EvSec22, a SNARE Protein, Regulates Hyphal Growth, Stress Tolerance, and Nematicidal Pathogenicity in Esteya vermicola
Abstract
1. Introduction
2. Materials and Methods
2.1. Vector Construction and Fungal Transformation
2.2. Infectivity Assay of B. xylophilus
2.3. Conidia Count
2.4. Growth Assay on Solid Medium
2.5. Hyphal Septal Distance Measurement
2.6. Abiotic Stress Assay
2.7. Data Analysis
3. Result
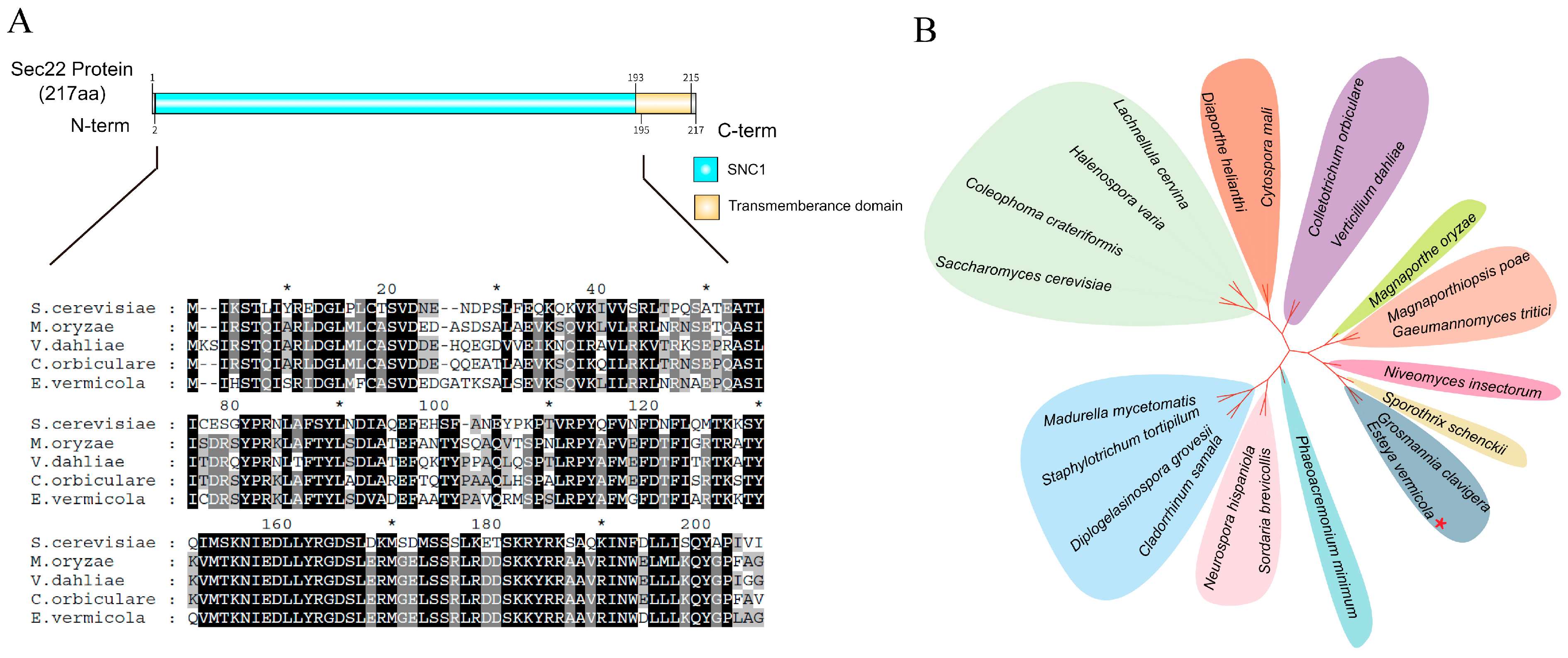
3.1. Sequence Analysis of EvSec22 in E. vermicola CBS115803
3.2. Optimization of E. vermicola Transformation Methods and Construction of EvSec22 Mutants and Complementary Strains
3.3. EvSec22 Mutants Impaired the Infectivity of E. vermicola Against B. xylophilus
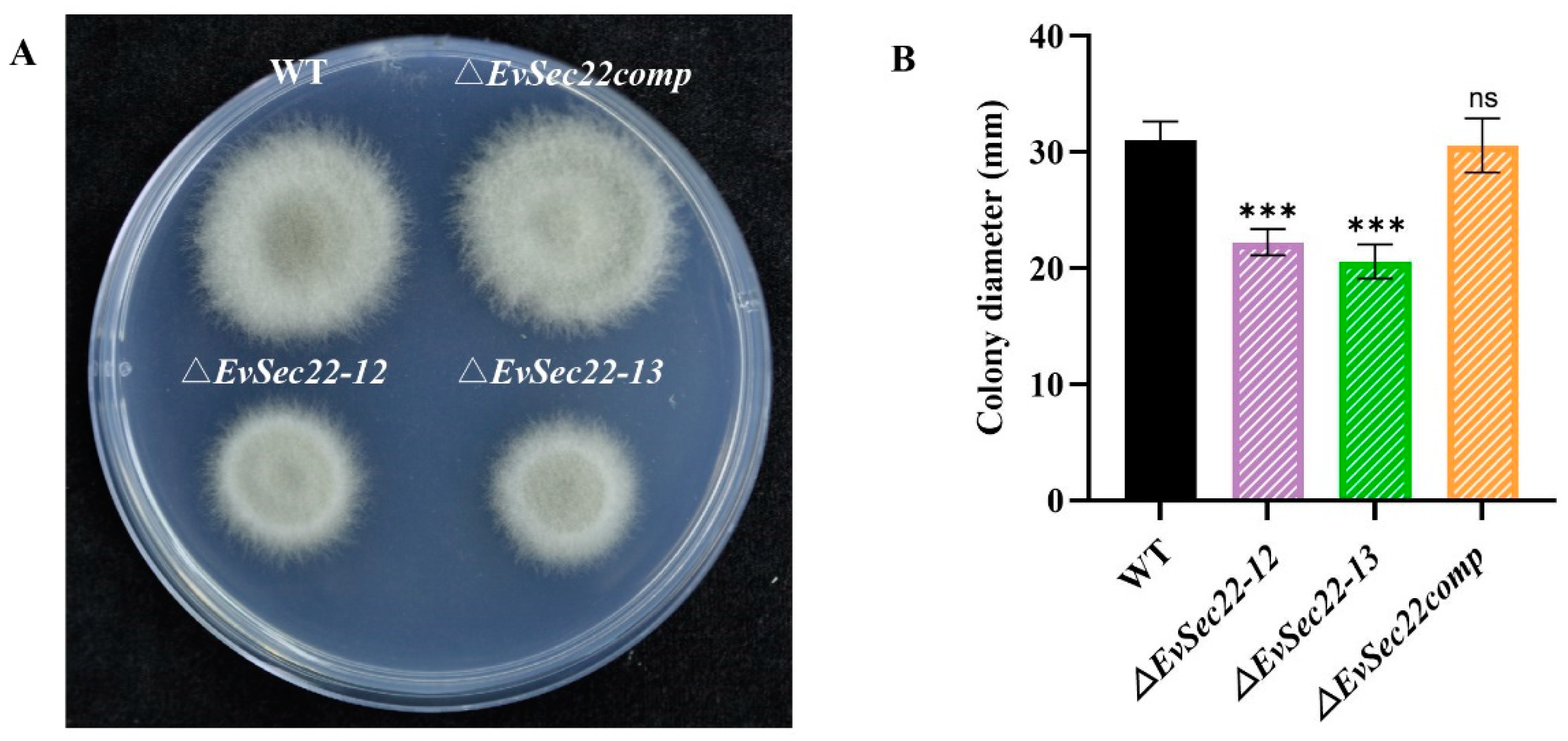
3.4. Loss of EvSec22 Leads to Slower Hyphal Growth and Hyphal Septal Spacing in E. vermicola
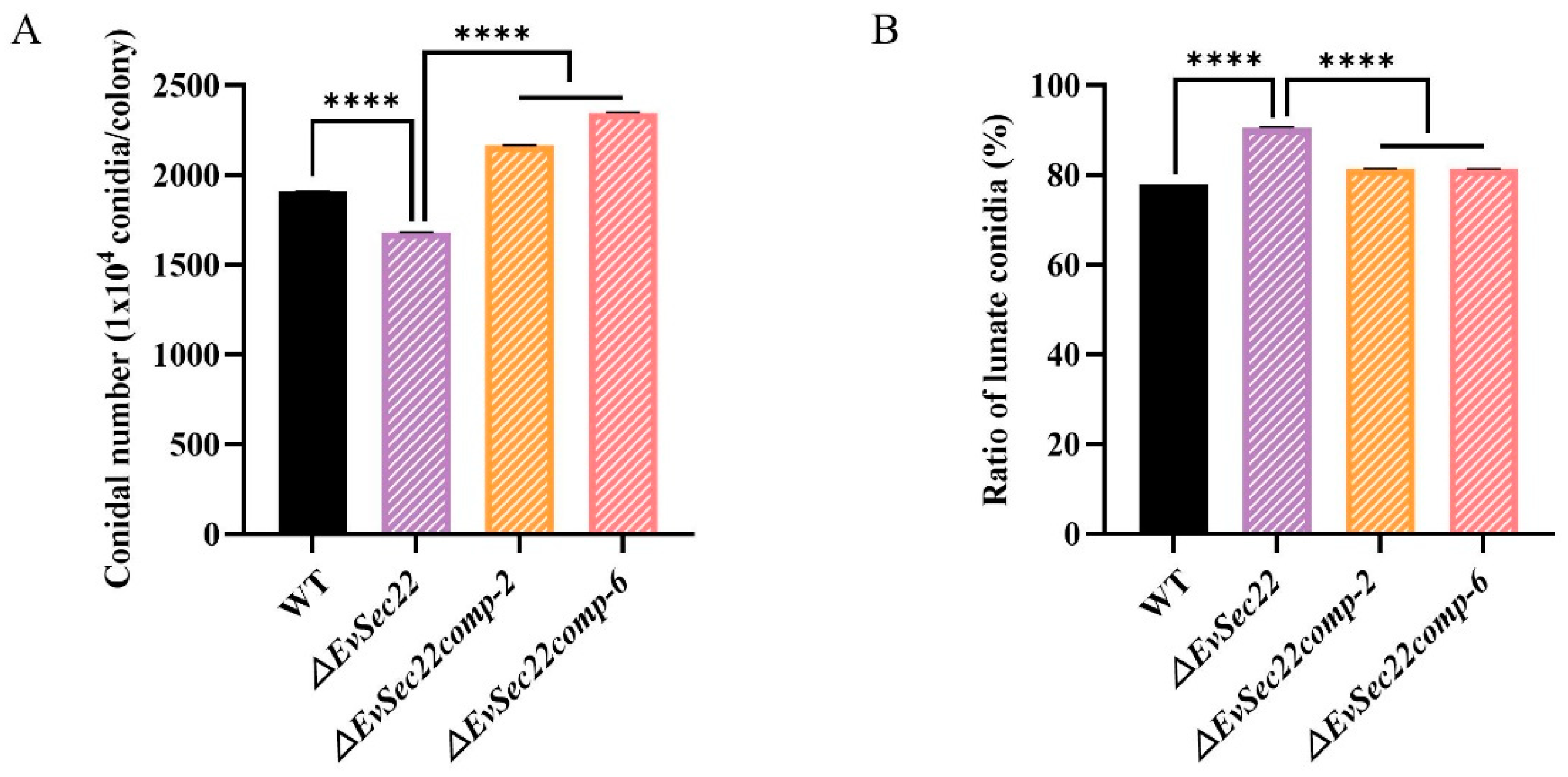
3.5. EvSec22 Deletion Reduces Total Conidia Count but Increases the Proportion of Lunate-Shaped Conidia
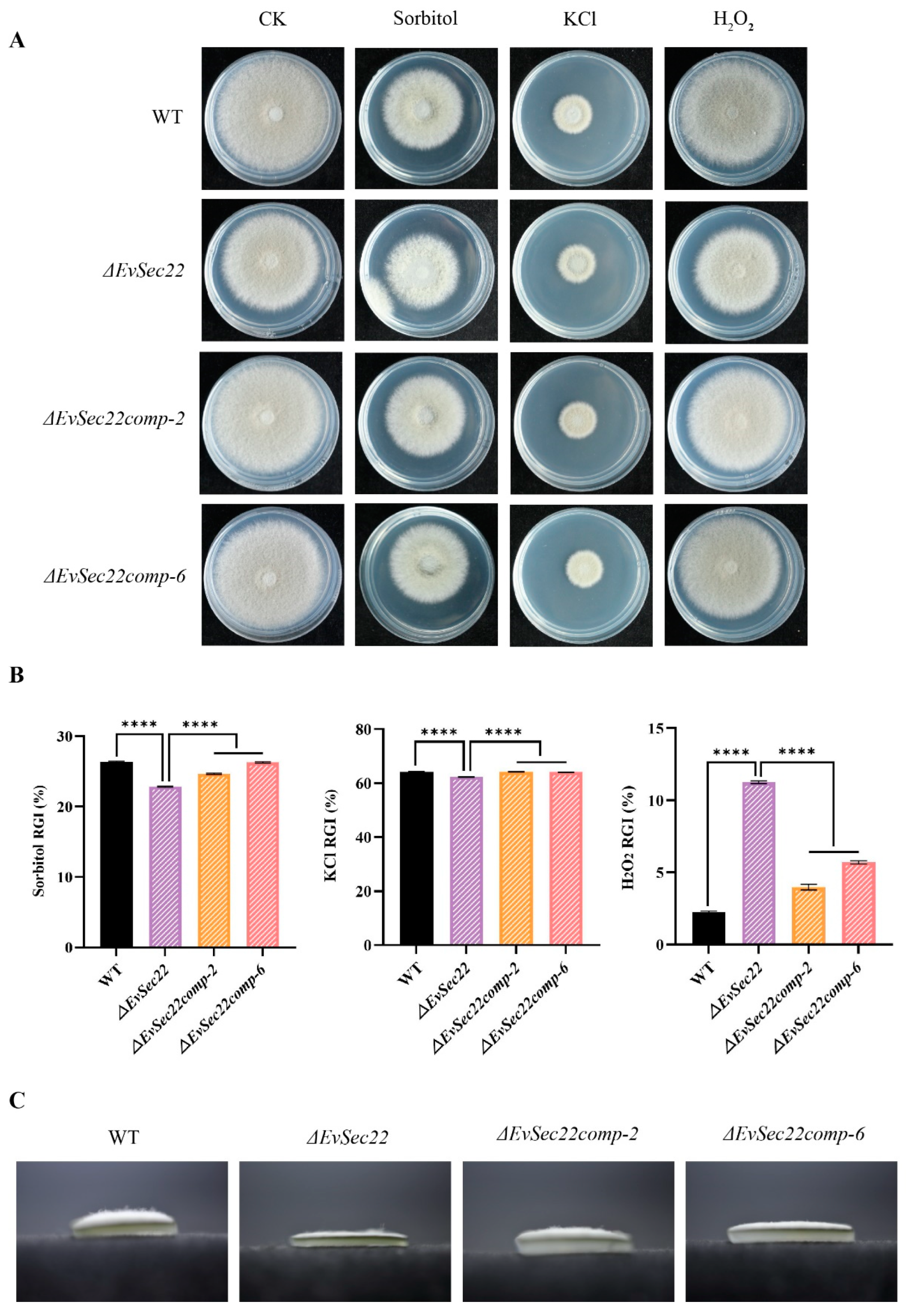
3.6. Deletion of EvSec22 Affects E. vermicola’s Tolerance to Abiotic Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickle, W.R.; Golden, A.M.; Mamiya, Y.; Wergin, W.P. On the taxonomy and morphology of the pine wood nematode, Bursaphelenchus xylophilus (Steiner &Buhrer 1934) Nickle 1970. J. Nematol. 1981, 13, 385–392. [Google Scholar] [PubMed]
- Mamiya, Y. Pine wood nematode, Bursaphelenchus lignicolus Mamiya and Kiyohara, as a causal agent of pine wilting disease. Rev. Plant Prot. Res. 1972, 5, 46–60. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Hunt, D. Pine wilt disease: A worldwide threat to forest ecosystems. Nematology 2009, 1, 315–316. [Google Scholar] [CrossRef]
- Robertson, L.; Arcos, S.C.; Escuer, M.; Merino, R.S.; Espárrago, G.; Abelleira, A.; Navas, A. Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 2011, 13, 755–757. [Google Scholar] [CrossRef]
- Zhao, L.L.; Mota, M.; Vieira, P.; Butcher, R.A.; Sun, J.H. Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014, 30, 299–308. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, Q.X.; Song, B.A. Chemical nematicides: Recent research progress and outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.L.; Yu, A.X.; Wang, G.D.; Zheng, F.; Jia, J.L.; Xu, H.H. Chitosan-based nanoparticles of avermectin to control pine wood nematodes. Int. J. Biol. Macromol. 2018, 112, 258–263. [Google Scholar] [CrossRef]
- Affokpon, A.; Coyne, D.L.; Htay, C.C.; Agbèdè, R.D.; Lawouin, L.; Coosemans, J. Biocontrol potential of native Trichoderma isolates against root-knot nematodes in West African vegetable production systems. Soil Biol. Biochem. 2011, 43, 600–608. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere Interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef]
- Liou, J.Y.; Shih, J.Y.; Tzean, S.S. Esteya, a new nematophagous genus from Taiwan, attacking the pinewood nematode (Bursaphelenchus xylophilus). Mycol. Res. 1999, 103, 242–248. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fang, Z.M.; Sun, B.S.; Gu, L.J.; Zhang, K.Q.; Sung, C.K. High infectivity of an endoparasitic fungus strain, Esteya vermicola, against nematodes. J. Microbiol. 2008, 46, 380–389. [Google Scholar] [CrossRef]
- Lin, F.; Ye, J.L.; Wang, H.G.; Zhang, A.J.; Zhao, B.G. Host deception: Predaceous fungus, Esteya vermicola, entices pine wood nematode by mimicking the scent of pine tree for nutrient. PLoS ONE 2013, 8, e71676. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Fang, Z.M.; Wang, Z.; Zhang, D.L.; Gu, L.J.; Lee, M.R.; Liu, L.; Sung, C.K. Biological control of the pinewood nematode Bursaphelenchus xylophilus by application of the endoparasitic fungus Esteya vermicola. BioControl 2011, 56, 91–100. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J. Securing SNAREs for assembly. J. Biol. Chem. 2020, 295, 10136–10137. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef]
- Fasshauer, D.; Sutton, R.B.; Brunger, A.T.; Jahn, R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 1998, 95, 15781–15786. [Google Scholar] [CrossRef]
- Shao, K.K.; Li, F.; Yang, Y.; Wang, N.; Gao, X.D.; Nakanishi, H. Characteristics of SNARE proteins are defined by distinctive properties of SNARE motifs. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129658. [Google Scholar] [CrossRef]
- Aalto, M.K.; Ronne, H.; Keränen, S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993, 12, 4095–4104. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Waheed, A.; Hussain, Q.; Shen, L.; Wang, J.; Liu, G. SNARE protein Snc1 is essential for vesicle trafficking, membrane fusion and protein secretion in fungi. Cells 2023, 12, 1547. [Google Scholar] [CrossRef]
- Ballensiefen, W.; Ossipov, D.; Schmitt, H.D. Recycling of the yeast v-SNARE Sec22p involves COPI-proteins and the ER transmembrane proteins Ufe1p and Sec20p. J. Cell Sci. 1998, 111, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lau, M.S.Y.; Banfield, D.K. Multiple ER-Golgi SNARE transmembrane domains are dispensable for trafficking but required for SNARE recycling. Mol. Biol. Cell 2016, 27, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, L.; Zhang, D.D.; Short, D.P.G.; Zhou, L.; Song, S.S.; Liu, Y.; Wang, D.; Kong, Z.Q.; Cui, W.Y.; et al. SNARE-encoding genes VdSec22 and VdSso1 mediate protein secretion required for full virulence in Verticillium dahliae. Mol. Plant Microbe Interact. 2018, 31, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Irieda, H.; Maeda, H.; Akiyama, K.; Hagiwara, A.; Saitoh, H.; Uemura, A.; Terauchi, R.; Takano, Y. Colletotrichum orbiculare secretes virulence effectors to a biotrophic interface at the primary hyphal neck via exocytosis coupled with SEC22-mediated traffic. Plant Cell 2014, 26, 2265–2281. [Google Scholar] [CrossRef]
- Song, W.W.; Dou, X.Y.; Qi, Z.Q.; Wang, Q.; Zhang, X.; Zhang, H.F.; Guo, M.; Dong, S.M.; Zhang, Z.G.; Wang, P.; et al. R-SNARE homolog MoSec22 is required for conidiogenesis, cell wall integrity, and pathogenesis of Magnaporthe oryzae. PLoS ONE 2010, 5, e13193. [Google Scholar] [CrossRef]
- Zhou, S.Z.; Liu, S.; Guo, C.C.; Wei, H.W.; He, Z.H.; Liu, Z.Q.; Li, X.Y. The C2H2 transcription factor Con7 regulates vegetative growth, cell wall integrity, oxidative stress, asexual sporulation, appressorium and hyphopodium formation, and pathogenicity in Colletotrichum graminicola and Colletotrichum siamense. J. Fungi 2024, 10, 495. [Google Scholar] [CrossRef]
- Scott, B.; Eaton, C.J. Role of reactive oxygen species in fungal cellular differentiations. Curr. Opin. Microbiol. 2008, 11, 488–493. [Google Scholar] [CrossRef]
- Xie, C.J.; Li, Q.L.; Yang, X.Y. Characterization of VdASP F2 secretory factor from Verticillium dahliae by a fast and easy gene knockout system. Mol. Plant Microbe Interact. 2017, 30, 444–454. [Google Scholar] [CrossRef]
- Wang, H.H.; Wang, Y.B.; Yin, C.; Gao, J.; Tao, R.; Sun, Y.L.; Wang, C.Y.; Wang, Z.; Li, Y.X.; Sung, C.K. In vivo infection of Bursaphelenchus xylophilus by the fungus Esteya vermicola. Pest Manag. Sci. 2020, 76, 2854–2864. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Z.J.; Zheng, X.Y.; Yuan, J.J.; Zou, R.; Wang, Y.L.; Peng, X.; Xie, C.J. The essential role of arginine biosynthetic genes in lunate conidia formation, conidiation, mycelial growth, and virulence of nematophagous fungus, Esteya vermicola CBS115803. Pest Manag. Sci. 2024, 80, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, B.J.; Cui, X.Y.; Hou, J.; Zhang, N. Biocontrol activities of grey mould of grapes with the volatile organic compounds generated by yeast HXMG-1 isolated from grapes. J. Plant Dis. Prot. 2024, 131, 1387–1397. [Google Scholar] [CrossRef]
- Guo, C.M.; Yang, X.; Shi, H.L.; Chen, C.; Hu, Z.J.; Zheng, X.Y.; Yang, X.Y.; Xie, C.J. Identification of VdASP F2-interacting protein as a regulator of microsclerotial formation in Verticillium dahliae. Microb. Biotechnol. 2022, 15, 2040–2054. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.K.; Sun, H.H.; Ying, S.H.; Feng, M.G. Characterization of the Hog1 MAPK pathway in the entomopathogenic fungus Beauveria bassiana. Environ. Microbiol. 2017, 19, 1808–1821. [Google Scholar] [CrossRef]
- Adnan, M.; Fang, W.; Sun, P.; Zheng, Y.; Abubakar, Y.S.; Zhang, J.; Lou, Y.; Zheng, W.; Lu, G.D. R-SNARE FgSec22 is essential for growth, pathogenicity and DON production of Fusarium graminearum. Curr. Genet. 2020, 66, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Zhou, D.X.; Bai, N.; Liu, Q.Q.; Zhao, N.; Yang, J.K. SNARE protein AoSec22 orchestrates mycelial growth, vacuole assembly, trap formation, stress response, and secondary metabolism in Arthrobotrys oligospora. J. Fungi 2023, 9, 75. [Google Scholar] [CrossRef]
- Traeger, S.; Nowrousian, M. Functional analysis of developmentally regulated genes chs7 and sec22 in the Ascomycete Sordaria macrospora. G3 Genes Genomes Genet. 2015, 5, 1233–1245. [Google Scholar] [CrossRef][Green Version]
- Angelova, M.B.; Pashova, S.B.; Spasova, B.K.; Vassilev, S.V.; Slokoska, L.S. Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol. Res. 2005, 109, 150–158. [Google Scholar] [CrossRef]
- Ferrigo, D.; Scarpino, V.; Vanara, F.; Causin, R.; Raiola, A.; Blandino, M. Influence of H2O2-induced oxidative stress on in vitro growth and moniliformin and fumonisins accumulation by Fusarium proliferatum and Fusarium subglutinans. Toxins 2021, 13, 653. [Google Scholar] [CrossRef]
- Liu, Y.W.; Gong, X.D.; Li, M.X.; Si, H.L.; Zhou, Q.H.; Liu, X.C.; Fan, Y.; Zhang, X.Y.; Han, J.M.; Gu, S.Q.; et al. Effect of osmotic stress on the growth, development and pathogenicity of Setosphaeria turcica. Front. Microbiol. 2021, 12, 706349. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Zou, R.; Peng, X.; Wang, Y.; Cheng, Z.; Ye, T.; Han, L.; Xie, C. EvSec22, a SNARE Protein, Regulates Hyphal Growth, Stress Tolerance, and Nematicidal Pathogenicity in Esteya vermicola. J. Fungi 2025, 11, 295. https://doi.org/10.3390/jof11040295
Yuan J, Zou R, Peng X, Wang Y, Cheng Z, Ye T, Han L, Xie C. EvSec22, a SNARE Protein, Regulates Hyphal Growth, Stress Tolerance, and Nematicidal Pathogenicity in Esteya vermicola. Journal of Fungi. 2025; 11(4):295. https://doi.org/10.3390/jof11040295
Chicago/Turabian StyleYuan, Jingjie, Run Zou, Xuan Peng, Yilan Wang, Zhongwu Cheng, Tengqing Ye, Lihui Han, and Chengjian Xie. 2025. "EvSec22, a SNARE Protein, Regulates Hyphal Growth, Stress Tolerance, and Nematicidal Pathogenicity in Esteya vermicola" Journal of Fungi 11, no. 4: 295. https://doi.org/10.3390/jof11040295
APA StyleYuan, J., Zou, R., Peng, X., Wang, Y., Cheng, Z., Ye, T., Han, L., & Xie, C. (2025). EvSec22, a SNARE Protein, Regulates Hyphal Growth, Stress Tolerance, and Nematicidal Pathogenicity in Esteya vermicola. Journal of Fungi, 11(4), 295. https://doi.org/10.3390/jof11040295





