Gastrointestinal and Intra-Abdominal Mucormycosis in Non-Haematological Patients—A Comprehensive Review
Abstract
1. Introduction
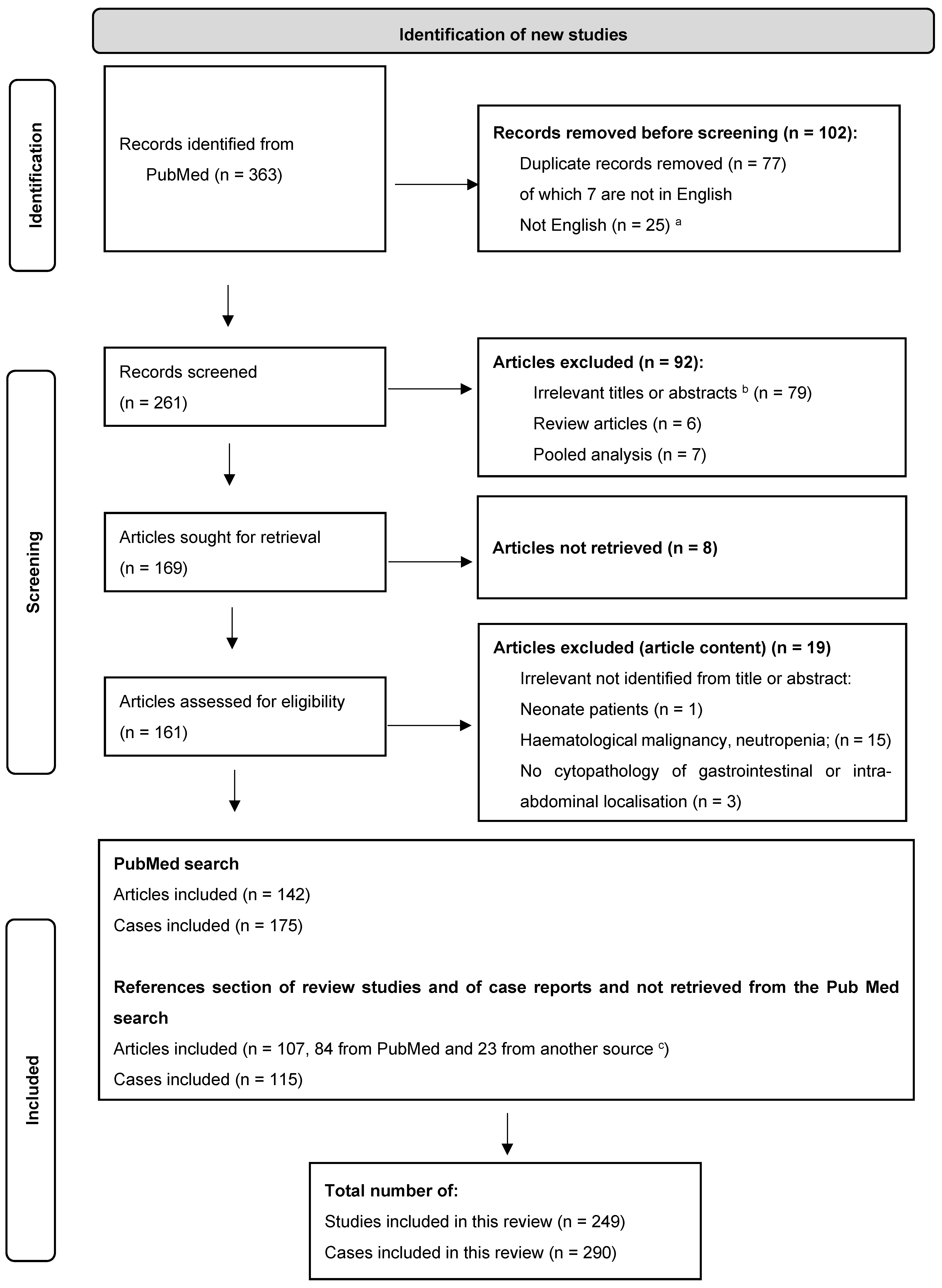
2. Patients and Methods
2.1. Search
2.2. Definitions
2.3. Statistics
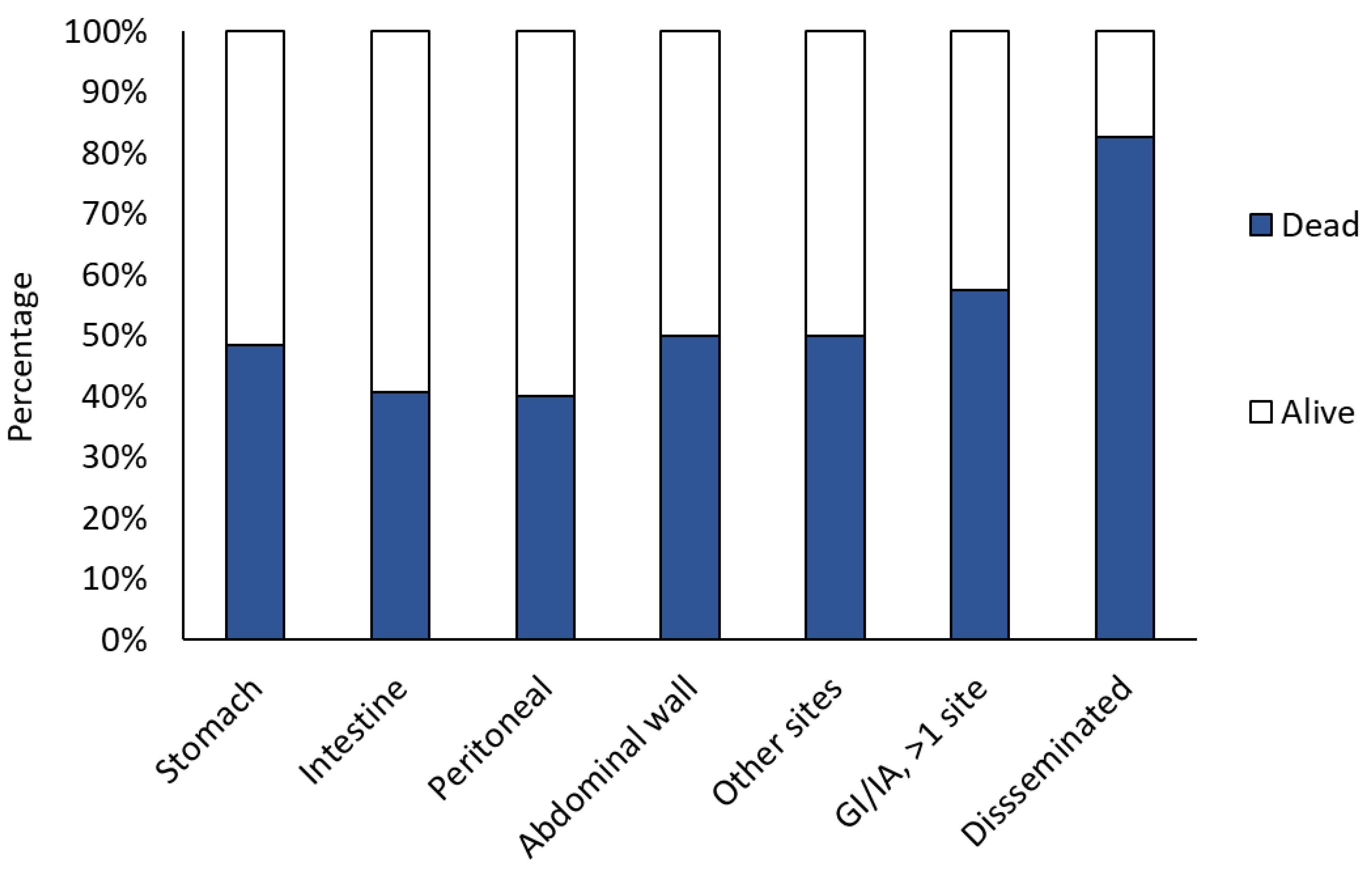
3. Results
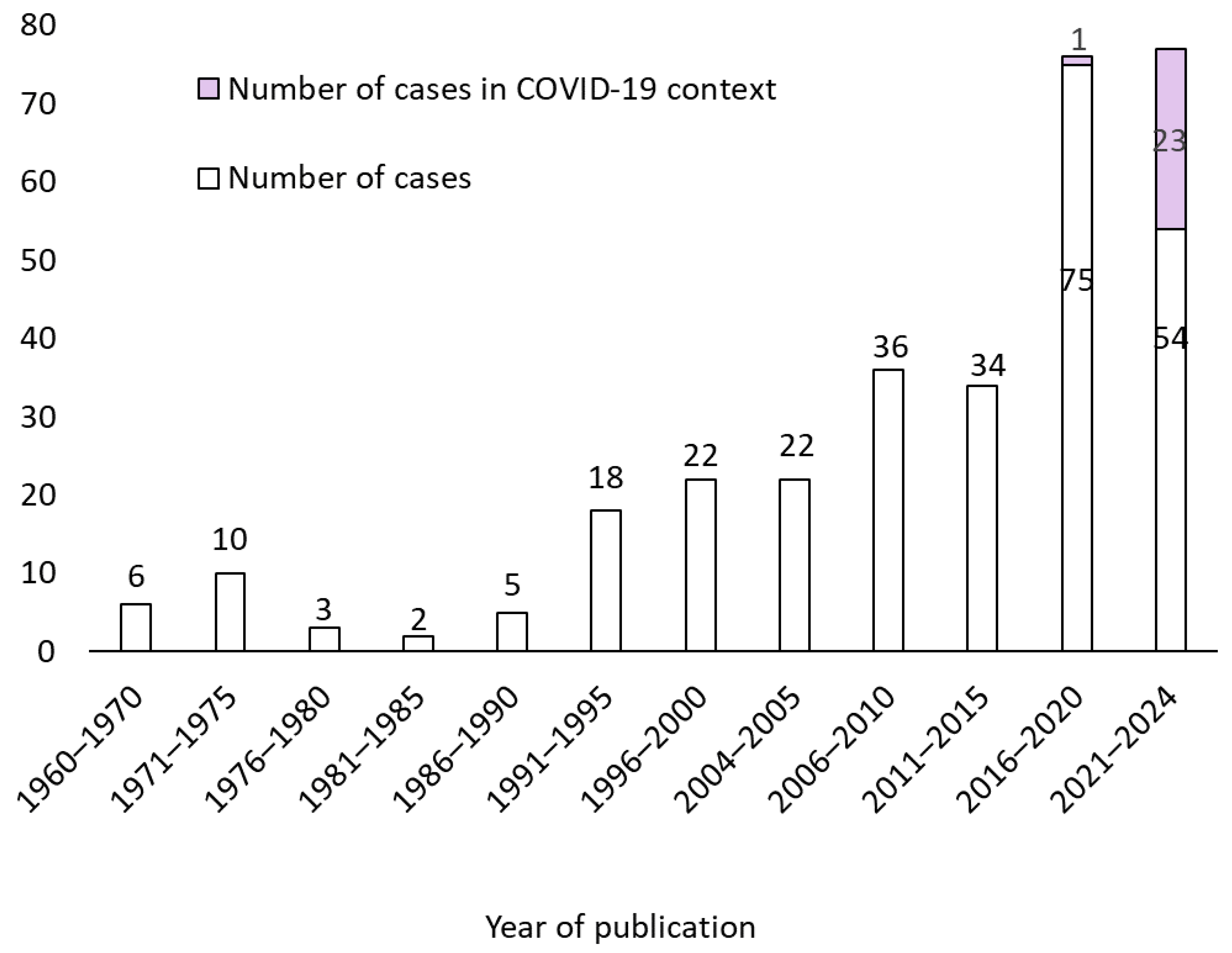
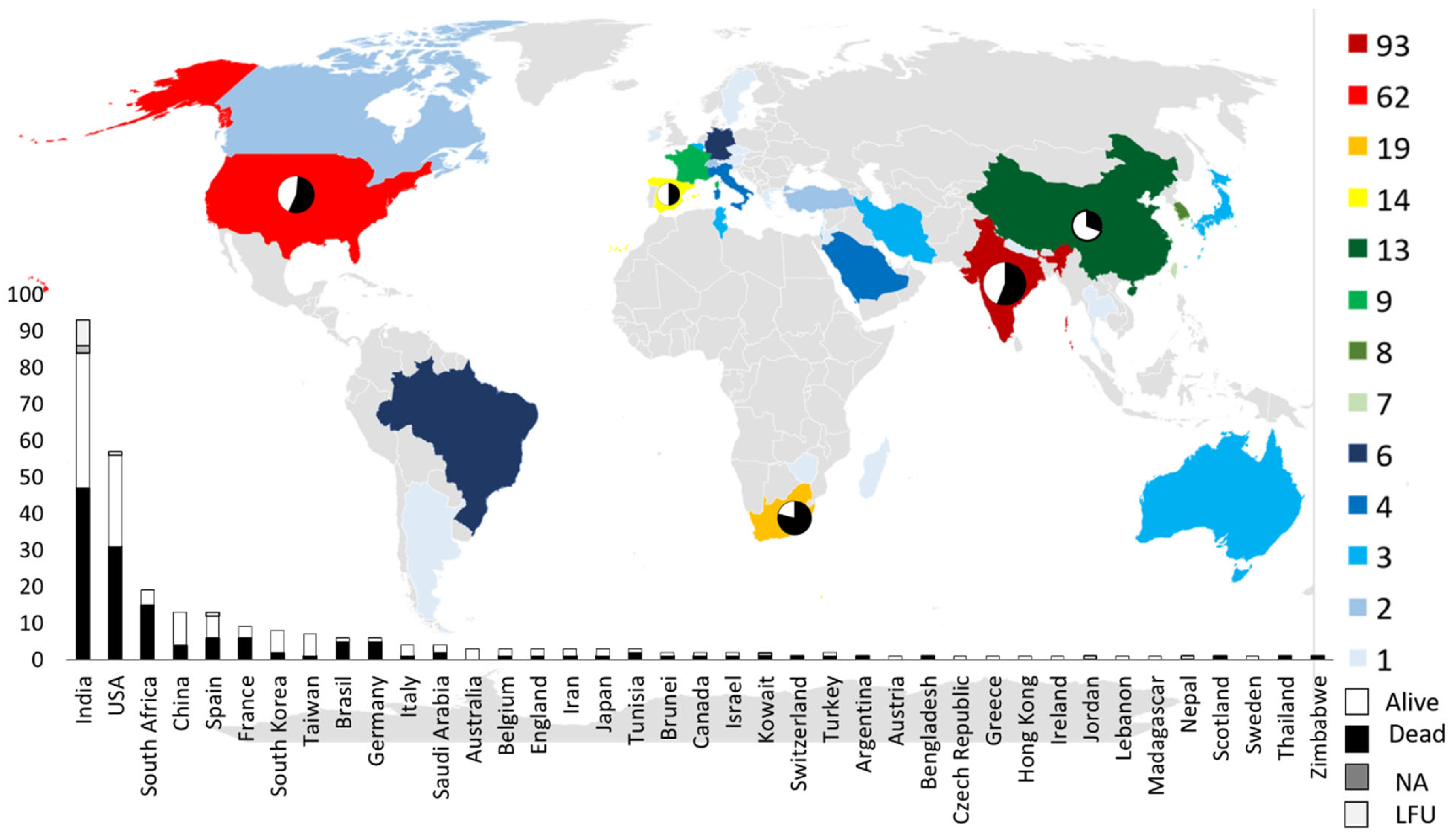
3.1. Demographic Data and Underling Conditions
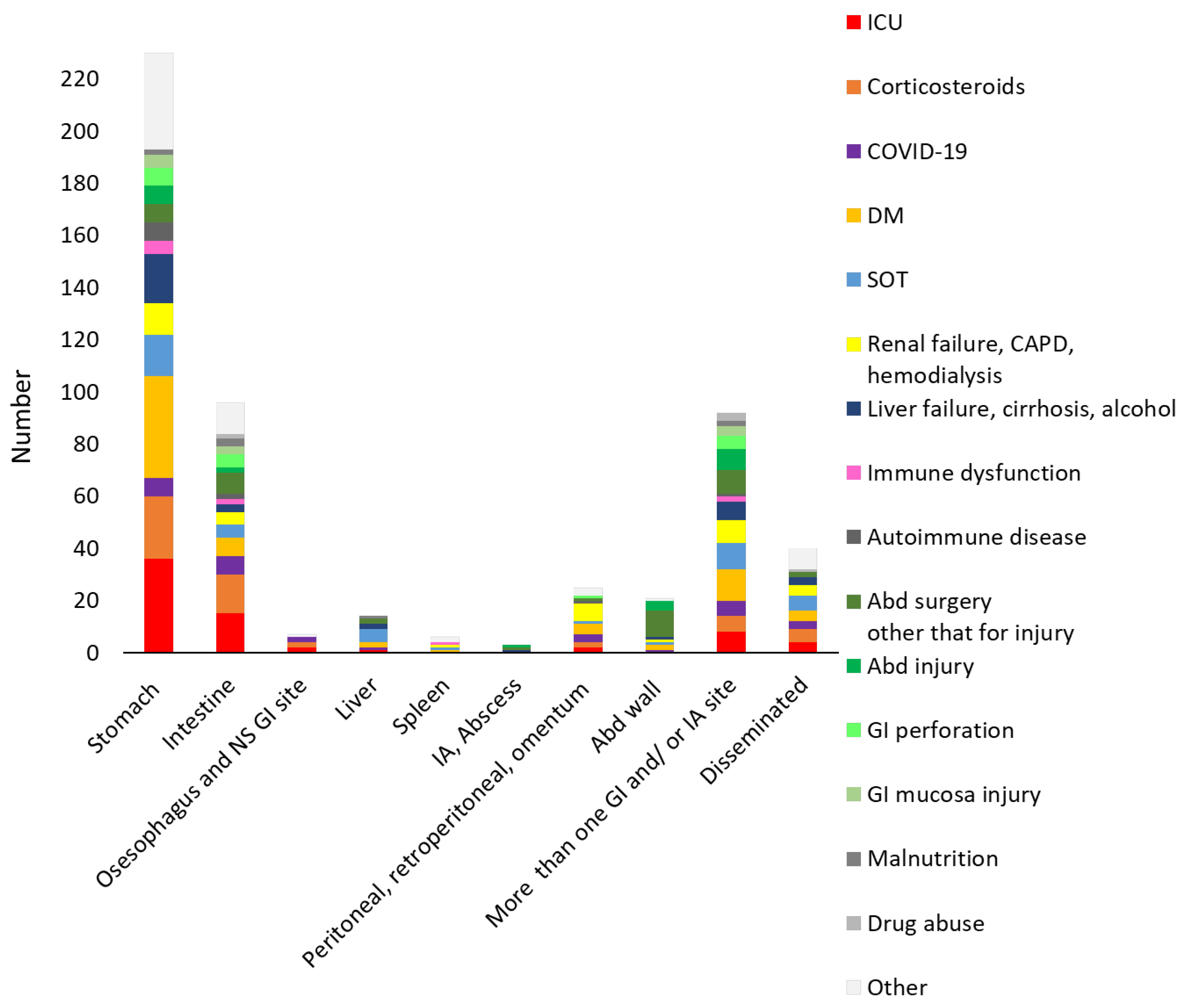
3.2. Clinical and Paraclinical Presentation
3.3. Clinical Presentation
3.4. Diagnostic
3.5. Management and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitale, R.G.; de Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; Wendy, W.; van de Sande, W.W.; Dolatabadi, S.; et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Alqarihi, A.; Kontoyiannis, D.P.; Ibrahim, A.S. Mucormycosis in 2023: An update on pathogenesis and management. Front. Cell Infect. Microbiol. 2023, 13, 1254919. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Wessel, V.C.; Bodey, G.P.; Rolston, K.V. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 2000, 30, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Carter, R.A.; Crippa, F.; Wald, A.; Corey, L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2002, 34, 909–917. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Bouchuiguir-Wafa, K.; Garbino, J. Emerging invasive zygomycosis in a tertiary care center: Epidemiology and associated risk factors. Int. J. Infect. Dis. 2010, 14 (Suppl. S3), e100–e103. [Google Scholar] [CrossRef]
- Bitar, D.; Lortholary, O.; Le Strat, Y.; Nicolau, J.; Coignard, B.; Tattevin, P.; Che, D.; Dromer, F. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg. Infect. Dis. 2014, 20, 1149–1155. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Ahmadi, B.; Rezaei-Matehkolaei, A.; Zarrinfar, H.; Skiada, A.; Mirhendi, H.; Nashibi, R.; Niknejad, F.; Nazeri, M.; Rafiei, A.; et al. Mucormycosis in Iran: A six-year retrospective experience. J. Mycol. Med. 2018, 28, 269–273. [Google Scholar] [CrossRef]
- Prakash, H.; Chakrabarti, A. Epidemiology of Mucormycosis in India. Microorganisms 2021, 9, 523. [Google Scholar] [CrossRef]
- Danion, F.; Letscher-Bru, V.; Guitard, J.; Sitbon, K.; Delliere, S.; Angoulvant, A.; Desoubeaux, G.; Botterel, F.; Bellanger, A.P.; Gargala, G.; et al. Coronavirus Disease 2019-Associated Mucormycosis in France: A Rare but Deadly Complication. Open Forum Infect Dis. 2022, 9, ofab566. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J., Jr.; Ibrahim, A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [PubMed]
- Tribble, D.R.; Warkentien, T.; Rodriguez, C.; Trauma Infectious Diseases Outcomes Study Group of the Infectious Disease Clinical Research Program. Mucormycosis after a tornado in Joplin, Missouri. N. Engl. J. Med. 2013, 368, 1067. [Google Scholar]
- Warkentien, T.E.; Shaikh, F.; Weintrob, A.C.; Rodriguez, C.J.; Murray, C.K.; Lloyd, B.A.; Ganesan, A.; Aggarwal, D.; Carson, M.L.; Tribble, D.R. Impact of Mucorales and Other Invasive Molds on Clinical Outcomes of Polymicrobial Traumatic Wound Infections. J. Clin. Microbiol. 2015, 53, 2262–2270. [Google Scholar]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, M.; Ferry, A.; Chaussard, M.; Hamane, S.; et al. Detection of Circulating Mucorales DNA in Critically Ill Burn Patients: Preliminary Report of a Screening Strategy for Early Diagnosis and Treatment. Clin. Infect. Dis. 2016, 63, 1312–1317. [Google Scholar]
- Dang, J.; Goel, P.; Choi, K.J.; Massenzio, E.; Landau, M.J.; Pham, C.H.; Huang, S.; Yenikomshian, H.A.; Spellberg, B.; Gillenwater, T.J. Mucormycosis following burn injuries: A systematic review. Burns 2023, 49, 15–25. [Google Scholar]
- Drogari-Apiranthitou, M.; Skiada, A.; Panayiotides, I.; Vyzantiadis, T.A.; Poulopoulou, A.; Christofidou, M.; Antoniadou, A.; Roilides, E.; Iosifidis, E.; Mamali, V.; et al. Epidemiology of Mucormycosis in Greece; Results from a Nationwide Prospective Survey and Published Case Reports. J. Fungi 2023, 9, 425. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl. S1), S23–S34. [Google Scholar]
- Serris, A.; Danion, F.; Lanternier, F. Disease Entities in Mucormycosis. J. Fungi 2019, 5, 23. [Google Scholar] [CrossRef]
- Dioverti, M.V.; Cawcutt, K.A.; Abidi, M.; Sohail, M.R.; Walker, R.C.; Osmon, D.R. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses 2015, 58, 714–718. [Google Scholar]
- Kaur, H.; Ghosh, A.; Rudramurthy, S.M.; Chakrabarti, A. Gastrointestinal mucormycosis in apparently immunocompetent hosts-A review. Mycoses 2018, 61, 898–908. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar]
- Rammaert, B.; Lanternier, F.; Zahar, J.R.; Dannaoui, E.; Bougnoux, M.E.; Lecuit, M.; Lortholary, O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl. S1), S44–S54. [Google Scholar]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018, 56 (Suppl. S1), 93–101. [Google Scholar]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar]
- Didehdar, M.; Chegini, Z.; Moradabadi, A.; Anoushirvani, A.A.; Tabaeian, S.P.; Yousefimashouf, M.; Shariati, A. Gastrointestinal mucormycosis: A periodic systematic review of case reports from 2015 to 2021. Microb. Pathog. 2022, 163, 105388. [Google Scholar]
- Song, Y.; Qiao, J.; Giovanni, G.; Liu, G.; Yang, H.; Wu, J.; Chen, J. Mucormycosis in renal transplant recipients: Review of 174 reported cases. BMC Infect. Dis. 2017, 17, 283. [Google Scholar]
- Mustafa, A.; Mahmood, Y.; Hasan, A.; Asaad, H.; Gharib, D.; Hama Hussein, K.; Hasan, K.M.; Ismaeil, D.A.; Hiwa, D.S.; Ali, R.M.; et al. Gastric Mucormycosis: A Systematic Review with Metadata. Barw. Med. J. 2024, 2, 65–84. [Google Scholar]
- Sutherland, J.C.; Jones, T.H. Gastric mucormycosis: Report of case in a Swazi. S. Afr. Med. J. 1960, 34, 161. [Google Scholar]
- Kahn, L.B. Gastric Mucormycosis: Report of a Case with a Review of the Literature. S. Afr. Med. J. 1963, 37, 1265–1269. [Google Scholar] [PubMed]
- Abramowitz, I. Fatal Perforations of the Stomach Due to Mucormycosis of the Gastro-Intestinal Tract. S. Afr. Med. J. 1964, 38, 93–94. [Google Scholar] [PubMed]
- Dannheimer, I.P.; Fouche, W.; Nel, C. Gastric mucormycosis in a diabetic patient. S. Afr. Med. J. 1974, 48, 838–839. [Google Scholar] [PubMed]
- Lawson, H.H.; Schmaman, A. Gastric phycomycosis. Br. J. Surg. 1974, 61, 743–746. [Google Scholar]
- Schulman, A.; Bornman, P.; Kaplan, C.; Morton, P.; Rose, A. Gastrointestinal mucormycosis. Gastrointest. Radiol. 1979, 4, 385–388. [Google Scholar]
- Brullet, E.; Andreu, X.; Elias, J.; Roig, J.; Cervantes, M. Gastric mucormycosis in a patient with acquired immunodeficiency syndrome. Gastrointest. Endosc. 1993, 39, 106–107. [Google Scholar]
- Winkler, S.; Susani, S.; Willinger, B.; Apsner, R.; Rosenkranz, A.R.; Potzi, R.; Berlakovich, G.A.; Pohanka, E. Gastric mucormycosis due to Rhizopus oryzae in a renal transplant recipient. J. Clin. Microbiol. 1996, 34, 2585–2587. [Google Scholar]
- Uchida, T.; Okamoto, M.; Fujikawa, K.; Yoshikawa, D.; Mizokami, A.; Mihara, T.; Kondo, A.; Ohba, K.; Kurohama, K.; Nakashima, M.; et al. Gastric mucormycosis complicated by a gastropleural fistula: A case report and review of the literature. Medicine 2019, 98, e18142. [Google Scholar]
- Corley, D.A.; Lindeman, N.; Ostroff, J.W. Survival with early diagnosis of invasive gastric mucormycosis in a heart transplant patient. Gastrointest. Endosc. 1997, 46, 452–454. [Google Scholar]
- Knoop, C.; Antoine, M.; Vachiery, J.L.; Depre, G.; Alonso-Vega, C.; Struelens, M.; Van Laethem, J.L.; Lingier, P.; Nagy, N.; Jacobs, F.; et al. Gastric perforation due to mucormycosis after heart-lung and heart transplantation. Transplantation 1998, 66, 932–935. [Google Scholar]
- Sharma, M.C.; Gill, S.S.; Kashyap, S.; Kataria, R.; Gupta, D.K.; Sahni, P.; Acharya, S.K. Gastrointestinal mucormycosis--an uncommon isolated mucormycosis. Indian J. Gastroenterol. 1998, 17, 131–133. [Google Scholar] [PubMed]
- Sheu, B.S.; Lee, P.C.; Yang, H.B. A giant gastric ulcer caused by mucormycosis infection in a patient with renal transplantation. Endoscopy 1998, 30, S60–S61. [Google Scholar] [PubMed]
- Barroso, F.; Forcelledo, J.L.; Mayorga, M.; Pena, F.; Marques, F.F.; de la Pena, J. A fatal case of gastric mucormyosis in a heart transplant recipient. Endoscopy 1999, 31, S2. [Google Scholar]
- Cherney, C.L.; Chutuape, A.; Fikrig, M.K. Fatal invasive gastric mucormycosis occurring with emphysematous gastritis: Case report and literature review. Am. J. Gastroenterol. 1999, 94, 252–256. [Google Scholar]
- Al-Rikabi, A.C.; Al-Dohayan, A.D.; Al-Boukai, A.A. Invasive mucormycosis in benign gastric ulcer. Saudi Med. J. 2000, 21, 287–290. [Google Scholar]
- Pickeral, J.J., 3rd; Silverman, J.F.; Sturgis, C.D. Gastric zygomycosis diagnosed by brushing cytology. Diagn. Cytopathol. 2000, 23, 51–54. [Google Scholar]
- Geramizadeh Bita, A.S. Invasive gastric mucormycosis: Report of a case and review of the litterature. Med. J. Islam. Republic Iran 2001, 14, 397–398. [Google Scholar]
- Tinmouth, J.; Baker, J.; Gardiner, G. Gastrointestinal mucormycosis in a renal transplant patient. Can. J. Gastroenterol. 2001, 15, 269–271. [Google Scholar]
- Paulo De Oliveira, J.E.; Milech, A. A fatal case of gastric mucormycosis and diabetic ketoacidosis. Endocr. Pract. 2002, 8, 44–46. [Google Scholar]
- Park, Y.S.; Lee, J.D.; Kim, T.H.; Joo, Y.H.; Lee, J.H.; Lee, T.S.; Kim, E.K. Gastric mucormycosis. Gastrointest. Endosc. 2002, 56, 904–905. [Google Scholar]
- Shahapure, A.G.; Patankar, R.V.; Bhatkhande, R. Gastric mucormycosis. Indian J. Gastroenterol. 2002, 21, 231–232. [Google Scholar] [PubMed]
- Vera, A.; Hubscher, S.G.; McMaster, P.; Buckels, J.A. Invasive gastrointestinal zygomycosis in a liver transplant recipient: Case report. Transplantation 2002, 73, 145–147. [Google Scholar] [PubMed]
- Maravi-Poma, E.; Rodriguez-Tudela, J.L.; de Jalon, J.G.; Manrique-Larralde, A.; Torroba, L.; Urtasun, J.; Salvador, B.; Montes, M.; Mellado, E.; Rodríguez-Albarrán, F.; et al. Outbreak of gastric mucormycosis associated with the use of wooden tongue depressors in critically ill patients. Intensive Care Med. 2004, 30, 724–728. [Google Scholar] [PubMed]
- Stamm, B. Mucormycosis of the stomach in a patient with multiple trauma. Histopathology 2005, 47, 222–223. [Google Scholar]
- Prasad, N.; Ram, R.; Satti Reddy, V.; Dakshinamurty, K.V. Non-fatal gastric mucormycosis in a renal transplant patient and review of the literature. Transpl. Infect. Dis. 2006, 8, 237–241. [Google Scholar]
- Devlin, S.M.; Hu, B.; Ippoliti, A. Mucormycosis presenting as recurrent gastric perforation in a patient with Crohn’s disease on glucocorticoid, 6-mercaptopurine, and infliximab therapy. Dig. Dis. Sci. 2007, 52, 2078–2081. [Google Scholar]
- Ho, Y.H.; Wu, B.G.; Chen, Y.Z.; Wang, L.S. Gastric Mucormycosis in an Alcoholic with Review of the Literature. Tzu Chi Med. J. 2007, 19, 169–172. [Google Scholar]
- Vaiphei, K.; Suri, V.; Bhalla, A. Fever, jaundice, altered sensorium, with multiple systemic manifestations. Indian J. Gastroenterol. 2007, 26, 82–86. [Google Scholar]
- Chung, C.S.; Wang, W.L.; Liu, K.L.; Lin, J.T.; Wang, H.P. Green ulcer in the stomach: Unusual mucormycosis infection. Gastrointest. Endosc. 2008, 68, 566–567. [Google Scholar]
- Shiva Prasad, B.N.; Shenoy, A.; Nataraj, K.S. Primary gastrointestinal mucormycosis in an immunocompetent person. J. Postgrad. Med. 2008, 54, 211–213. [Google Scholar]
- Azhar, A.; Calubiran, O.V.; Kilaru, R.M.; Lincoln, J.A.; Moshenyat, I.; Basti, K.; Visconti, E. Patient With Abdominal Pain-Concealing Gastrointestinal Mucormycosis. Gastroenterol. Hepatol. 2009, 5, 657–661. [Google Scholar]
- Nandu, V.; Nagral, A.; Khubchandani, S.; Agrawal, C. A rare cause of upper GI bleeding. Gut 2009, 58, 1039. [Google Scholar] [CrossRef] [PubMed]
- Small, M.; Gill, J.; Reed, J.; Poetter, D.; Goldsmith, S. Isolated gastric mucormycosis—A rare cause of a large gastric ulceration in an immunocompetent host. Am. J. Gastroenterol. 2010, 105, S172. [Google Scholar] [CrossRef]
- Feng, W.; Tseng, C.; Lin, C.; Tseng, K. Nonfatal gastric mucormycosis associated with emphysematous gastritis. Tzu Chi Med. J. 2010, 22, 146–148. [Google Scholar] [CrossRef]
- Johnson, C.B.; Ahmeti, M.; Tyroch, A.H.; Zuckerman, M.J.; Hakim, M.N. Gastric mucormycosis as a cause of life-threatening upper gastrointestinal bleeding in a trauma patient. Am. Surg. 2010, 76, E76–E77. [Google Scholar] [CrossRef]
- Paydar, S.; Baezzat, S.; Fazelzadeh, A.; Geramizadeh, B. A case of gastric zygomycosis in a diabetic patient successfully treated with total gastrectomy. Middle East. J. Dig. Dis. 2010, 2, 46–48. [Google Scholar]
- Pruthvi, B.C.R.C.; Rupashree, S.; Deepak, S.; Vikram, S.; Jayaprakash, B.R.N.; Rao, L. Gastric mucormycosis masquerading asmalignancy in an immunocompetent host. Arab. J. Gastroenterol. 2010, 11, 227–229. [Google Scholar] [CrossRef]
- Shenoi, S.; Emery, H.M. Successful treatment of invasive gastric mucormycosis in a child with systemic lupus erythematosus. Lupus 2010, 19, 646–649. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Ngan, A.H.; Tung, E.T.; Leung, S.Y.; To, K.K.; Cheng, V.C.; Yuen, K.Y. Lichtheimia hongkongensis sp. nov., a novel Lichtheimia spp. associated with rhinocerebral, gastrointestinal, and cutaneous mucormycosis. Diagn. Microbiol. Infect. Dis. 2010, 66, 274–284. [Google Scholar] [CrossRef]
- Chhaya, V.; Gupta, S.; Arnaout, A. Mucormycosis causing giant gastric ulcers. Endoscopy 2011, 43 (Suppl. S2), E289–E290. [Google Scholar] [CrossRef]
- Chang, C.F.; Huang, T.Y.; Lin, T.Y. Unusual giant gastric cardia ulcer. Intern. Emerg. Med. 2018, 13, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Roy, M.; Singh, T.; Singh, T. Gastric mucormycosis in an immunocompetent patient. J. Med. Soc. 2012, 26, 192–194. [Google Scholar]
- Naqvi, H.A.; Nadeem Yousaf, M.; Chaudhary, F.S.; Mills, L. Gastric Mucormycosis: An Infection of Fungal Invasion into the Gastric Mucosa in Immunocompromised Patients. Case Rep. Gastrointest. Med. 2020, 2020, 8876125. [Google Scholar]
- Muthuswamy, M. Giant necrotic ulcer: Invasive gastric mucormycosis. Am. J. Gastroenterol. 2012, 107, S300. [Google Scholar] [CrossRef]
- Ryan, O.; Frohlich, S.; Crotty, T.B.; Ryan, D. Rhizopus microsporus infection in an immunocompetent host: A case of immunoparalysis? Anaesth. Intensive Care. 2012, 40, 367–368. [Google Scholar]
- Corey, K.E.; Gupta, N.K.; Agarwal, S.; Xiao, H.D. Case records of the Massachusetts General Hospital. Case 32-2013. A 55-year-old woman with autoimmune hepatitis, cirrhosis, anorexia, and abdominal pain. N. Engl. J. Med. 2013, 369, 1545–1553. [Google Scholar] [CrossRef]
- Irtan, S.; Lamerain, M.; Lesage, F.; Verkarre, V.; Bougnoux, M.E.; Lanternier, F.; Zahar, J.R.; Salvi, N.; Talbotec, C.; Lortholary, O.; et al. Mucormycosis as a rare cause of severe gastrointestinal bleeding after multivisceral transplantation. Transpl. Infect. Dis. 2013, 15, E235–E238. [Google Scholar] [CrossRef]
- Katta, J.G.S.; Narach, T.; Poetter, D.; Kulkarni, P.; Reed, J.; Goldsmith, S.F. Gastric mucormycosis managed with combination antifungal therapy and no surgical debridement. Infect. Dis. Clin. Pract. 2013, 21, 265–268. [Google Scholar] [CrossRef]
- Machicado, J.Y.M.; Wolf, D. Gastric mucormycosis presenting as gastrotrointestinal bleeding in a trauma patient. Am. J. Gastroenterol. 2013, 108, S228–S229. [Google Scholar] [CrossRef]
- Bini, R.; Addeo, A.; Maganuco, L.; Fontana, D.; Viora, T.; Leli, R. The role of surgery in a case of diffuse mucormycosis with haematemesis and gastric necrosis. Ann. R. Coll. Surg. Engl. 2014, 96, e31–e33. [Google Scholar] [CrossRef]
- Kaiser, P.; Maggio, E.M.; Pfammatter, T.; Misselwitz, B.; Flury, S.; Schneider, P.M.; Dutkowski, P.; Breitenstein, S.; Müllhaupt, B.; Clavien, P.A.; et al. Histopathological evidence of invasive gastric mucormycosis after transarterial chemoembolization and liver transplantation. Infection 2014, 42, 779–783. [Google Scholar] [PubMed]
- Lee, S.H.; Son, Y.G.; Sohn, S.S.; Ryu, S.W. Successful treatment of invasive gastric mucormycosis in a patient with alcoholic liver cirrhosis: A case report. Exp. Ther. Med. 2014, 8, 401–404. [Google Scholar] [PubMed]
- Kulkarni, R.V.; Thakur, S.S. Invasive Gastric Mucormycosis—A Case Report. Indian J. Surg. 2015, 77 (Suppl. S1), 87–89. [Google Scholar] [PubMed]
- Nandwani, A.; Jha, P.K.; Duggal, R.; Kher, V. Invasive gastric mucormycosis and cytomegalovirus infection in an ABO incompatible renal transplant recipient. Indian J. Nephrol. 2015, 25, 373–376. [Google Scholar]
- Nasta, A.M.B.K.; Ranjan, S. An unusual case of gastric gangrene, diaphragmatic gangrene and autosplenectomy due to mucormycosis in a diabetic patient. Int. J. Case Rep. Images 2015, 5, 95–98. [Google Scholar]
- Raviraj, K.S.; Miglani, P.; Garg, A.; Agarwal, P.K. Gastric Mucormycosis with Hemolytic Uremic Syndrome. J. Assoc. Physicians India 2015, 63, 75–76. [Google Scholar]
- Lin, P.Y.; Tang, J.H.; Chang, C.C. A rare cause of GI bleeding in a 56-year-old man. Gut 2017, 66, 1074. [Google Scholar]
- Mittal, T.; Pulle, M.V.; Dey, A.; Malik, V.K. Gastric Mucormycosis Presenting as Gastrocolic Fistula: A Rare Entity. Indian J. Surg. 2016, 78, 511–512. [Google Scholar]
- Ravi, D.; Naqvi, A.; Odashiro, A.; Alowami, S. Gastrointestinal mucormycosis in a patient with COPD and diabetes mellitus. Can. J. Pathol. 2016, 8, 13. [Google Scholar]
- Tathe, S.P.; Dani, A.A.; Chawhan, S.M.; Meshram, S.A.; Randale, A.A.; Raut, W.K. Gastric mucormycosis: Diagnosis by imprint cytology. Diagn. Cytopathol. 2016, 44, 820–822. [Google Scholar]
- Chow, K.L.; McElmeel, D.P.; Brown, H.G.; Tabriz, M.S.; Omi, E.C. Invasive gastric mucormycosis: A case report of a deadly complication in an immunocompromised patient after penetrating trauma. Int. J. Surg. Case Rep. 2017, 40, 90–93. [Google Scholar] [PubMed]
- Chugh, P.; Sasken, H.; Azeez, S.; Kassab, M. Gastric Mucormycosis as a Cause of Upper Gastrointestinal Bleeding in an Immunocompetent Patient. Am. J. Gastroenterol. 2017, 112, S1059–S1060. [Google Scholar]
- Galvan Fernandez, J.; Jimenez Cuenca, M.I.; Molpeceres Martinez, I.; Alvarez-Quinones, M.L. A rare cause of emphysematous infectious gastritis. Rev. Esp. Enferm. Dig. 2017, 109, 368. [Google Scholar]
- Grimaldi, D.; Pradier, O.; Hotchkiss, R.S.; Vincent, J.L. Nivolumab plus interferon-gamma in the treatment of intractable mucormycosis. Lancet Infect. Dis. 2017, 17, 18. [Google Scholar]
- Metussin, D.; Telisinghe, P.U.; Chong, P.L.; Chong, V.H. Gastrointestinal: Gastric mucormycosis. J. Gastroenterol. Hepatol. 2017, 32, 1537. [Google Scholar]
- Nasa, M.; Sharma, Z.; Lipi, L.; Sud, R. Gastric Angioinvasive Mucormycosis in Immunocompetent Adult, A Rare Occurrence. J. Assoc. Physicians India 2017, 65, 103–104. [Google Scholar]
- Sanchez Velazquez, P.; Pera, M.; Gimeno, J.; Zapatero, A.; Nolla, J.; Pera, M. Mucormycosis: An unusual cause of gastric perforation and severe bleeding in immunocompetent patients. Rev. Esp. Enferm. Dig. 2017, 109, 223–225. [Google Scholar]
- Suhaildeen, K.; Majhi, U.; Seshadri, R.A.; Murhekar, K. Gastric Mucormycosis Masquerading as Gastric Malignancy. Indian J. Surg. Oncol. 2017, 8, 407–410. [Google Scholar]
- Abreu, B.; Duarte, M.L.; Santos, L.R.D.; Sementilli, A.; Figueiras, F.N. A rare case of gastric mucormycosis in an immunocompetent patient. Rev. Soc. Bras. Med. Trop. 2018, 51, 401–402. [Google Scholar]
- Alfano, G.; Fontana, F.; Francesca, D.; Assirati, G.; Magistri, P.; Tarantino, G.; Ballarin, R.; Rossi, G.; Franceschini, E.; Codeluppi, M.; et al. Gastric Mucormycosis in a Liver and Kidney Transplant Recipient: Case Report and Concise Review of Literature. Transplant. Proc. 2018, 50, 905–909. [Google Scholar]
- Kim, H.N.; Han, S.A.; Park, H.Y.; Kim, H.W.; Hong, R.; Choi, N.G.; Shin, M.H.; Yoon, N.R.; Kim, H.L.; Chung, J.H.; et al. Successful treatment of invasive gastric mucormycosis in a kidney transplant recipient. J. Korean Soc. Transplant. 2018, 32, 104–107. [Google Scholar] [CrossRef]
- Termos, S.; Othman, F.; Alali, M.; Al Bader, B.M.S.; Alkhadher, T.; Hassanaiah, W.F.; Taqi, A.; Sapkal, A. Total Gastric Necrosis Due to Mucormycosis: A Rare Case of Gastric Perforation. Am. J. Case Rep. 2018, 19, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Gautam, A.R.; Paudyal, B.; Sigdel, K.R.; Basnyat, B. Case Report: Gastric Mucormycosis—A rare but important differential diagnosis of upper gastrointestinal bleeding in an area of Helicobacter pylori endemicity. Wellcome Open Res. 2019, 4, 5. [Google Scholar] [CrossRef]
- Gani, I.; Doroodchi, A.; Falkenstrom, K.; Berry, H.; Lee, W.; Mulloy, L.; Saeed, M.; Kapoor, R. Gastric Mucormycosis in a Renal Transplant Patient Treated with Isavuconazole Monotherapy. Case Rep. Transplant. 2019, 2019, 9839780. [Google Scholar] [CrossRef]
- Guzman Rojas, P.; Robalino Gonzaga, E.; Catania, J.; Moqete, M. An unexpected cause of gastric ulcer. Am. J. Gastroenterol. 2019, 114, S1506–S1507. [Google Scholar] [CrossRef]
- Lankarani, D.; Maka, R.R.; Aziz, H.; Maklad, M.; Jayaraj, M.; Ohning, G. Medical management of gastric mucormycosis in an immunocompetent patient. 2746. Am. J. Gastroenterol. 2019, 114, S1517–S1518. [Google Scholar] [CrossRef]
- Malek, A.; De la Hoz, A.; Arduino, R.; Aisenberg, G.M. Disseminated tuberculosis and gastric mucormycosis coinfection. IDCases 2019, 18, e00595. [Google Scholar] [CrossRef]
- Peng, H.; Xiao, J.; Wan, H.; Shi, J.; Li, J. Severe Gastric Mycormycosis Infection Followed by Cytomegalovirus Pneumonia in a Renal Transplant Recipient: A Case Report and Concise Review of the Literature. Transplant. Proc. 2019, 51, 556–560. [Google Scholar] [CrossRef]
- Sharaan, A.; Alziadat, M.R.; Syed, Z.; Samuel, A.; Yelisetti, R.; Mathew, J.; Ismail, M. Often a deadly fungus; mucor must be among us: A case of angioinvasive gastric mucormycosis with a positive outcome. Am. J. Respir. Crit. Care Med. 2019, 199, A6582. [Google Scholar]
- Monte Junior, E.S.D.; Santos, M.; Ribeiro, I.B.; Luz, G.O.; Baba, E.R.; Hirsch, B.S.; Funari, M.P.; de Moura, E.G.H. Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report. Clin. Endosc. 2020, 53, 746–749. [Google Scholar] [CrossRef]
- Hameed, T.; Jain, S.K.; Ansari, F.M.; Nizam, A.; Dua, A. Spontaneous Gastric Necrosis: A Rare Presentation of Invasive Mucormycosis in an Immunocompetent Adult. Case Rep. Infect. Dis. 2020, 2020, 7514051. [Google Scholar]
- Jaju, M.; Sagar, R.; Srivastav, S.; Singh, R.; Kumar, V.; Faisal, M. Different types of mucormycosis: Case series. Int. J. Res. Med. Sci. 2000, 8, 2284–2296. [Google Scholar]
- Jung, H.; Kim, G.J.; Oh, T.H. Successful Management of a Rare Gastric Mucormycosis Presenting with Massive Melena in a Polytrauma Patient. Int. Med. Case Rep. J. 2020, 13, 531–535. [Google Scholar]
- Rivas, C.; Gonzalez, M.; Rapoport, G.; Chacon, I. Mucormycosis in an Immunocompromised Patient. Am. J. Gastroenterol. 2020, 115, S1581. [Google Scholar]
- Sharma, D. Successful management of emphysematous gastritis with invasive gastric mucormycosis. BMJ Case Rep. 2020, 13, e231297. [Google Scholar]
- Ghuman, S.S.; Sindhu, P.; Buxi, T.B.S.; Sheth, S.; Yadav, A.; Rawat, K.S.; Sud, S. CT appearance of gastrointestinal tract mucormycosis. Abdom. Radiol. 2021, 46, 1837–1845. [Google Scholar]
- Huang, H.; Xie, L.; Zheng, Z.; Yu, H.; Tu, L.; Cui, C.; Yu, J. Mucormycosis-induced upper gastrointestinal ulcer perforation in immunocompetent patients: A report of two cases. BMC Gastroenterol. 2021, 21, 311. [Google Scholar]
- Rai, A.; Gajula, B.; Kumar, N.; Malik, A. Gastric Perforation Secondary to Fungal Gastritis in an Immuno-Competent Adult. Cureus 2021, 13, e13156. [Google Scholar]
- Yuvaraj, M.; Mathapati, P.M.; Seena, C.R.; Ramaswami, S. Gastric mucormycosis with splenic invasion a rare abdominal complication of COVID-19 pneumonia. J. Clin. Imaging Sci. 2021, 11, 62. [Google Scholar]
- Bhaskar, B.K.; Gutte, S.H.; Gurjar, M.; Saran, S.; Rahul, R.; Sengar, P. A Rare Case Report of Intra-abdominal Mucormycosis Complicating Acute Pancreatitis. Indian J. Crit. Care Med. 2022, 26, 736–738. [Google Scholar]
- Chauhan, N.K.; Agarwal, A.; Dutt, N.; Yadav, T.; Kochar, R. Pulmonary embolism and gastric bleed with disseminated mucormycosis—Treading dangerous waters. Monaldi Arch. Chest Dis. 2022, 93, 2418. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, L.; Topcu, U.; Manay, M.; Esen, B.H.; Bektas, S.N.; Aydin, S.; Özdemir, B.; Khostelidi, S.N.; Klimko, N.; Cornely, O.; et al. COVID-19-associated mucormycosis: A systematic review and meta-analysis of 958 cases. Clin. Microbiol. Infect. 2023, 29, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Khsiba, A.; Moalla, M.; Nechi, S.; Bani, A.; Elloumi, A.; Jemal, S.; Azouz, M.M.; Medhioub, M.; Hamzaoui, L. Fatal invasive gastric mucormycosis: Two case reports. Clin. Case Rep. 2022, 10, e6330. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Choudhury, A.; Sasani, A.; Mandavdhare, H. Two tales of gastric mucormycosis S3683. Am. J. Gastroenterol. 2022, 117, S2310. [Google Scholar] [CrossRef]
- Safwan, M.; Khan, S.; Belthazar, A.; Sasidharan, M. Primary invasive gastric mucormycosis presenting as Meleney’s gangrene: A case report and review of literature. Libyan J. Med. Sci. 2021, 5, 171–173. [Google Scholar] [CrossRef]
- Albtoosh, A.S.; Shaf’ei, M.; Al Hayek, S.; Ramdan, L.I.; Abu Shahin, N.; Alzyoud, M.; Farah, R. A successfully treated gastric mucormycosis in an immunocompetent patient: Case report and literature review. Clin. Case Rep. 2023, 11, e7540. [Google Scholar] [CrossRef]
- Arora, S.; Singh, A.; Prasad, P.; Rahul Singh, R. Case Reports on Black Fungus of the Gastrointestinal Tract: A New Complication in COVID-19 Patients. Korean J. Gastroenterol. 2023, 81, 221–225. [Google Scholar] [CrossRef]
- Bhowmik, S.; Jadav, D.; Aggarwal, D.; Shekhawat, R.S. Gastric mucormycosis. Autops. Case Rep. 2023, 13, e2023421. [Google Scholar] [CrossRef]
- Khanna, D.; Sahu, P. Primary gastric mucormycosis after COVID. Gastrointest. Endosc. 2023, 97, 1159–1160. [Google Scholar] [CrossRef]
- Kim, J.; Wee, D.; Behin, D.; Brandt, L. Gastric mucormycosis in an immunosuppressed patient following dual pancreas-kidney ransplant. Am. J. Gastroenterol. 2023, 118, S2624. [Google Scholar] [CrossRef]
- Malakar, S.; Elhence, A.; Prasad, P.; Ghoshal, U.C.; Pandey, G.; Mohindra, S. Primary gastric mucormycosis presenting with post-Coronavirus disease-19 upper gastrointestinal bleed. Indian J. Gastroenterol. 2023, 42, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Marco, D.N.; Gonzalez-Munoz, B.; Doti, P.I. Gastric mucormycosis presenting as fever of unknown origin in an immunocompetent host after heatstroke. Pol. Arch. Intern. Med. 2023, 133, 16513. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, V.; Amarapurkar, A.D.; Rane, P. Mucormycosis Masquerading as Gastric Tumor. ACG Case Rep. J. 2024, 11, e01401. [Google Scholar] [CrossRef] [PubMed]
- de Lucena, L.A.; de Oliveira, F.G.; de Andrade, M.M.P.; de Paula, K.C.; Praxedes, M.R.G.; de Oliveira, R.A. Mucormycosis-induced hypercalcemia: A case report. CEN Case Rep. 2024, 13, 66–71. [Google Scholar] [CrossRef]
- He, D.; Wang, Z. Multiple Gastric Ulcers in An Immunosuppressed Patient with COVID-19 Infection caused by Mucormycosis. J. Gastrointestin. Liver Dis. 2024, 33, 16. [Google Scholar] [CrossRef]
- Huang, W.; Li, L.; Gao, J.; Kang, L. Gastric mucormycosis presenting as diffuse thickening of the gastric wall with enhancement. Rev. Esp. Enferm. Dig. 2024, 116, 167–168. [Google Scholar] [CrossRef]
- Ji, R. Mucormycosis mimicking portal hypertensive haemorrhage as a complication of alcoholic liver cirrhosis: A case report. BMC Infect. Dis. 2024, 24, 136. [Google Scholar]
- Lalwani, S.; Govindasamy, M.; Gupta, M.; Siraj, F.; Varma, V.; Mehta, N.; Kumaran, V.; Mohan, N.; Chopra, P.; Arora, A.; et al. Gastrointestinal mucormycosis—Four cases with different risk factors, involving different anatomical sites. Indian J. Gastroenterol. 2012, 31, 139–143. [Google Scholar] [CrossRef]
- Perez Fernandez, A.; Rubio Mateos, J.M.; Sanchez Fernandez, M.J. Gastric ulcer due to mucormycosis in a critical patient. Rev. Esp. Enferm. Dig. 2020, 112, 576–577. [Google Scholar] [CrossRef]
- Raju, B.; Santhanakumar, K.S.; Kesavachandran, U. Gastrointestinal involvement of unusual Mucormycete Syncephalastrum racemosum in a diabetic patient with adenocarcinoma: Rare case presentation with review of literature. Infection 2020, 48, 791–797. [Google Scholar] [CrossRef]
- Taams, M.; Bade, P.G.; Thomson, S.R. Post-traumatic abdominal mucormycosis. Injury 1992, 23, 390–392. [Google Scholar] [PubMed]
- Saltmarsh, G.; Plurad, D.; Bricker, S.; Bongard, F.; Neville, A.; Putnam, B.; Kim, D. Gastric necrosis and recurrent small bowel perforation resulting from gastrointestinal mucormycosis. Am. Surg. 2014, 80, E293–E294. [Google Scholar] [PubMed]
- Ju, J.H.; Park, H.S.; Shin, M.J.; Yang, C.W.; Kim, Y.S.; Choi, Y.J.; Song, H.J.; Kim, S.W.; Chung, I.S.; Bang, B.K. Successful treatment of massive lower gastrointestinal bleeding caused by mixed infection of cytomegalovirus and mucormycosis in a renal transplant recipient. Am. J. Nephrol. 2001, 21, 232–236. [Google Scholar] [PubMed]
- Kaneko, Y.; Oinuma, K.I.; Terachi, T.; Arimura, Y.; Niki, M.; Yamada, K.; Kakeya, H.; Mizutani, T. Successful Treatment of Intestinal Mycosis Caused by a Simultaneous Infection with Lichtheimia ramosa and Aspergillus calidoustus. Intern. Med. 2018, 57, 2421–2424. [Google Scholar]
- Nidhi, M.; Sadia, K.; Khatri, A.; Arnab, G.; Khan, N.A. Gastrointestinal Mucormycosis in a two-year-old child: A clinical and radiological enigma. Med. Mycol. Case Rep. 2019, 26, 5–9. [Google Scholar]
- Hyvernat, H.; Dunais, B.; Burel-Vandenbos, F.; Guidicelli, S.; Bernardin, G.; Gari-Toussaint, M. Fatal peritonitis caused by Rhizopus microsporus. Med. Mycol. 2010, 48, 1096–1098. [Google Scholar]
- Wotiye, A.B.; Ks, P.; Ayele, B.A. Invasive intestinal mucormycosis in a 40-year old immunocompetent patient—A rarely reported clinical phenomenon: A case report. BMC Gastroenterol. 2020, 20, 61. [Google Scholar]
- Liu, Q.; Chen, P.; Xin, L.; Zhang, J.; Jiang, M. A rare intestinal mucormycosis caused by Lichtheimia ramosa in a patient with diabetes: A case report. Front. Med. 2024, 11, 1435239. [Google Scholar]
- Mungazi, S.G.; Zambuko, B.; Muchuweti, D.; Muguti, E.G.; Mlotshwa, S. Fatal haemorrhagic duodenal mucormycosis in a non-immunocompromised host: A case report. Med. Mycol. Case Rep. 2017, 17, 1–3. [Google Scholar]
- Sun, M.; Hou, X.; Wang, X.; Chen, G.; Zhao, Y. Gastrointestinal Mucormycosis of the Jejunum in an Immunocompetent Patient: A Case Report. Medicine 2017, 96, e6360. [Google Scholar]
- Poyuran, R.; Dharan, B.S.; Sandhyamani, S.; Narasimhaiah, D. Mucormycosis-induced ileocecal perforation: A case report and review of literature. J. Postgrad. Med. 2020, 66, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Anitha, S.; Thomas, A.; Kanavi, J.V.; Thomas, A. Intestinal Perforation Secondary to Mucormycosis Associated with Puerperal Sepsis. Cureus 2021, 13, e17428. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jayashree, M.; Chakrabarti, A.; Sodhi, K.S.; Kanojia, R.P.; Mitra, S. Invasive Gastrointestinal Mucormycosis: A Master Masquerader. Pediatr. Infect. Dis. J. 2018, 37, 1067–1070. [Google Scholar] [CrossRef]
- Aruni, A., Sr.; Tandup, C.; Chowdhury, A., Sr.; Roy, A.; Jha, B. Illicit Drug Use a Risk Factor for Ileal Mucormycosis Presenting with Acute Abdomen. Cureus 2020, 12, e12213. [Google Scholar] [CrossRef]
- Himaal Dev, G.J.; Venkategowda, P.M.; Sutar, A.R.; Shankar, V. Intestinal mucormycosis in an adult with H1N1 pneumonia on extracorporeal membrane oxygenation. Ann. Card. Anaesth. 2021, 24, 92–94. [Google Scholar]
- Manda, D.; Sen, I.; Thakral, P.; Das, S.S.; Cb, V.; Malik, D. Invasive Fungal Infection in COVID-19-Recovered Patient Detected on 18F-FDG-Labeled Leukocytes PET/CT Scan. Clin. Nucl. Med. 2022, 47, e177–e179. [Google Scholar] [CrossRef]
- Zulpi, P.K.; Kulkarni, V.K.; Rani, H.; Halgeri, A.; Agnihotri, A. Double Whammy Due to Coronavirus Disease-2019: Invasive Small Bowel Mucormycosis with Recent-Onset Diabetic Ketoacidosis Presenting as Diffuse Fecal Peritonitis in a 12-Year-Old Girl. J. Indian Assoc. Pediatr. Surg. 2023, 28, 59–61. [Google Scholar] [CrossRef]
- Chethan, K.; Prasad, S.; Ramachandra, L. Ileocolic mucormycosis—An unusual cause of a mass in the right iliac fossa. Can. J. Infect. Dis. Med. Microbiol. 2012, 23, e65–e66. [Google Scholar] [CrossRef][Green Version]
- Yadav, S.; Sharma, A.; Kothari, N.; Bhatia, P.K.; Goyal, S.; Goyal, A. Mucormycosis: A Case Series of Patients Admitted in Non-COVID-19 Intensive Care Unit of a Tertiary Care Center during the Second Wave. Indian J. Crit. Care Med. 2021, 25, 1193–1196. [Google Scholar]
- Sarkardeh, M.; Meftah, E.; Mohammadzadeh, N.; Koushki, J.; Sadrzadeh, Z. COVID-19 and Intestinal Ischemia: A Multicenter Case Series. Front. Med. 2022, 9, 879996. [Google Scholar] [CrossRef]
- Martinello, M.; Nelson, A.; Bignold, L.; Shaw, D. “We are what we eat”! Invasive intestinal mucormycosis: A case report and review of the literature. Med. Mycol. Case Rep. 2012, 1, 52–55. [Google Scholar] [PubMed]
- Kumar, C.; Jain, P.; Wadhwa, N.; Diwaker, P.; Nirupma Panikar, K. Nosocomial Jejunal Mucormycosis—An Unusual Cause of Perforation Peritonitis. Iran. J. Pathol. 2017, 12, 295–300. [Google Scholar] [PubMed]
- Budhiraja, R.; Bhargava, S.; Sood, N. Jejunal stricture due to mucormycosis. Trop. Doct. 2019, 49, 318–320. [Google Scholar]
- Bhana, M.; Laher, N.; McGrath, N.G.; Moeng, M.S. Small bowel mucormycosis: An unexpected case in a penetrating trauma survivor. Int. J. Surg. Case Rep. 2023, 113, 109071. [Google Scholar]
- Watson, M.; Fong, R.; Schoemaker, D.; Due, S. Black fungus: Gastrointestinal invasive mucormycosis causing small bowel perforation, gastric and peristomal ulceration. ANZ J. Surg. 2024, 94, 252–253. [Google Scholar]
- Paliwal, P.; Rahar, S.; Sharma, A.; Gupta, D.; Ahuja, A.; Chauhan, D.S. Intestinal mucormycosis in a patient with COVID-19: A case report. Indian J. Pathol. Microbiol. 2022, 65, 475–477. [Google Scholar]
- Sriperumbuduri, S.; Kalidindi, K.; Megha, H.; Guditi, S.; Taduri, G. An unusual case of gastrointestinal mucormycosis in a patient with nephrotic syndrome. Indian J. Nephrol. 2017, 27, 145–147. [Google Scholar]
- Singh, R.P.; Gupta, N.; Kaur, T.; Gupta, A. Rare case of gastrointestinal mucormycosis with colonic perforation in an immunocompetent patient with COVID-19. BMJ Case Rep. 2021, 14, e244096. [Google Scholar]
- Nagy-Agren, S.E.; Chu, P.; Smith, G.J.; Waskin, H.A.; Altice, F.L. Zygomycosis (mucormycosis) and HIV infection: Report of three cases and review. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 10, 441–449. [Google Scholar]
- Cebisli, E.; Ulgen-Tekerek, N.; Dursun, O.; Koker, A.; Kisaoglu, A.; Artan, R.; Soyucen, E.; Elpek, G.O. Intestinal Mucormycosis in a Child with Maple Syrup Urine Disease After Orthotopic Liver Transplant. Exp. Clin. Transplant. 2023, 21, 375–379. [Google Scholar]
- Saraf, P.; Naresh Bharti, J. Mucormycosis presenting as intestinal perforation: A pathologist perspective. Arab. J. Gastroenterol. 2024, 25, 234–236. [Google Scholar] [PubMed]
- Eiser, A.R.; Slifkin, R.F.; Neff, M.S. Intestinal mucormycosis in hemodialysis patients following deferoxamine. Am. J. Kidney Dis. 1987, 10, 71–73. [Google Scholar] [PubMed]
- Yinadsawaphan, T.; Ngamskulrungroj, P.; Chalermwai, W.; Dhitinanmuang, W.; Angkasekwinai, N. Gastrointestinal mucormycosis due to Rhizopus microsporus following Streptococcus pyogenes toxic shock syndrome in an HIV patient: A case report. BMC Infect. Dis. 2020, 20, 817. [Google Scholar]
- Li, C.; Zhu, H.; Tan, Y.; Liu, D. Gastrointestinal bleeding due to duodenal mucormycosis in an immunocompetent host mimicking malignancy. Rev. Esp. Enferm. Dig. 2019, 111, 961–962. [Google Scholar]
- Evert, K.; Dienemann, T.; Brochhausen, C.; Lunz, D.; Lubnow, M.; Ritzka, M.; Keil, F.; Trummer, M.; Scheiter, A.; Salzberger, B.; et al. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Arch. 2021, 479, 97–108. [Google Scholar]
- Marco del Pont, J.; De Cicco, L.; Gallo, G.; Llera, J.; De Santibanez, E.; D’Agostino, D. Hepatic arterial thrombosis due to Mucor species in a child following orthotopic liver transplantation. Transpl. Infect. Dis. 2000, 2, 33–35. [Google Scholar]
- Mekeel, K.L.; Hemming, A.W.; Reed, A.I.; Matsumoto, T.; Fujita, S.; Schain, D.C.; Nelson, D.R.; Dixon, L.R.; Fujikawa, T. Hepatic mucormycosis in a renal transplant recipient. Transplantation 2005, 79, 1636. [Google Scholar]
- Zhan, H.X.; Lv, Y.; Zhang, Y.; Liu, C.; Wang, B.; Jiang, Y.Y.; Liu, X.M. Hepatic and renal artery rupture due to Aspergillus and Mucor mixed infection after combined liver and kidney transplantation: A case report. Transplant. Proc. 2008, 40, 1771–1773. [Google Scholar]
- Abboud, C.S.; Bergamasco, M.D.; Baia, C.E.; Lallee, M.P.; Zan, A.S.; Zamorano, M.M.; Pereira, O.I.; Mies, S. Case report of hepatic mucormycosis after liver transplantation: Successful treatment with liposomal amphotericin B followed by posaconazole sequential therapy. Transplant. Proc. 2012, 44, 2501–2502. [Google Scholar]
- Chaudhary, R.J.; Choudhary, N.S.; Saraf, N.; Gautam, D.; Piplani, T.; Thiagrajan, S.; Bhangui, P.; Saigal, S.; Rastogi, A.; Soin, A.S. Delayed Graft Dysfunction due to Invasive Hepatic Mucormycosis After Living Donor Liver Transplantation. J. Clin. Exp. Hepatol. 2020, 10, 629–632. [Google Scholar]
- Teira, R.; Trinidad, J.M.; Eizaguirre, B.; Ortiz, J.; Santamaria, J.M. Zygomycosis of the spleen in a patient with the acquired immunodeficiency syndrome. Mycoses 1993, 36, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Huang, X.; Li, Y.; Wang, J. Isolated splenic mucormycosis secondary to diabetic ketoacidosis: A case report. BMC Infect. Dis. 2022, 22, 596. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Ghosh, A.; Prasad, G.S.; David, J.K.; Gupta, S.; Das, A.; Sakhuja, V.; Panda, N.K.; Singh, S.K.; Das, S.; et al. Apophysomyces elegans: An emerging zygomycete in India. J. Clin. Microbiol. 2003, 41, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Singh, S.K.; Kakkar, N.; Jain, S.; Kalra, N.; Sakia, U.N. Splenic and renal mucormycosis in a healthy host: Successful management by aggressive treatment. Trop. Gastroenterol. 2010, 31, 57–58. [Google Scholar]
- O’Connor, C.; Farrell, C.; Fabre, A.; Eaton, D.; Redmond, K.; McSharry, D.G.; Conneely, J.B.; Shields, C.J.; Egan, J.J.; Hannan, M.M. Near-fatal mucormycosis post-double lung transplant presenting as uncontrolled upper gastrointestinal haemorrhage. Med. Mycol. Case Rep. 2018, 21, 30–33. [Google Scholar] [CrossRef]
- Jain, M.; Tyagi, R.; Tyagi, R.; Jain, G. Post-COVID-19 Gastrointestinal Invasive Mucormycosis. Indian J. Surg. 2022, 84, 545–547. [Google Scholar] [CrossRef]
- Okhuysen, P.C.; Rex, J.H.; Kapusta, M.; Fife, C. Successful treatment of extensive posttraumatic soft-tissue and renal infections due to Apophysomyces elegans. Clin. Infect. Dis. 1994, 19, 329–331. [Google Scholar] [CrossRef]
- Durila, M.; Pavlicek, P.; Hadacova, I.; Nahlovsky, J.; Janeckova, D. Endogenous Heparinoids May Cause Bleeding in Mucor Infection and can be Detected by Nonactivated Thromboelastometry and Treated by Recombinant Activated Factor VII: A Case Report. Medicine 2016, 95, e2933. [Google Scholar] [CrossRef]
- Sedlacek, M.; Cotter, J.G.; Suriawinata, A.A.; Kaneko, T.M.; Zuckerman, R.A.; Parsonnet, J.; Block, C.A. Mucormycosis peritonitis: More than 2 years of disease-free follow-up after posaconazole salvage therapy after failure of liposomal amphotericin, B. Am. J. Kidney Dis. 2008, 51, 302–306. [Google Scholar] [CrossRef]
- Polo, J.R.; Luno, J.; Menarguez, C.; Gallego, E.; Robles, R.; Hernandez, P. Peritoneal mucormycosis in a patient receiving continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 1989, 13, 237–239. [Google Scholar] [CrossRef]
- Fergie, J.E.; Fitzwater, D.S.; Einstein, P.; Leggiadro, R.J. Mucor peritonitis associated with acute peritoneal dialysis. Pediatr. Infect. Dis. J. 1992, 11, 498–500. [Google Scholar] [PubMed]
- Adam, R.D.; Hunter, G.; DiTomasso, J.; Comerci, G., Jr. Mucormycosis: Emerging prominence of cutaneous infections. Clin. Infect. Dis. 1994, 19, 67–76. [Google Scholar] [PubMed]
- Khan, Z.U.; Chugh, T.D. Invasive fungal infections in Kuwait: A retrospective study. Indian J. Chest Dis. Allied Sci. 2000, 42, 279–287. [Google Scholar] [PubMed]
- Monecke, S.; Hochauf, K.; Gottschlich, B.; Ehricht, R. A case of peritonitis caused by Rhizopus microsporus. Mycoses 2006, 49, 139–142. [Google Scholar]
- Pimentel, J.D.; Dreyer, G.; Lum, G.D. Peritonitis due to Cunninghamella bertholletiae in a patient undergoing continuous ambulatory peritoneal dialysis. J. Med. Microbiol. 2006, 55 Pt 1, 115–118. [Google Scholar]
- Nayak, S.; Satish, R.; Gokulnath Savio, J.; Rajalakshmi, T. Peritoneal mucormycosis in a patient on CAPD. Perit. Dial. Int. 2007, 27, 216–217. [Google Scholar]
- Bhutada, K.; Borkar, S.S.; Mendiratta, D.K.; Shende, V.R. Successful treatment of peritonitis by C. bertholletiae in a chronic kidney failure patient on continuous ambulatory peritoneal dialysis after kidney rejection. Singap. Med. J. 2012, 53, e106–e109. [Google Scholar]
- Pamidimukkala, U.; Sudhaharan, S.; Kancharla, A.; Vemu, L.; Challa, S.; Karanam, S.D.; Chavali, P.; Prakash, H.; Ghosh, A.K.; Gupta, S.; et al. Mucormycosis due to Apophysomyces species complex- 25 years’ experience at a tertiary care hospital in southern India. Med. Mycol. 2020, 58, 425–433. [Google Scholar]
- Dalgic, B.; Bukulmez, A.; Sari, S. Pyogenic liver abscess and peritonitis due to Rhizopus oryzae in a child with Papillon-Lefevre syndrome. Eur. J. Pediatr. 2011, 170, 803–805. [Google Scholar]
- Verma, G.R.; Lobo, D.R.; Walker, R.; Bose, S.M.; Gupta, K.L. Disseminated mucormycosis in healthy adults. J. Postgrad. Med. 1995, 41, 40–42. [Google Scholar]
- Kaushik, R. Primary cutaneous zygomycosis in India. Indian J. Surg. 2012, 74, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Kumar, P.; Padhye, A.A.; Chatha, L.; Singh, S.K.; Das, A.; Wig, J.D.; Kataria, R.N. Primary cutaneous zygomycosis due to Saksenaea vasiformis and Apophysomyces elegans. Clin. Infect. Dis. 1997, 24, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.S.; Raman, A.; Nair, A. Nosocomial zygomycotic post-surgical necrotizing fasciitis in a healthy adult caused by Apophysomyces elegans in south India. J. Med. Vet. Mycol. 1997, 35, 61–63. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Khilnani, G.C.; Aggarwal, S.; Kumar, S.; Banerjee, U.; Xess, I. Primary cutaneous mucormycosis in an immunocompetent host: Report of a case. Surg. Today 2003, 33, 319–322. [Google Scholar] [CrossRef]
- Thami, G.P.; Kaur, S.; Bawa, A.S.; Chander, J.; Mohan, H.; Bedi, M.S. Post-surgical zygomycotic necrotizing subcutaneous infection caused by Absidia corymbifera. Clin. Exp. Dermatol. 2003, 28, 251–253. [Google Scholar] [CrossRef]
- Kerbaul, F.; Guidon, C.; Collart, F.; Lepidi, H.; Cayatte, B.; Bonnet, M.; Bellezza, M.; Métras, D.; Gouin, F. Abdominal wall mucormycosis after heart transplantation. J. Cardiothorac. Vasc. Anesth. 2004, 18, 822–823. [Google Scholar] [CrossRef]
- Padmaja, I.J.; Ramani, T.V.; Kalyani, S. Cutaneous zygomycosis: Necrotising fascitis due to Saksenaea vasiformis. Indian J. Med. Microbiol. 2006, 24, 58–60. [Google Scholar] [CrossRef]
- Belfiori, R.; Terenzi, A.; Marchesini, L.; Repetto, A. Absidia Corymbifera in an immune competent accident victim with multiple abdominal injuries: Case report. BMC Infect. Dis. 2007, 7, 46. [Google Scholar] [CrossRef]
- Tilak, R.; Raina, P.; Gupta, S.K.; Tilak, V.; Prakash, P.; Gulati, A.K. Cutaneous zygomycosis: A possible postoperative complication in immunocompetent individuals. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 596–599. [Google Scholar] [CrossRef]
- Tapish, S.; Taha, M.; Naresh, G.; Neeraj, D.; Malik Vinod, K. Primary mucormycosis of abdominal wall: A rare fungal infection in a immunocompetent patient. Indian J. Surg. 2010, 72 (Suppl. S1), 306–308. [Google Scholar] [CrossRef]
- Alharbi, M.; Jhinger, R.K.; Wuerz, T.; Walkty, A. Marked peripheral eosinophilia due to prolonged administration of posaconazole. JMM Case Rep. 2017, 4, e005100. [Google Scholar]
- Ram, R.; Swarnalatha, G.; Prasad, N.; Dakshinamurty, K.V. Exit site infection due to Zygomycosis resulting in abdominal wall necrosis in a continuous ambulatory peritoneal dialysis patient. Nephrol. Dial. Transplant. 2007, 22, 266–267. [Google Scholar]
- Patel, A.K.; Vora, H.J.; Patel, K.K.; Patel, B. Elderly diabetic patient with surgical site mucormycosis extending to bowel. J. Glob. Infect. Dis. 2010, 2, 186–188. [Google Scholar]
- Zhao, L.; Wang, C.X.; Zhang, L.; Tu, X.A.; Wang, W.; Chen, Y.; Liu, L.S. Mucormycosis extending from the surgical wound to the transplanted kidney: Case report and literature review. Exp. Clin. Transplant. 2012, 10, 403–405. [Google Scholar]
- Paonam, S.; Bag, S.; Mavuduru, R.S.; Agarwal, M.M.; Mandal, A.K. Isolated bilateral renal mucormycosis masquerading as renal abscess in an immunocompetent individual: A lesson learnt. Case Rep. Urol. 2014, 2014, 304380. [Google Scholar]
- Narayanaswamy, S.; Goradia, R.; Bellurkar, A.; Patwardhan, S. Isolated renal mucormycosis masquerading as emphysematous pyelonephritis. BMJ Case Rep. 2023, 16, e254501. [Google Scholar]
- Stein, A.; Schmaman, A. Rupture of the stomach due to mucormycosis. S Afr. J. Surg. 1965, 3, 123–129. [Google Scholar]
- Deal, W.B.; Johnson, J.E., 3rd. Gastric phycomycosis. Report of a case and review of the literature. Gastroenterology 1969, 57, 579–586. [Google Scholar]
- Branton, M.H.; Johnson, S.C.; Brooke, J.D.; Hasbargen, J.A. Peritonitis due to Rhizopus in a patient undergoing continuous ambulatory peritoneal dialysis. Rev. Infect. Dis. 1991, 13, 19–21. [Google Scholar]
- Norden, G.; Bjorck, S.; Persson, H.; Svalander, C.; Li, X.G.; Edebo, L. Cure of zygomycosis caused by a lipase-producing Rhizopus rhizopodiformis strain in a renal transplant patient. Scand. J. Infect. Dis. 1991, 23, 377–382. [Google Scholar]
- Vadeboncoeur, C.; Walton, J.M.; Raisen, J.; Soucy, P.; Lau, H.; Rubin, S. Gastrointestinal mucormycosis causing an acute abdomen in the immunocompromised pediatric patient—Three cases. J. Pediatr. Surg. 1994, 29, 1248–1249. [Google Scholar] [PubMed]
- Hosseini, M.; Lee, J. Gastrointestinal mucormycosis mimicking ischemic colitis in a patient with systemic lupus erythematosus. Am. J. Gastroenterol. 1998, 93, 1360–1362. [Google Scholar] [PubMed]
- Guardia, J.A.; Bourgoignie, J.; Diego, J. Renal mucormycosis in the HIV patient. Am. J. Kidney Dis. 2000, 35, E24. [Google Scholar]
- Herbrecht, R.; Letscher-Bru, V.; Bowden, R.A.; Kusne, S.; Anaissie, E.J.; Graybill, J.R.; Noskin, G.A.; Oppenheim, B.A.; Andrès, E.; Pietrelli, L.A. Treatment of 21 cases of invasive mucormycosis with amphotericin B colloidal dispersion. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 460–466. [Google Scholar]
- Nannini, E.C.; Paphitou, N.I.; Ostrosky-Zeichner, L. Peritonitis due to Aspergillus and zygomycetes in patients undergoing peritoneal dialysis: Report of 2 cases and review of the literature. Diagn. Microbiol. Infect. Dis. 2003, 46, 49–54. [Google Scholar]
- Serna, J.H.; Wanger, A.; Dosekun, A.K. Successful treatment of mucormycosis peritonitis with liposomal amphotericin B in a patient on long-term peritoneal dialysis. Am. J. Kidney Dis. 2003, 42, E14–E17. [Google Scholar]
- Alkhunaizi, A.M.; Amir, A.A.; Al-Tawfiq, J.A. Invasive fungal infections in living unrelated renal transplantation. Transplant. Proc. 2005, 37, 3034–3037. [Google Scholar]
- Sethi, J.; Ramachandran, R.; Kohli, H.S.; Gupta, K.L. Isolated renal mucormycosis in a patient with Idiopathic CD4 lymphocytopenia. BMJ Case Rep. 2018, 2018, bcr201825234. [Google Scholar]
- Deja, M.; Wolf, S.; Weber-Carstens, S.; Lehmann, T.N.; Adler, A.; Ruhnke, M.; Tintelnot, K. Gastrointestinal zygomycosis caused by Mucor indicus in a patient with acute traumatic brain injury. Med. Mycol. 2006, 44, 683–687. [Google Scholar]
- Taj-Aldeen, S.J.; Almaslamani, M.; Theelen, B.; Boekhout, T. Phylogenetic analysis reveals two genotypes of the emerging fungus Mucor indicus, an opportunistic human pathogen in immunocompromised patients. Emerg. Microbes Infect. 2017, 6, e63. [Google Scholar]
- Manchikalapati, P.; Canon, C.L.; Jhala, N.; Eloubeidi, M.A. Gastrointestinal zygomycosis complicating heart and lung transplantation in a patient with Eisenmenger’s syndrome. Dig. Dis. Sci. 2005, 50, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Choi, H.J.; Yoo, J.; Kang, S.J.; Lee, K.Y. Emphysematous gastritis associated with invasive gastric mucormycosis: A case report. J. Korean Med. Sci. 2007, 22, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Berne, J.D.; Villarreal, D.H.; McGovern, T.M.; Rowe, S.A.; Moore, F.O.; Norwood, S.H. A fatal case of posttraumatic gastric mucormycosis. J. Trauma 2009, 66, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Thomson, S.R.; Tudor, G.; Khan, Z.; Mjoli, M. Ileosigmoid knot complicated by gastric and splenic mucormycosis: A lethal combination. BMJ Case Rep. 2009, 2009, bcr10.2008.1049. [Google Scholar] [CrossRef]
- Mezhir, J.J.; Mullane, K.M.; Zarling, J.; Satoskar, R.; Pai, R.K.; Roggin, K.K. Successful nonoperative management of gastrointestinal mucormycosis: Novel therapy for invasive disease. Surg. Infect. 2009, 10, 447–451. [Google Scholar] [CrossRef]
- Van Sickels, N.; Hoffman, J.; Stuke, L.; Kempe, K. Survival of a patient with trauma-induced mucormycosis using an aggressive surgical and medical approach. J. Trauma. 2011, 70, 507–509. [Google Scholar] [CrossRef]
- Rudler, M.; Barret, M.; Poynard, T.; Thabut, D. Gastric mucormycosis: A rare cause of gastrointestinal bleeding in cirrhosis. Clin. Res. Hepatol. Gastroenterol. 2012, 36, e32–e33. [Google Scholar] [CrossRef]
- Tan, J.; Manickam, R.; Pisharam, J.; Telisinghe, P.; Chong, V.H. Mucormycosis—A possible trigger pathogen for encapsulating peritoneal sclerosis. Perit. Dial. Int. 2012, 32, 479–481. [Google Scholar] [CrossRef]
- Enani, M.A.; Alharthi, B.N.; Dewanjee, N.; Bhat, N.A.; Fagih, M. Spontaneous gastric ulcer perforation and acute spleen infarction caused by invasive gastric and splenic mucormycosis. J. Glob. Infect. Dis. 2014, 6, 122–124. [Google Scholar] [CrossRef]
- Gaut, D.; Cone, B.D.; Gregson, A.L.; Agopian, V.G. Gastrointestinal Mucormycosis After Orthotopic Liver Transplantation Presenting as Femoral Nerve Palsy: A Case Report and Review of the Literature. Transplant. Proc. 2017, 49, 1608–1614. [Google Scholar] [CrossRef]
- Izaguirre-Anariba, D.E.; Chee, F.; Thet, Z.; Lanza, J. An Interesting Case of a 57-Year-Old Male with an Upper Gastrointestinal Bleeding and Obstructive Uropathy with Bilateral Hydronephrosis Diagnosed with Systemic Mucormycosis. Case Rep. Infect. Dis. 2018, 2018, 6283701. [Google Scholar]
- Aggarwal, P.; Patel, H.; Gonzalez, L.; Brown, L.; Agarwal, A.; Speeg, K. Invasive Colonic Mucormycosis in an Immunocompromised Postliver Transplant Patient. ACG Case Rep. J. 2020, 7, e00450. [Google Scholar]
- Reis, F.P.D.; Campos, S.V.; Aiello, V.D.; Duarte, M.I.S.; Samano, M.N.; Pego-Fernandes, P.M. Gastrointestinal mucormycosis post lung transplantation. Braz. J. Infect. Dis. 2019, 23, 368–370. [Google Scholar] [PubMed]
- Rotundo, L. A fatal case of disseminated gastric mucormycosis with emphysematous gastritis. Am. J. Gastroenterol. 2019, 114, S1505–S1506. [Google Scholar]
- Sehmbey, G.; Malik, R.; Kosa, D.; Srinivasan, I.; Chuang, K.Y.; Bellapravalu, S. Gastric Ulcer and Perforation due to Mucormycosis in an Immunocompetent Patient. ACG Case Rep. J. 2019, 6, e00154. [Google Scholar]
- Shankaralingappa, S. Unsuspected invasive gastrointestinal mucormycosis masquerading as inflammatory bowel disease: A pathologist’s perspective. Indian J. Pathol. Microbiol. 2019, 62, 332–334. [Google Scholar]
- Petrochko, J.M.; Abrahamian, G.; Cigarroa, F.; Thomas, E. Colonic mucormycosis in solid organ transplantation: Case report and review of the literature (colonic mucormycosis after DDLT). Transpl. Infect. Dis. 2020, 22, e13362. [Google Scholar]
- Shah, C.; Zimmerman, S.; McKinney, J.; Ebers, A. Colonic mucormycosis in an immunocompetent patient with endocarditis. IDCases 2020, 20, e00773. [Google Scholar]
- Chiang, T.H.; Lee, Y.W.; Tan, J.H.; Kao, C.C.; Chang, C.C.; Fang, K.C. Mucormycosis causing massive lower gastrointestinal bleeding: A case report. BMC Gastroenterol. 2021, 21, 272. [Google Scholar]
- Hammami, F.; Koubaa, M.; Chakroun, A.; Smaoui, F.; Marrakchi, C.; Hentati, N.; Mzali, R.; Rekik, K.; Jemaa, M.B. Survival of an immuno-competent patient from splenic and gastric mucormycosis-case report and review of the literature. J. Mycol. Med. 2021, 31, 101174. [Google Scholar]
- Martin-Blais, R.; Pathak, S.; Fitzwater, S.; Dawson, D.W.; Sisk, A.E.; Farmer, D.G.; Venick, R.; Yeganeh, N. Intestinal mucormycosis initially identified by next-generation sequencing of cell-free, D.N.A. Transpl. Infect. Dis. 2021, 23, e13656. [Google Scholar]
- Varshney, V.K.; Swami, A.; Thirunavukkarasu, B.; Agarwal, A.; Baid, G. Synchronous Small Bowel Gangrene with Pyelonephritis Secondary to Mucormycosis: A Disastrous Complication of COVID-19 Pandemic. Cureus 2021, 13, e15911. [Google Scholar]
- Kyuno, D.; Kubo, T.; Tsujiwaki, M.; Sugita, S.; Hosaka, M.; Ito, H.; Harada, K.; Takasawa, A.; Kubota, Y.; Takasawa, K.; et al. COVID-19-associated disseminated mucormycosis: An autopsy case report. World J. Clin. Cases. 2022, 10, 10358–10365. [Google Scholar] [PubMed]
- Ito, H.; Kakizaki, R.; Harada, K.; Kyuno, D.; Kubo, T.; Bunya, N.; Kasai, T.; Uemura, S.; Narimatsu, E. Disseminated mucormycosis in a patient with severe COVID-19 on venovenous extracorporeal membrane oxygenation: A case report. IDCases 2022, 29, e01578. [Google Scholar]
- Meshram, H.S.; Kumar, D.; Kute, V.B. Rare and Unusual Follow-up Sequelae of Coronavirus Disease 2019: Splenic Mucormycosis in a Renal Transplant Recipient. Transplant Proc. 2022, 54, 1554–1556. [Google Scholar]
- Ralaizanaka, B.M.; Razafindrazoto, C.I.; Bolot, E.; Bors, G.; Housson-Wetzel, S.; Razafimahefa, S.H.; Ramanampamonjy, R.M.; Claude, P. Gastrointestinal Mucormycosis-Induced Massive Lower Gastrointestinal Bleeding, Rectal Perforation, and Pulmonary Embolism: A Long Diagnostic Pathway in a Case Report. Clin. Exp. Gastroenterol. 2022, 15, 145–151. [Google Scholar]
- Banerjee, N.; Lodha, M.; Kompally, P.; Chawla, S. Post-COVID-19 Intestinal and Mesenteric Mucormycosis. Am. Surg. 2023, 89, 2770–2773. [Google Scholar]
- Rathi, C.; Shinde, R.K.; Dighe, S.P.; Lamture, Y.; Mahawar, R. Gastric Necrosis: A Rare Complication of Gastric Mucormycosis. Cureus 2023, 15, e35810. [Google Scholar]
- Li, K.W.; Wen, T.F.; Li, G.D. Hepatic mucormycosis mimicking hilar cholangiocarcinoma: A case report and literature review. World J. Gastroenterol. 2010, 16, 1039–1042. [Google Scholar]
- Rabin, E.R.; Lundberg, G.D.; Mitchell, E.T. Mucormycosis in severely burned patients. Report of two cases with extensive destruction of the face and nasal cavity. N. Engl. J. Med. 1961, 264, 1286–1289. [Google Scholar]
- Virmani, R.; Connor, D.H.; McAllister, H.A. Cardiac mucormycosis. A report of five patients and review of 14 previously reported cases. Am. J. Clin. Pathol. 1982, 78, 42–47. [Google Scholar] [PubMed]
- Straatsma, B.R.; Zimmerman, L.E.; Gass, J.D. Phycomycosis. A clinicopathologic study of fifty-one cases. Lab. Investig. 1962, 11, 963–985. [Google Scholar] [PubMed]
- Agger, W.A.; Maki, D.G. Mucormycosis. A complication of critical care. Arch. Intern. Med. 1978, 138, 925–927. [Google Scholar]
- Gupta, K.L.; Joshi, K.; Pereira, B.J.; Singh, K. Disseminated mucormycosis presenting with acute renal failure. Postgrad. Med. J. 1987, 63, 297–299. [Google Scholar]
- Nakamura, M.; Weil, W.B., Jr.; Kaufman, D.B. Fatal fungal peritonitis in an adolescent on continuous ambulatory peritoneal dialysis: Association with deferoxamine. Pediatr. Nephrol. 1989, 3, 80–82. [Google Scholar]
- Singh, N.; Gayowski, T.; Singh, J.; Yu, V.L. Invasive gastrointestinal zygomycosis in a liver transplant recipient: Case report and review of zygomycosis in solid-organ transplant recipients. Clin. Infect. Dis. 1995, 20, 617–620. [Google Scholar]
- Stoebner, P.E.; Gaspard, C.; Mourad, G.; Beraud, J.J.; Meynadier, J.; Meunier, L. Fulminant mucormycosis in a renal transplant recipient. Acta Derm. Venereol. 2000, 80, 305. [Google Scholar]
- Tsaousis, G.; Koutsouri, A.; Gatsiou, C.; Paniara, O.; Peppas, C.; Chalevelakis, G. Liver and brain mucormycosis in a diabetic patient type II successfully treated with liposomial amphotericin B. Scand. J. Infect. Dis. 2000, 32, 335–337. [Google Scholar]
- Sehgal, A.; Raghavendran, M.; Kumar, D.; Srivastava, A.; Dubey, D.; Kumar, A. Rhinocerebral mucormycosis causing basilar artery aneurysm with concomitant fungal colonic perforation in renal allograft recipient: A case report. Transplantation 2004, 78, 949–950. [Google Scholar]
- Gimeno-García, A.; Parra-Blanco, A.; Nicolás-Pérez, D.; Manzano-Sanz, C.; Méndez-Medina, R.; Quintero, E. Mucormycosis with gastric involvement in a patient with severe atheromatous vascular disease. Dig. Endosc. 2006, 18, 144–146. [Google Scholar]
- Alexander, B.D.; Schell, W.A.; Siston, A.M.; Rao, C.Y.; Bower, W.A.; Balajee, S.A.; Howell, D.N.; Moore, Z.S.; Noble-Wang, J.; Rhyne, J.A.; et al. Fatal Apophysomyces elegans infection transmitted by deceased donor renal allografts. Am. J. Transplant. 2010, 10, 2161–2167. [Google Scholar] [PubMed]
- Arena, V.; De-Giorgio, F.; Pennacchia, I.; Manna, R.; Vetrugno, G.; Stigliano, E.; Milic, N.; Gasbarrini, G.; Abenavoli, L. Haemophagocytic syndrome associated with mucormycosis infection. Int. J. Immunopathol. Pharmacol. 2012, 25, 751–755. [Google Scholar] [PubMed]
- Gurevich, M.; Levi, I.; Steinberg, R.; Shonfeld, T.; Shapiro, R.; Israeli, M.; Sprecher, H.; Shalit, I.; Mor, E. Mucormycosis in a liver allograft: Salvage re-transplantation and targeted immunosuppressive management. Transpl. Infect. Dis. 2012, 14, E97–E101. [Google Scholar]
- Nam, Y.; Jung, J.; Park, S.S.; Kim, S.J.; Shin, S.J.; Choi, J.H.; Kim, M.; Yoon, H.E. Disseminated mucormycosis with myocardial involvement in a renal transplant recipient. Transpl. Infect. Dis. 2015, 17, 890–896. [Google Scholar]
- Alqhamdi, S.; Idress, B.; Alharbi, A.; Aljurais, N. Case report: Disseminated pulmonary mucormycosis involving spleen in diabetic patient with aggressive surgical approach. Int. J. Surg. Case Rep. 2019, 54, 42–46. [Google Scholar]
- Sami, C.A.; Rashed, H.M.; Khan, A.H.; Barai, L.; Arafat, S.M. Disseminated mucormycosis: An unusual case of ascites with bone marrow invasion. IDCases 2022, 29, e01553. [Google Scholar]

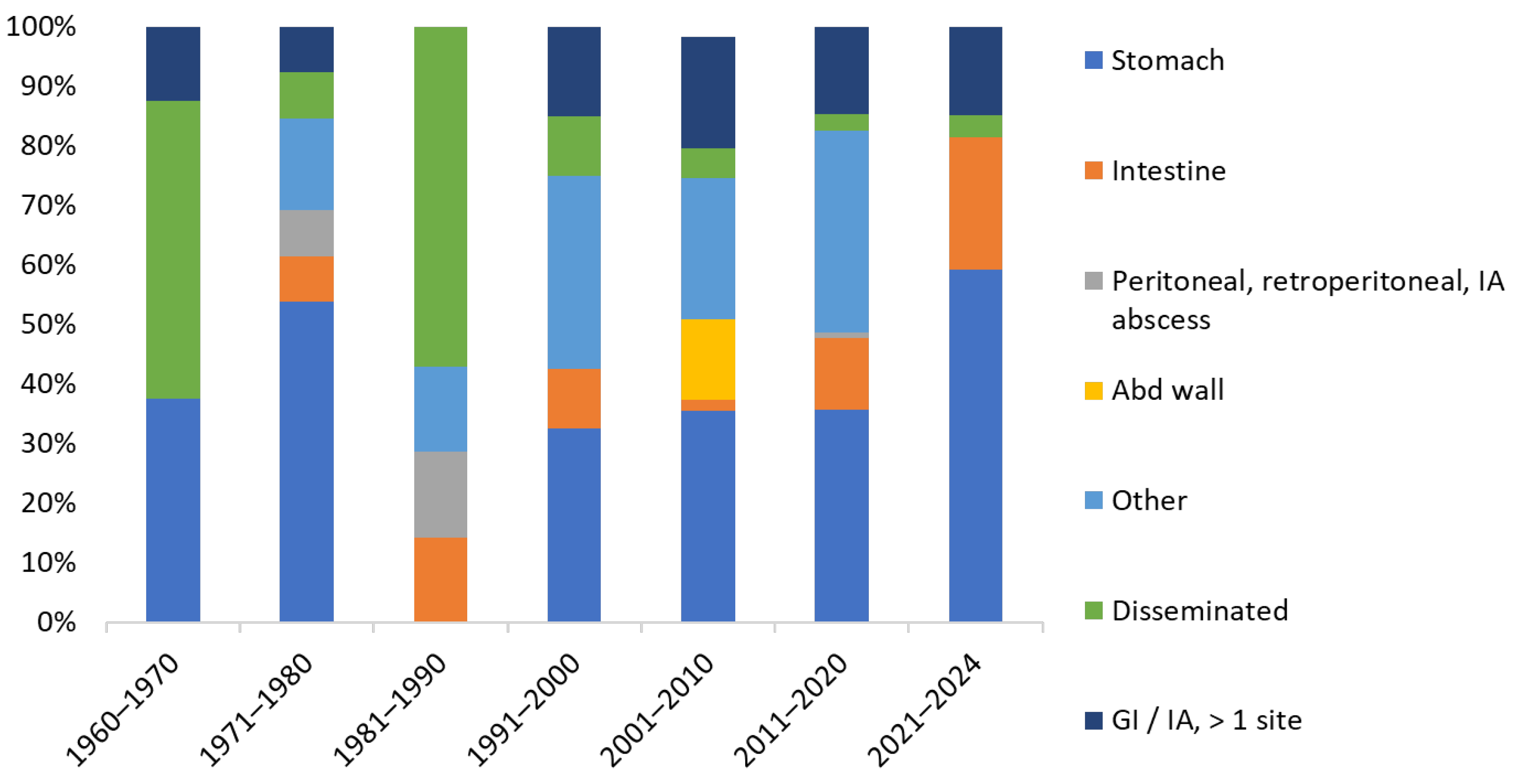
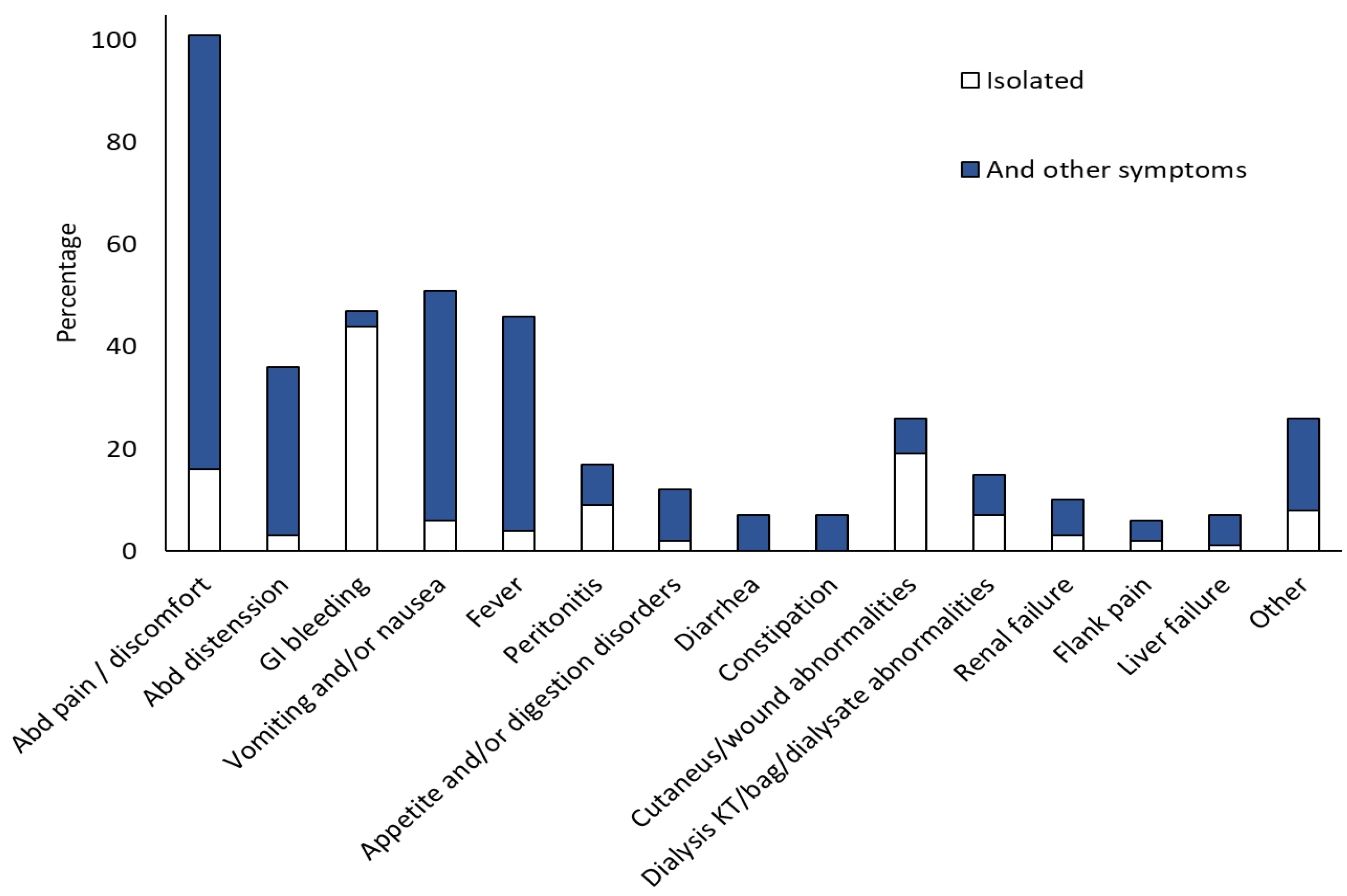
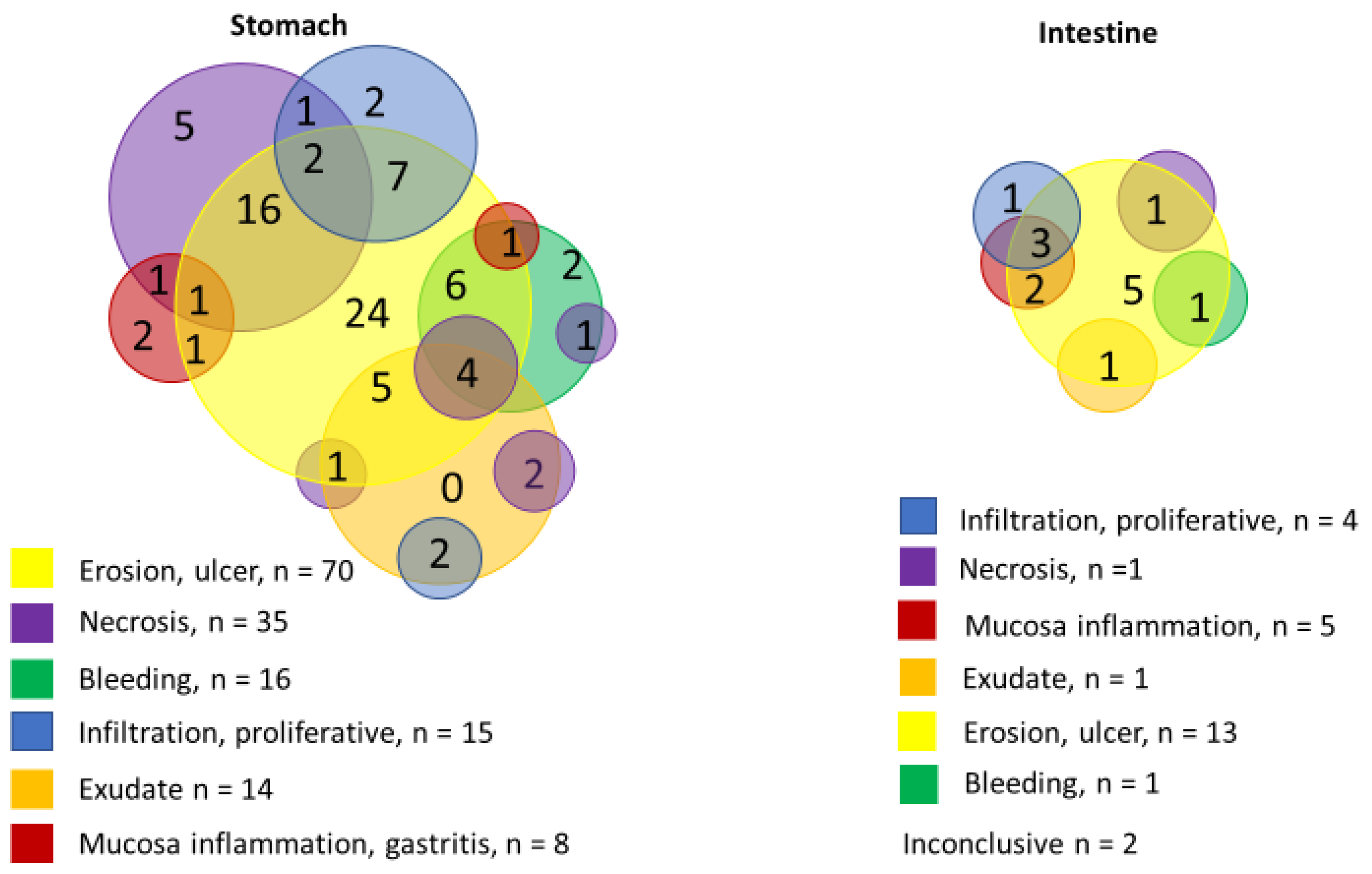
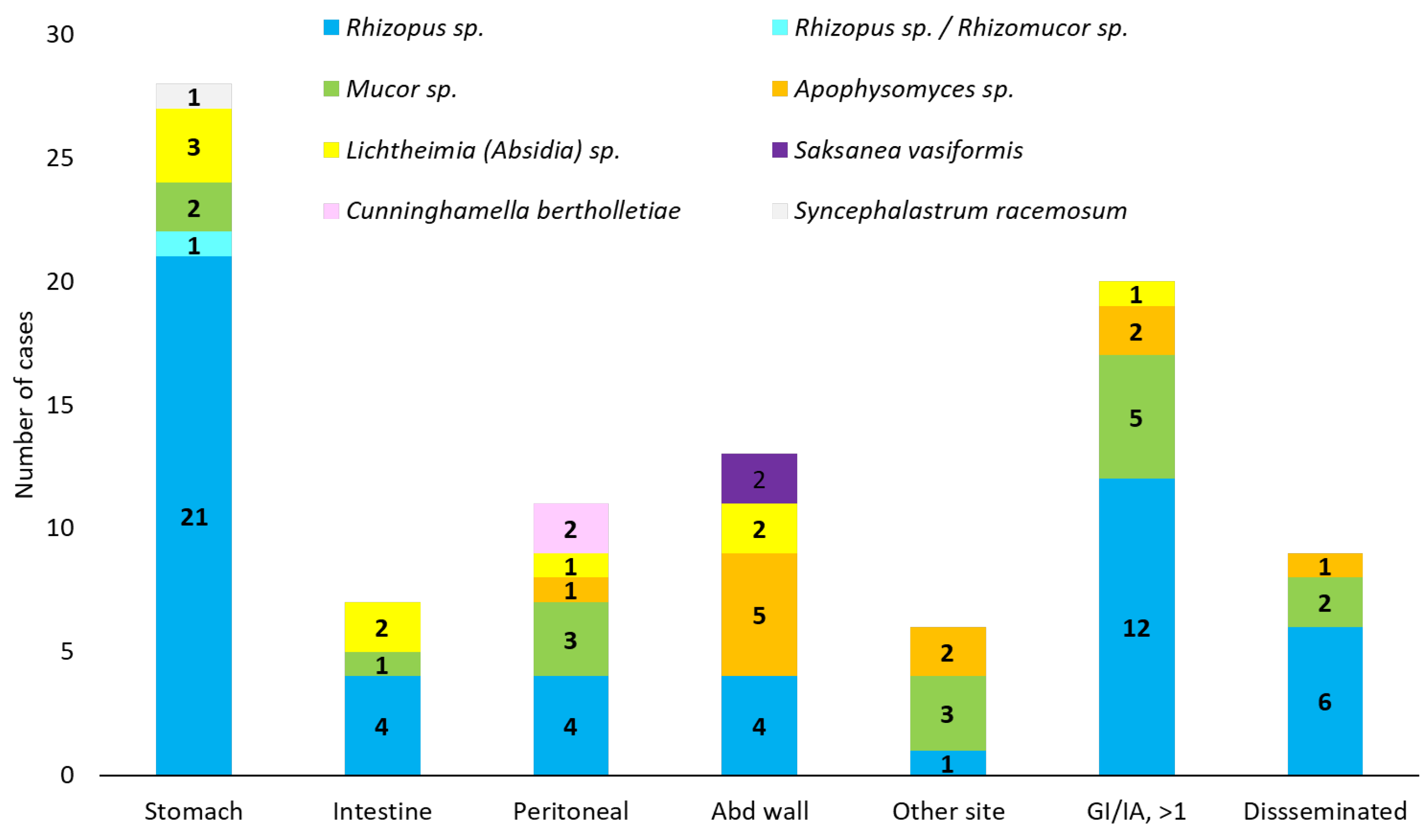

| Underlying Disease/Condition | Cases | |
|---|---|---|
| Number | Percentage | |
| Diabetes mellitus, of which with ketoacidosis (n = 10) | 70 10 | 24.8 3.5 |
| Solid organ transplantation Kidney only Liver only Other: kidney + liver (n = 4); heart (n = 3); heart + lung (n = 2), kidney + pancreas, lung, multi-organs (n = 1) | 46 20 14 12 | 16.3 7.1 5 4.2 |
| Autoimmune disease Inflammatory bowel disease (n = 4) Systemic lupus erythematosus (n = 3), Inflammatory vasculitis (n = 2), Sarcoidosis and rheumatic heart disease (n = 1) | 11 | 3.9 |
| Renal failure/dysfunction, of which 10 occurred in diabetic patients CAPD Haemodialysis, No replacement for kidney dysfunction (n = 4) | 36 21 11 | 12.8 7.5 3.9 |
| Hepatic failure/dysfunction, of which Cirrhosis | 19 8 | 6.7 2.8 |
| Impaired immune response: HIV infection, of which AIDS, CD4 count > 200/mm3 (n = 4) CD4 low count or dysfunction (n = 3) Splenic absence or splenectomy (n = 2) Other impaired immune response: Papillon–Lefevre syndrome, Down’s syndrome, low absolute lymphocyte count, and monocyte HLA-DR dysfunction (n = 1) | 18 10 6 | 6.4 3.5 2.1 |
| COVID-19 | 24 | 8.5 |
| Alcohol abuse, of which hepatic failure/dysfunction (n = 2) and cirrhosis (n = 1) | 23 | 8.1 |
| Drug abuse | 9 | 3.2 |
| Malnutrition (none without other underlying condition) | 5 | 1.8 |
| Abdominal injury Traffic accident Blunt/stab wound Fall/professional accident (n = 4) Gunshot/bombing (n = 3) | 22 9 6 | 7.8 3.2 2.1 |
| Abdominal surgery, of which for gastrointestinal perforation/peritonitis | 42 18 | 14.9 6.4 |
| Stay in ICU, of which Septic shock/sepsis Metabolic acidosis a Multi-organ failure, Mechanical Ventilation ECMO | 67 25 12 16 22 5 | 23.7 8.9 4.2 5.7 7.8 1.8 |
| Corticosteroids (excluding TOS immunosuppression), of which auto-immune disease treatment (n = 5) | 45 | 16 |
| Other b | 50 | 17.7 |
| None | 6 | 2.1 |
| Stomach (n = 111) | Intestine (n = 33) | Peritoneal (n = 13) | Abd. Wall (n = 15) | Other Sites c (n = 10) | GI/IA, >1 Site (n = 56) | Disseminated (n = 16) | |
|---|---|---|---|---|---|---|---|
| Abdominal pain/discomfort | 59 (53.15%) | 12 (36.36%) | 5 (38.46%) | 3 (20%) | 2 (20%) | 18 (32.14%) | 1 (5.88%) |
| Abdominal distension | 11 (9.91%) | 13 (39.39%) | 2 (15.38%) | 0 | 0 | 8 (14.28%) | 2 (11.76%) |
| GI bleeding | 52 (46.85%) | 12 (36.36%) | 0 | 0 | 5 (50) | 13 (23.21%) | 2 (11.76%) |
| Vomiting and/or nausea | 27 (24.32%) | 13 (39.39%) | 2 (15.38%) | 1 (6.67%) | 0 | 6 (10.71%) | 2 (11.76%) |
| Fever | 15 (13.51%) | 3 (23.08%) | 2 (15.38%) | 6 (40%) | 2 (20%) | 1 (1.78%) | 5 (29.41%) |
| Peritonitis | 1 (6.67%) | 6 (18.18%) | 1 (7.69%) | 0 | 0 | 6 (10.71%) | 2 (11.76%) |
| Appetite/digestion disorders | 10 (9.01%) | 1 (3.03%) | 0 | 0 | 1 (10%) | 0 | 0 |
| Diarrhoea | 1 (0.90%) | 2 (6.06%) | 1 (7.69%) | 0 | 0 | 2 (3,57%) | 0 |
| Constipation | 2 (1.80%) | 4 (12.12%) | 0 | 0 | 0 | 1 (1.78%) | 0 |
| Cutaneous/wound abnormalities | 0 | 2 (6.06%) | 1 (7.69%) | 14 (93.33%) | 1 (10%) | 8 (14.28%) | 0 |
| Dialysis catheter or bag or dialysate abnormalities a | 0 | 0 | 4 (30.77%) | 0 | 0 | 4 (7.14%) | 0 |
| Renal failure | 0 | 0 | 0 | 0 | 1 (10%) | 7 (12.5%) | 2 (11.76%) |
| Flank pain | 0 | 0 | 0 | 0 | 2 (20%) | 4 (7.14%) | 0 |
| Liver failure | 2 (1.80%) | 0 | 1 (7.69%) | 0 | 0 | 2 (3.57%) | 2 (11.76%) |
| Other b | 3 (2.70%) | 1 (3.03%) | 0 | 0 | 2 (20%) | 5 (8.92%) | 12 (70.59%) |
| Identification | No (%) |
|---|---|
| Rhizopus | 52 (54.7) |
| Rhizopus sp. | 23 (24.2) |
| R. microsporus | 12 (12.6) |
| R. microsporus var. rhizopodiformis | 6 (6.3) |
| R. microsporus var. microsporus | 1 (1) |
| R. microsporus var. azygosporus | 1 (1) |
| R. oryzae (arrhizus) | 7 (7.4) |
| R. oryzae or R. sexualis | 1 (1) |
| R. stolonifer | 1 (1) |
| Rhizomucor sp./Rhizopus sp. | 1 (1) |
| Mucor | 16 (16.8) |
| Mucor sp. | 13 (13.7) |
| M. indicus | 2 (2.1) |
| M. ramosissimus | 1 (1) |
| Apophysomyces | 11 (11.6) |
| A. elegans | 10 (10.5) |
| A. variabilis | 1 (1) |
| Lichtheimia (former Absidia) | 9 (9.5) |
| Lichtheimia (former Absidia) sp. | 2 (2.1) |
| L. corymbifera | 3 (3.6) |
| L. ramosa | 3 (3.6) |
| L. hongkongensis | 1 (1) |
| Cunninghamella bertholletiae | 2 (2.1) |
| Saksanea vasiformis | 2 (2.1) |
| Syncephalastrum racemosum | 1 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, B.; Lefevre Utile, A.; Jaureguiberry, S.; Angoulvant, A. Gastrointestinal and Intra-Abdominal Mucormycosis in Non-Haematological Patients—A Comprehensive Review. J. Fungi 2025, 11, 298. https://doi.org/10.3390/jof11040298
Henry B, Lefevre Utile A, Jaureguiberry S, Angoulvant A. Gastrointestinal and Intra-Abdominal Mucormycosis in Non-Haematological Patients—A Comprehensive Review. Journal of Fungi. 2025; 11(4):298. https://doi.org/10.3390/jof11040298
Chicago/Turabian StyleHenry, Benoît, Alain Lefevre Utile, Stephane Jaureguiberry, and Adela Angoulvant. 2025. "Gastrointestinal and Intra-Abdominal Mucormycosis in Non-Haematological Patients—A Comprehensive Review" Journal of Fungi 11, no. 4: 298. https://doi.org/10.3390/jof11040298
APA StyleHenry, B., Lefevre Utile, A., Jaureguiberry, S., & Angoulvant, A. (2025). Gastrointestinal and Intra-Abdominal Mucormycosis in Non-Haematological Patients—A Comprehensive Review. Journal of Fungi, 11(4), 298. https://doi.org/10.3390/jof11040298






