Proteomic Analysis of the Fusarium graminearum Secretory Proteins in Wheat Apoplast Reveals a Cell-Death-Inducing M43 Peptidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Conditions for Growing
2.2. Apoplast Fluid Extraction and Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.3. Bioinformatics Analysis
2.4. Agrobacterium-Mediated Transient Expression in N. benthamiana
2.5. 3,3′-Diaminobenzidine (DAB) and Trypan Blue Straining
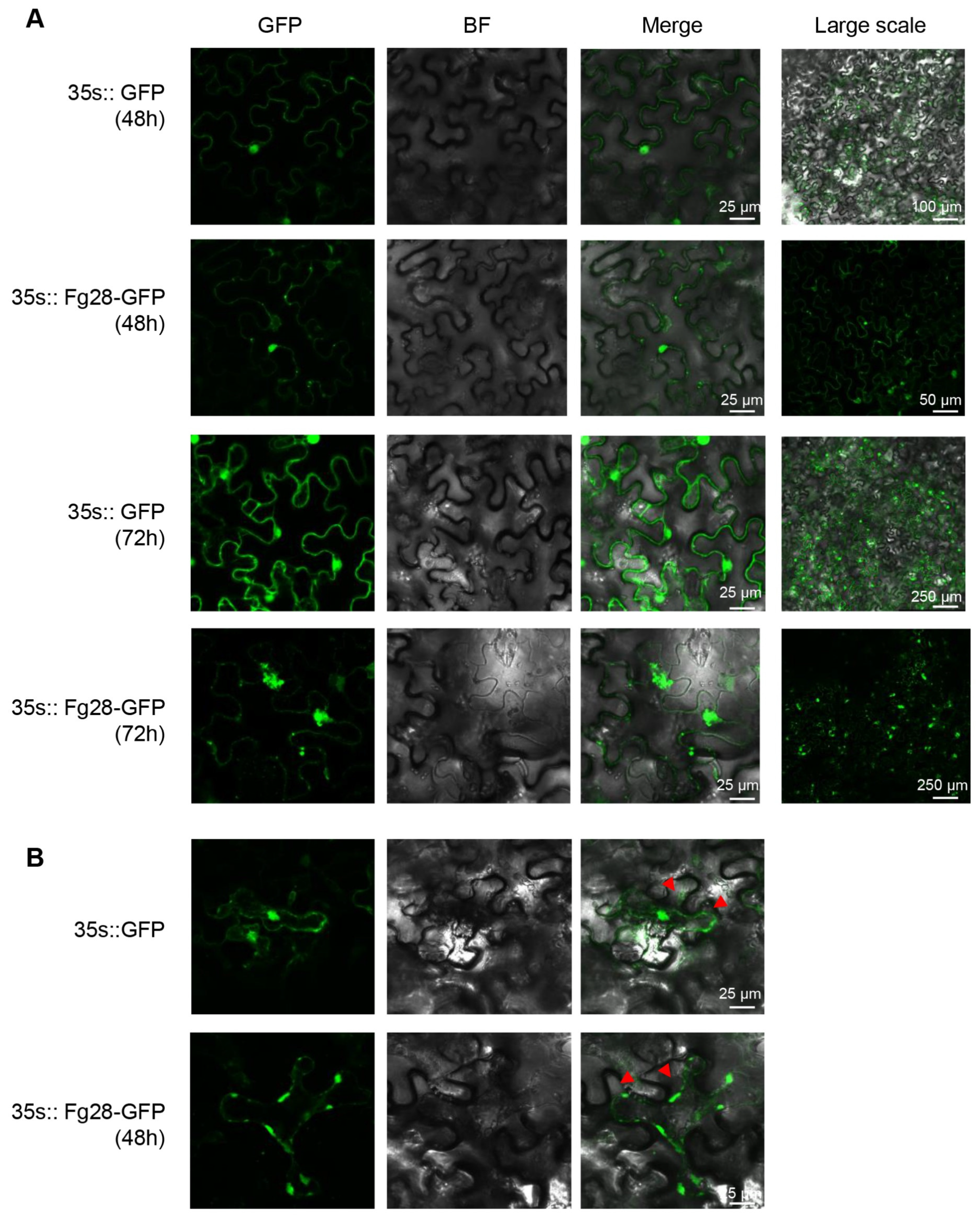
2.6. Subcellular Localization of Fg28
2.7. Yeast Signal Sequence Trap System and Secretion Assay
2.8. Gene Knockout and Pathogenicity Testing
2.9. RNA Extraction and RT-qPCR Analysis
2.10. Statistical Analyses
3. Results
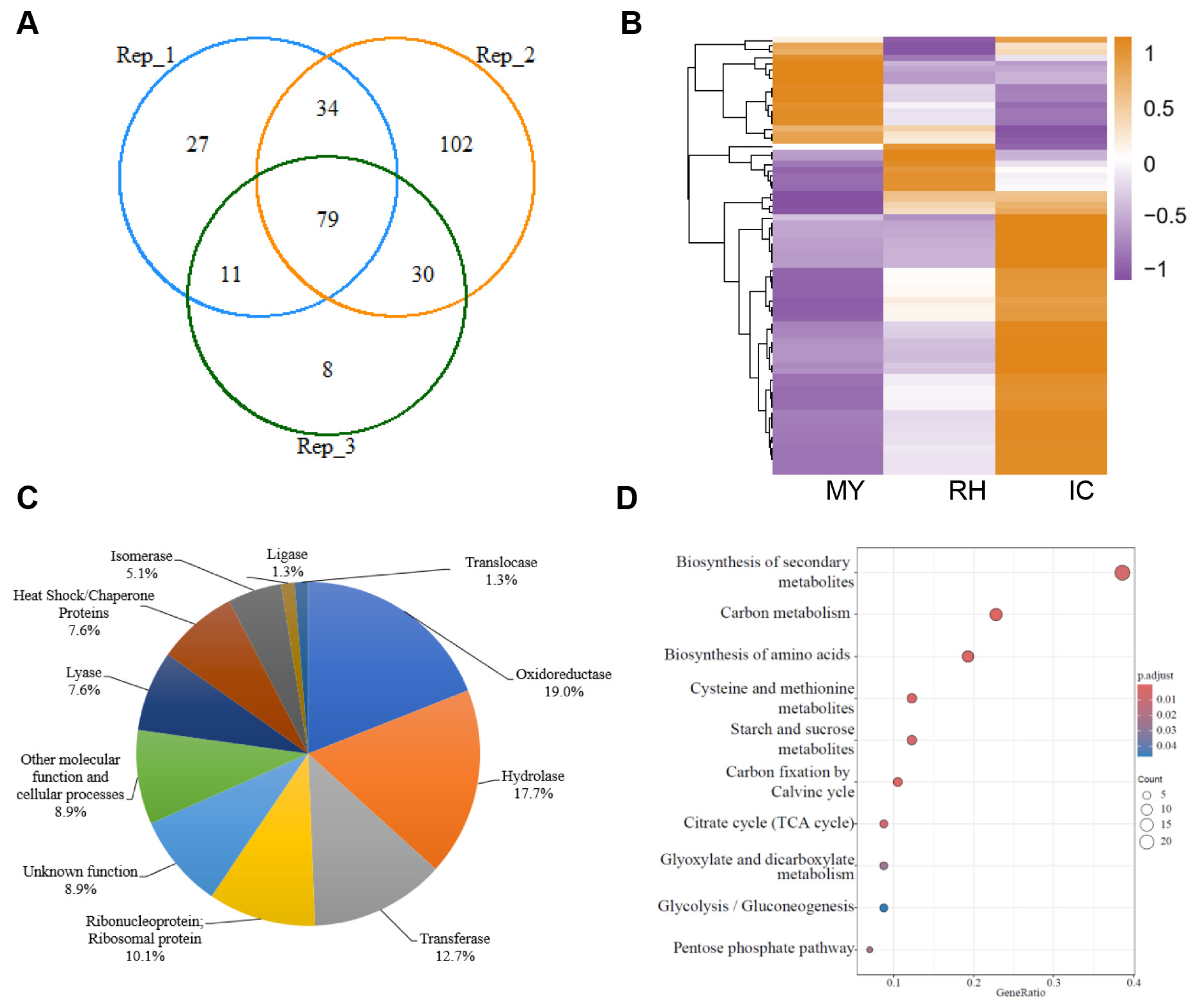
3.1. Proteomic Analysis of the F. graminearum Secretome in the Apoplast Fluid of Wheat Coleoptiles
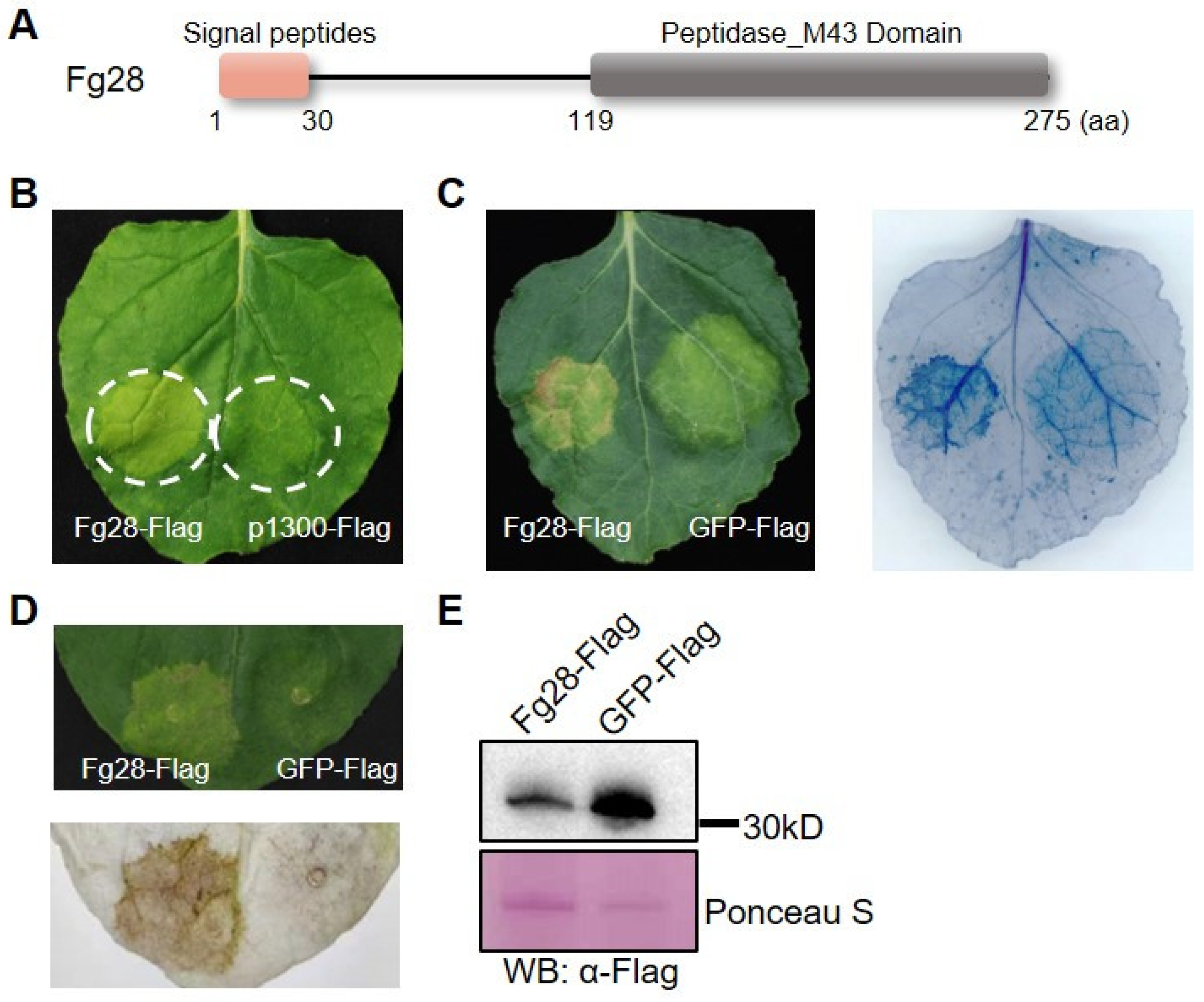
3.2. Fg28 Induces Cell Death in N. benthamiana
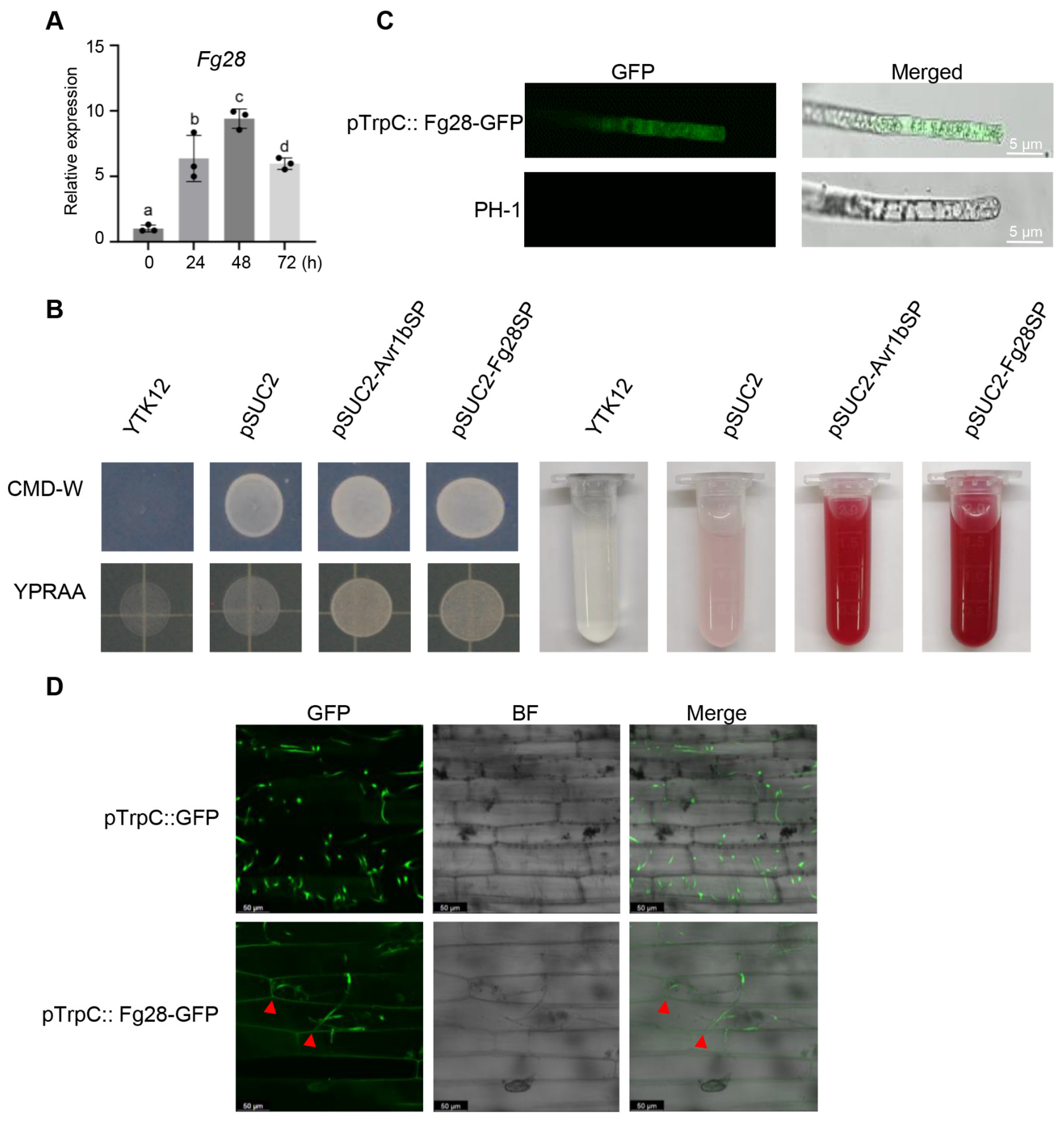
3.3. Fg28 Is Highly Up-Regulated and Secreted During the Early Stages of F. graminearum Infection
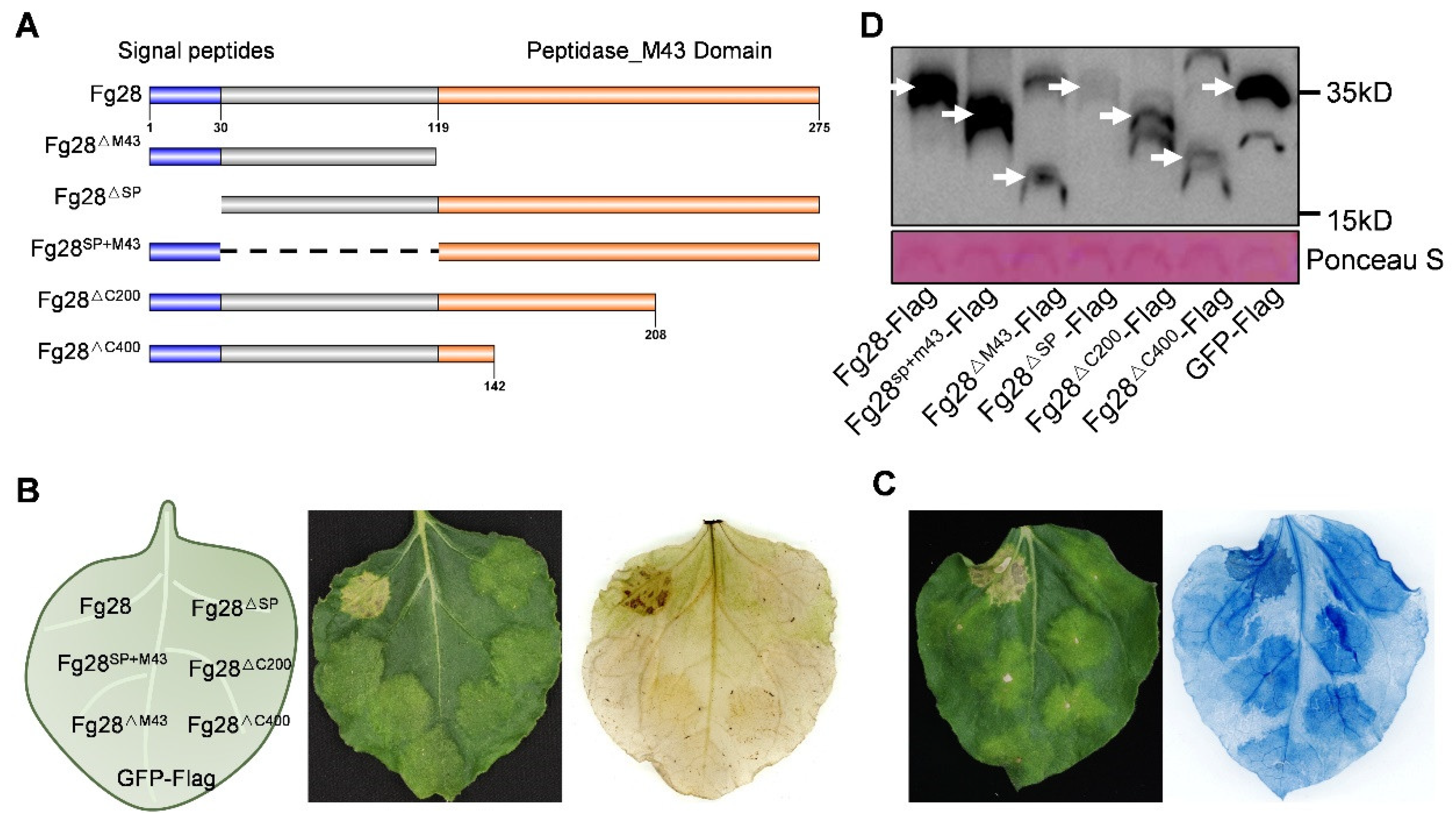
3.4. Full Length of Fg28 Required to Induce Cell Death
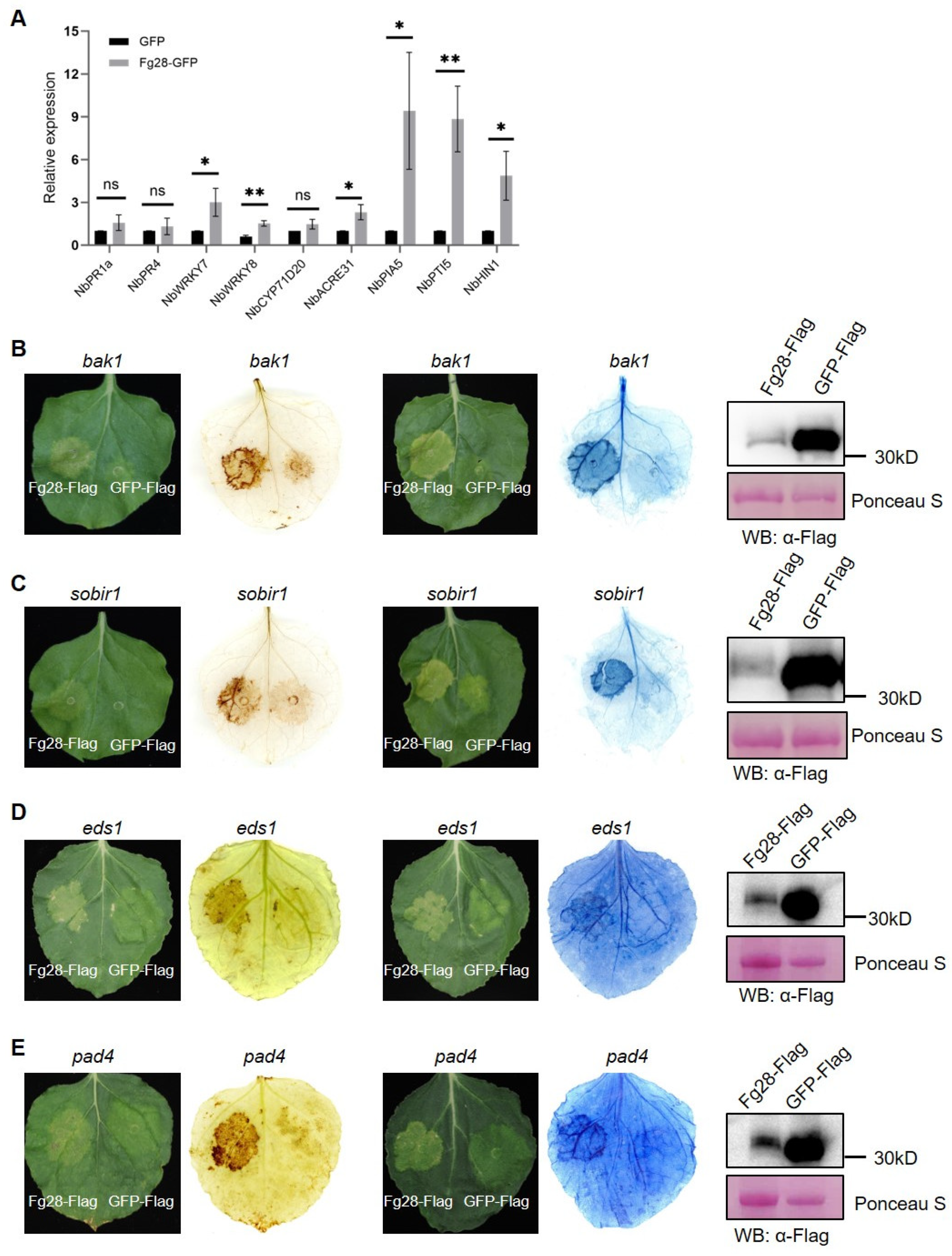
3.5. Fg28 Induces Plant Immune Response and Is Independent of BAK1/SOBIR1 and EDS1/PAD4
3.6. Knockout Fg28 Have no Defect on the Virulence of F. graminearum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; Hao, Y.; Mergoum, M.; Bai, G.; Humphreys, G.; Cloutier, S.; Xia, X.; He, Z. Breeding wheat for resistance to Fusarium head blight in the Global North: China, USA, and Canada. Crop J. 2019, 7, 730–738. [Google Scholar]
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium head blight in wheat: Contemporary status and molecular approaches. 3 Biotech. 2020, 10, 172. [Google Scholar]
- Ma, Z.; Xie, Q.; Li, G.; Jia, H.; Zhou, J.; Kong, Z.; Li, N.; Yuan, Y. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020, 133, 1541–1568. [Google Scholar] [PubMed]
- Moonjely, S.; Ebert, M.; Paton-Glassbrook, D.; Noel, Z.A.; Roze, L.; Shay, R.; Watkins, T.; Trail, F. Update on the state of research to manage Fusarium head blight. Fungal Genet. Biol. 2023, 169, 103829. [Google Scholar]
- Walter, S.; Nicholson, P.; Doohan, F.M. Action and Reaction of Host and Pathogen during Fusarium Head Blight Disease. New Phytol. 2009, 185, 54–66. [Google Scholar]
- Yang, B.; Wang, Y.; Tian, M.; Dai, K.; Zheng, W.; Liu, Z.; Yang, S.; Liu, X.; Shi, D.; Zhang, H.; et al. Fg12 ribonuclease secretion contributes to Fusarium graminearum virulence and induces plant cell death. J. Integr. Plant Biol. 2021, 63, 365–377. [Google Scholar]
- Jiang, C.; Hei, R.; Yang, Y.; Zhang, S.; Wang, Q.; Wang, W.; Zhang, Q.; Yan, M.; Zhu, G.; Huang, P.; et al. An orphan protein of Fusarium graminearum modulates host immunity by mediating proteasomal degradation of TaSnRK1α. Nat. Commun. 2020, 11, 4382. [Google Scholar]
- Xiong, J.; Luo, M.; Chen, Y.; Hu, Q.; Fang, Y.; Sun, T.; Hu, G.; Zhang, C.J. Subtilisin-like proteases from Fusarium graminearum induce plant cell death and contribute to virulence. Plant Physiol. 2024, 195, 1681–1693. [Google Scholar]
- Voigt, C.A.; Schäfer, W.; Salomon, S. A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J. 2005, 42, 364–375. [Google Scholar]
- Niu, X.W.; Zheng, Z.Y.; Feng, Y.G.; Guo, W.Z.; Wang, X.Y. The Fusarium Graminearum virulence factor FGL targets an FKBP12 immunophilin of wheat. Gene 2013, 525, 77–83. [Google Scholar]
- Qi, P.F.; Zhang, Y.Z.; Liu, C.H.; Chen, Q.; Guo, Z.R.; Wang, Y.; Xu, B.J.; Jiang, Y.F.; Zheng, T.; Gong, X.; et al. Functional Analysis of FgNahG Clarifies the Contribution of Salicylic Acid to Wheat (Triticum aestivum) Resistance against Fusarium Head Blight. Toxins 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, S.; Dai, K.; Zheng, W.; Zhang, X.; Yang, B.; Ye, W.; Zheng, X.; Wang, Y. The effector Fg62 contributes to Fusarium graminearum virulence and induces plant cell death. Phytopathol. Res. 2023, 5, 12. [Google Scholar]
- Shang, S.; He, Y.; Hu, Q.; Fang, Y.; Cheng, S.; Zhang, C.J. Fusarium graminearum effector FgEC1 targets wheat TaGF14b protein to suppress TaRBOHD-mediated ROS production and promote infection. J. Integr. Plant Biol. 2024, 66, 2288–2303. [Google Scholar] [PubMed]
- Blümke, A.; Falter, C.; Herrfurth, C.; Sode, B.; Bode, R.; Schäfer, W.; Feussner, I.; Voigt, C.A. Secreted Fungal Effector Lipase Releases Free Fatty Acids to Inhibit Innate Immunity-Related Callose Formation during Wheat Head Infection. Plant Physiol. 2014, 165, 346–358. [Google Scholar]
- Xu, Q.; Hu, S.; Jin, M.; Xu, Y.; Jiang, Q.; Ma, J.; Zhang, Y.; Qi, P.; Chen, G.; Jiang, Y.; et al. The N-terminus of a Fusarium graminearum-secreted protein enhances broad-spectrum disease resistance in plants. Mol. Plant Pathol. 2022, 23, 1751–1764. [Google Scholar]
- Dora, S.; Terrett, O.M.; Sánchez-Rodríguez, C. Plant–microbe interactions in the apoplast: Communication at the plant cell wall. Plant Cell 2022, 34, 1532–1550. [Google Scholar]
- Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149, 167–192. [Google Scholar]
- Masachis, S.; Segorbe, D.; Turrà, D.; Leon-Ruiz, M.; Fürst, U.; El Ghalid, M.; Leonard, G.; López-Berges, M.S.; Richards, T.A.; Felix, G. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016, 1, 16043. [Google Scholar]
- Sun, Y.; Wang, Y.; Zhang, X.; Chen, Z.; Xia, Y.; Wang, L.; Sun, Y.; Zhang, M.; Xiao, Y.; Han, Z. Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature 2022, 610, 335–342. [Google Scholar]
- Jiang, H.; Xia, Y.; Zhang, S.; Zhang, Z.; Feng, H.; Zhang, Q.; Chen, X.; Xiao, J.; Yang, S.; Zeng, M. The CAP superfamily protein PsCAP1 secreted by Phytophthora triggers immune responses in Nicotiana benthamiana through a leucine-rich repeat receptor-like protein. New Phytol. 2023, 240, 784–801. [Google Scholar]
- Xu, Y.; Zhang, Y.; Zhu, J.; Sun, Y.; Guo, B.; Liu, F.; Huang, J.; Wang, H.; Dong, S.; Wang, Y.; et al. Phytophthora sojae apoplastic effector AEP1 mediates sugar uptake by mutarotation of extracellular aldose and is recognized as a MAMP. Plant Physiol. 2021, 187, 321–335. [Google Scholar] [PubMed]
- Lohaus, G.; Pennewiss, K.; Sattelmacher, B.; Hussmann, M.; Hermann Muehling, K. Is the infiltration-centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiol. Plant. 2001, 111, 457–465. [Google Scholar] [PubMed]
- Wang, W.; Scali, M.; Vignani, R.; Spadafora, A.; Sensi, E.; Mazzuca, S.; Cresti, M. Protein extraction for two-dimensional electrophoresis from olive leaf, a plant tissue containing high levels of interfering compounds. Electrophoresis 2003, 24, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Pirovani, C.P.; Carvalho, H.A.; Machado, R.C.; Gomes, D.S.; Alvim, F.C.; Pomella, A.W.; Gramacho, K.P.; Cascardo, J.C.; Pereira, G.A.; Micheli, F. Protein extraction for proteome analysis from cacao leaves and meristems, organs infected by Moniliophthora perniciosa, the causal agent of the witches’ broom disease. Electrophoresis 2008, 29, 2391–2401. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, Y.; Chen, Y.; Yang, Q.; Quan, Z.; Dai, R.; Qing, H. Rab21, a novel PS1 interactor, regulates γ-secretase activity via PS1 subcellular distribution. Mol. Neurobiol. 2018, 55, 3841–3855. [Google Scholar] [CrossRef]
- Qin, X.; Pi, X.; Wang, W.; Han, G.; Zhu, L.; Liu, M.; Cheng, L.; Shen, J.R.; Kuang, T.; Sui, S.F. Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits. Nat. Plants 2019, 5, 263–272. [Google Scholar] [CrossRef]
- Mentges, M.; Glasenapp, A.; Boenisch, M.; Malz, S.; Henrissat, B.; Frandsen, R.J.N.; Guldener, U.; Munsterkotter, M.; Bormann, J.; Lebrun, M.H.; et al. Infection cushions of Fusarium graminearum are fungal arsenals for wheat infection. Mol. Plant Pathol. 2020, 21, 1070–1087. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2024, 53, gkae909. [Google Scholar]
- Pan, L.-J.; Lin, L.; Liu, Y.-P.; Wen, S.-X.; Zhang, Z.-Y. The M43 domain-containing metalloprotease RcMEP1 in Rhizoctonia cerealis is a pathogenicity factor during the fungus infection to wheat. J. Integr. Agric. 2020, 19, 2044–2055. [Google Scholar]
- Jashni, M.K.; Dols, I.H.; Iida, Y.; Boeren, S.; Beenen, H.G.; Mehrabi, R.; Collemare, J.; de Wit, P.J. Synergistic action of a metalloprotease and a serine protease from Fusarium oxysporum f. sp. lycopersici cleaves chitin-binding tomato chitinases, reduces their antifungal activity, and enhances fungal virulence. Mol. Plant-Microbe Interact. 2015, 28, 996–1008. [Google Scholar]
- Sanz-Martín, J.M.; Pacheco-Arjona, J.R.; Bello-Rico, V.; Vargas, W.A.; Monod, M.; Díaz-Mínguez, J.M.; Thon, M.R.; Sukno, S.A. A highly conserved metalloprotease effector enhances virulence in the maize anthracnose fungus Colletotrichum graminicola. Mol. Plant Pathol. 2016, 17, 1048–1062. [Google Scholar] [CrossRef]
- Guo, F.; Pan, L.; Liu, H.; Lv, L.; Chen, X.; Liu, Y.; Li, H.; Ye, W.; Zhang, Z. Whole-genome metalloproteases in the wheat sharp eyespot pathogen Rhizoctonia cerealis and a role in fungal virulence. Int. J. Mol. Sci. 2022, 23, 10691. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.A.; Collins-Racie, L.A.; Colbert, M.; Duckett, M.; Golden-Fleet, M.; Kelleher, K.; Kriz, R.; LaVallie, E.R.; Merberg, D.; Spaulding, V. A genetic selection for isolating cDNAs encoding secreted proteins. Gene 1997, 198, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tang, C.; Wang, X.; Sun, S.; Zhao, J.; Kang, Z.; Wang, X. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nat. Commun. 2019, 10, 5571. [Google Scholar] [PubMed]
- Zhang, J.; Wang, F.; Liang, F.; Zhang, Y.; Ma, L.; Wang, H.; Liu, D. Functional analysis of a pathogenesis-related thaumatin-like protein gene TaLr35PR5 from wheat induced by leaf rust fungus. BMC Plant Biol. 2018, 18, 76. [Google Scholar]
- Moradi, S.; Sanjarian, F.; Safaie, N.; Mousavi, A.; Bakhshi Khaniki, G.R. A modified method for transformation of Fusarium graminearum. J. Crop Prot. 2013, 2, 297–304. [Google Scholar]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef]
- Yu, J.H.; Hamari, Z.; Han, K.H.; Seo, J.A.; Reyes-Dominguez, Y.; Scazzocchio, C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004, 41, 973–981. [Google Scholar]
- Jia, L.-J.; Wang, W.-Q.; Tang, W.-H. Wheat coleoptile inoculation by Fusarium graminearum for large-scale phenotypic analysis. Bio-Protoc. 2017, 7, e2439. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Sun, Y.; Liu, B.; Wang, S.; Xuan, Y.; Gao, Z. The secreted ribonuclease SRE1 contributes to Setosphaeria turcica virulence and activates plant immunity. Front. Microbiol. 2022, 13, 941991. [Google Scholar]
- Zhu, W.; Dong, H.; Xu, R.; You, J.; Yan, D.z.; Xiong, C.; Wu, J.; Bi, K. Botrytis cinerea BcCDI1 protein triggers both plant cell death and immune response. Front. Plant Sci. 2023, 14, 1136463. [Google Scholar]
- Hogenhout, S.A.; Van der Hoorn, R.A.; Terauchi, R.; Kamoun, S. Emerging concepts in effector biology of plant-associated organisms. Mol. Plant-Microbe Interact. 2009, 22, 115–122. [Google Scholar] [PubMed]
- Qian, H.; Wang, L.; Wang, B.; Liang, W. The secreted ribonuclease T2 protein FoRnt2 contributes to Fusarium oxysporum virulence. Mol. Plant Pathol. 2022, 23, 1346–1360. [Google Scholar] [PubMed]
- Ma, Z.; Song, T.; Zhu, L.; Ye, W.; Wang, Y.; Shao, Y.; Dong, S.; Zhang, Z.; Dou, D.; Zheng, X.; et al. A Phytophthora sojae Glycoside Hydrolase 12 Protein Is a Major Virulence Factor during Soybean Infection and Is Recognized as a PAMP. Plant Cell 2015, 27, 2057–2072. [Google Scholar]
- Paper, J.M.; Scott-Craig, J.S.; Adhikari, N.D.; Cuomo, C.A.; Walton, J.D. Comparative proteomics of extracellular proteins in vitro and in planta from the pathogenic fungus Fusarium graminearum. Proteomics 2007, 7, 3171–3183. [Google Scholar]
- Heese, A.; Hann, D.R.; Gimenez-Ibanez, S.; Jones, A.M.; He, K.; Li, J.; Schroeder, J.I.; Peck, S.C.; Rathjen, J.P. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 2007, 104, 12217–12222. [Google Scholar]
- Liebrand, T.W.; van den Berg, G.C.; Zhang, Z.; Smit, P.; Cordewener, J.H.; America, A.H.; Sklenar, J.; Jones, A.M.; Tameling, W.I.; Robatzek, S. Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA 2013, 110, 10010–10015. [Google Scholar]
- Gust, A.A.; Felix, G. Receptor like proteins associate with SOBIR1-type of adaptors to form bimolecular receptor kinases. Curr. Opin. Plant Biol. 2014, 21, 104–111. [Google Scholar]
- Sun, X.; Lapin, D.; Feehan, J.M.; Stolze, S.C.; Kramer, K.; Dongus, J.A.; Rzemieniewski, J.; Blanvillain-Baufumé, S.; Harzen, A.; Bautor, J. Pathogen effector recognition-dependent association of NRG1 with EDS1 and SAG101 in TNL receptor immunity. Nat. Commun. 2021, 12, 3335. [Google Scholar]
- Yang, C.; Liu, R.; Pang, J.; Ren, B.; Zhou, H.; Wang, G.; Wang, E.; Liu, J. Poaceae-specific cell wall-derived oligosaccharides activate plant immunity via OsCERK1 during Magnaporthe oryzae infection in rice. Nat. Commun. 2021, 12, 2178. [Google Scholar]
- Wang, Y.; Xu, Y.; Sun, Y.; Wang, H.; Qi, J.; Wan, B.; Ye, W.; Lin, Y.; Shao, Y.; Dong, S.; et al. Leucine-rich repeat receptor-like gene screen reveals that Nicotiana RXEG1 regulates glycoside hydrolase 12 MAMP detection. Nat. Commun. 2018, 9, 594. [Google Scholar]
- Kettles, G.J.; Bayon, C.; Sparks, C.A.; Canning, G.; Kanyuka, K.; Rudd, J.J. Characterization of an antimicrobial and phytotoxic ribonuclease secreted by the fungal wheat pathogen Zymoseptoria tritici. New Phytol. 2018, 217, 320–331. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhao, R.; Fang, Y.; Fan, Y.; Hu, Q.; Huang, W.; Ma, W.; Zhang, C. Proteomic Analysis of the Fusarium graminearum Secretory Proteins in Wheat Apoplast Reveals a Cell-Death-Inducing M43 Peptidase. J. Fungi 2025, 11, 240. https://doi.org/10.3390/jof11040240
Li P, Zhao R, Fang Y, Fan Y, Hu Q, Huang W, Ma W, Zhang C. Proteomic Analysis of the Fusarium graminearum Secretory Proteins in Wheat Apoplast Reveals a Cell-Death-Inducing M43 Peptidase. Journal of Fungi. 2025; 11(4):240. https://doi.org/10.3390/jof11040240
Chicago/Turabian StyleLi, Pengfeng, Ruihua Zhao, Ying Fang, Yujin Fan, Qianyong Hu, Wei Huang, Wujun Ma, and Cuijun Zhang. 2025. "Proteomic Analysis of the Fusarium graminearum Secretory Proteins in Wheat Apoplast Reveals a Cell-Death-Inducing M43 Peptidase" Journal of Fungi 11, no. 4: 240. https://doi.org/10.3390/jof11040240
APA StyleLi, P., Zhao, R., Fang, Y., Fan, Y., Hu, Q., Huang, W., Ma, W., & Zhang, C. (2025). Proteomic Analysis of the Fusarium graminearum Secretory Proteins in Wheat Apoplast Reveals a Cell-Death-Inducing M43 Peptidase. Journal of Fungi, 11(4), 240. https://doi.org/10.3390/jof11040240






