Current study | |
| DDJH-Pyri-R3 | / | S. sclerotiorum/Pyrimethanil/Current study | |
| DDJH-Pyri-R4 | / | S. sclerotiorum/Pyrimethanil/Current study | |
| CGS1/CGS2 | HBWH-10 | / | Penicillium digitatum/Pyrimethanil/12 |
| JXGZ-92 | / | Penicillium digitatum/Pyrimethanil/12 | |
| JXGZ-59 | / | Penicillium digitatum/Pyrimethanil/12 | |
| JXGZ-177 | / | Penicillium digitatum/Pyrimethanil/12 | |
| CGS1/CGS2 | PR1 | / | Aspergillus flavus/Pyrimethanil/24 |
| PR2 | / | Aspergillus flavus/Pyrimethanil/24 | |
| PR3 | / | Aspergillus flavus/Pyrimethanil/24 | |
| PR4 | / | Aspergillus flavus/Pyrimethanil/24 | |
| PR5 | / | Aspergillus flavus/Pyrimethanil/24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, Z.; Liu, T.; Gao, X.; Shi, Y.; Wu, H.; Liu, R.; Kan, Y.; Yu, H.; Zhou, F. The Resistance Mechanisms of Anilinopyrimidine Fungicide Pyrimethanil in Sclerotinia sclerotiorum. J. Fungi 2025, 11, 344. https://doi.org/10.3390/jof11050344
Wang Y, Chen Z, Liu T, Gao X, Shi Y, Wu H, Liu R, Kan Y, Yu H, Zhou F. The Resistance Mechanisms of Anilinopyrimidine Fungicide Pyrimethanil in Sclerotinia sclerotiorum. Journal of Fungi. 2025; 11(5):344. https://doi.org/10.3390/jof11050344
Chicago/Turabian StyleWang, Yanfen, Zeyuan Chen, Tiancheng Liu, Xupeng Gao, Yanchao Shi, Honghui Wu, Runqiang Liu, Yunchao Kan, Hao Yu, and Feng Zhou. 2025. "The Resistance Mechanisms of Anilinopyrimidine Fungicide Pyrimethanil in Sclerotinia sclerotiorum" Journal of Fungi 11, no. 5: 344. https://doi.org/10.3390/jof11050344
APA StyleWang, Y., Chen, Z., Liu, T., Gao, X., Shi, Y., Wu, H., Liu, R., Kan, Y., Yu, H., & Zhou, F. (2025). The Resistance Mechanisms of Anilinopyrimidine Fungicide Pyrimethanil in Sclerotinia sclerotiorum. Journal of Fungi, 11(5), 344. https://doi.org/10.3390/jof11050344






