Abstract
Epidithiodiketopiperazines (ETPs) are a class of fungal secondary metabolites (SMs) featuring a transannular disulfide bridge at the diketopiperazine (DKP) core. The complex structures and biological activities have attracted widespread attention from biologists and chemists. In this study, we identified five novel ETP derivatives 4–7 and 4′ from three gene deletion mutants of Trichoderma hypoxylon CGMCC 3.17906, including ΔtdaP, ΔtdaQ, and ΔtdaQΔtdaI. Their structures were characterized through NMR and HR-ESI-MS data analysis. Compounds 4 and 4′ have unique heteroatom substitutions at the α and α′ positions, compound 5 possesses a unique α, β′-disulfide bridge, and compounds 6 and 7 contain a C3′-(thio)carbonyl group. Based on structural elucidation and biosynthetic pathway of α, β′-disulfide bridged ETPs, we also proposed the formation of 4–7 and 4′. This study not only expands the chemical diversity of ETPs, but also offers new mechanistic insights into the biosynthetic pathways of fungal ETPs.
1. Introduction
Epidithiodiketopiperazines (ETPs) are a unique class of fungal secondary metabolites (SMs) featuring a diketopiperazine (DKP) core and a distinctive transannular disulfide bridge [1,2,3,4,5]. These compounds are renowned for their structural diversity and potent biological activities, such as antitumor, antimicrobial, and immunosuppressive properties [6,7,8,9]. Based on the positions of sulfur atoms attached to 2,5-DKP, ETPs can be divided into two subfamilies, i.e., α, α′-disulfide (C2, C2′) and α, β′-disulfide (C2, C3′) bridged subtypes. Hundreds of α, α′-disulfide bridged ETPs have been reported in previous studies, such as gliotoxin, acetylaranotin, sirodesmin PL, chaetocin, and chetoseminudin A [10,11,12,13,14,15]. In contrast, only a few ETPs feature an irregular di(poly)sulfide that is anchored at the α and β′ positions of the DKP core, such as pretrichodermamides, aspirochlorine, lasiodipline D, and phaeosphaone D [16,17,18,19,20]. In addition to the transannular α, β′-disulfide (ring F), compounds of pretrichodermamide family share a complex heterocyclic peptide framework characterized by a 6/6/6/(5)/6/7 ring system (rings A–F) (Figure 1A) [16]. The unique structural properties confer pretrichodermamides important biological activities. Previous studies have shown that pretrichodermamides play an important role in the agriculturally beneficial fungus Trichoderma to inhibit the growth of plant pathogens [9,21,22,23,24]. Moreover, the majority of pretrichodermamides have been extensively studied for their potent anticancer activities [6].
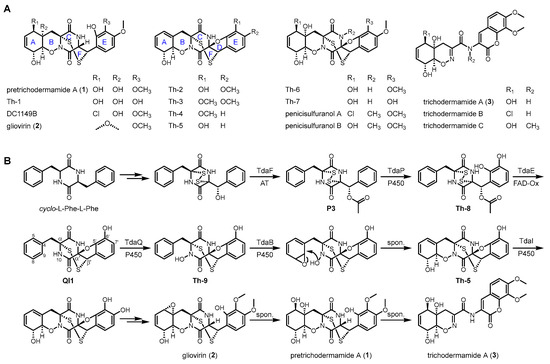
Figure 1.
Representative epidithiodiketopiperazine (ETP) derivatives identified in fungi (A) and the biosynthetic pathway of pretrichodermamide A (1) in Trichoderma hypoxylon (B).
The fungicolous fungus Trichoderma hypoxylon CGMCC 3.17906, which was isolated from the stroma of Hypoxylon anthochroum in Thailand, is one of the producers of pretrichodermamides [25]. In our previous studies, we have identified the prototype pretrichodermamide A (1), together with its precursor gliovirin (2) and the desulfurized derivative trichodermamide A (3) from T. hypoxylon and reported the complex biosynthesis by targeted deletions, feeding experiments, and biochemical investigations (Figure 1B and Figure S1) [16,26,27]. The formation of α, β′-disulfide requires the installation of α, α′-disulfide catalyzed by the flavin-containing enzymes TdaR, β′-acetoxy catalyzed by the acetyltransferase TdaF, and C5′, C6′-dihydroxy groups catalyzed by the cytochrome P450 monooxygenase (CYP) TdaP as prerequisites. Specifically, the flavin-containing enzyme TdaE harboring a noncanonical CXXQ motif plays a key role in the conversion of the α, α′- into the α, β′-disulfide via a reactive ortho-quinone methide intermediate [27]. At the late stage of α, β′-disulfide formation, the formation of 1–3 requires seven tailoring enzymes for the sequential 1,2-oxazine construction, phenyl modifications, cyclohexadiene oxidation, and furan opening (Figure 1B) [16].
More interestingly, the biosynthetic elucidation of 1 uncovered a highly flexible catalytic machinery of multiple enzymes, which enables the generation of even greater structural diversity than earlier studies (Figure 1A and Figure S2). Collectively, a total number of 47 structures containing 39 shunt products were identified in the single and double gene deletion mutants. However, considering the remarkable catalytic flexibility of Tda enzymes, it is plausible that more diverse products could potentially be generated in these gene deletion mutants. In this study, we optimized the cultivation conditions of three gene deletion mutants, including ΔtdaP, ΔtdaQ, and ΔtdaQΔtdaI, and identified five novel ETP derivatives (4–7 and 4′). We also proposed the formation of 4–7 and 4′ based on the biosynthetic pathway of pretrichodermamides. Our results expand the structural diversity of fungal ETPs and provide further insights into the complex biosynthetic pathways of α, β′-disulfide bridged ETPs.
2. Materials and Methods
2.1. Strain, Media, and Culture Conditions
T. hypoxylon was isolated from the stroma of H. anthochroum in Thailand and deposited in the China General Microbiological Culture Collection Center (CGMCC 3.17906, Beijing, China) [25]. The gene deletion mutants of T. hypoxylon used in this study, including Δtri5, ΔtdaPΔtri5, ΔtdaQΔtri5, Δtri5Δthlig4, and ΔtdaQΔtdaIΔtri5Δthlig4 mutants, designated as TYHL26, TYHL41, TYPL43, TYYH3, and TYPL52, respectively, are summarized in Table S1. All strains were cultivated at 26 °C on potato dextrose agar (PDA, BD Difco™, 39 g/L) for growth, and on rice medium for detection and isolation of differential SMs.
2.2. Large-Scale Fermentation, Extraction, and Isolation of Differential Compounds
To isolate 4 and 4′, ΔtdaPΔtri5 mutant TYHL41 was cultivated in a 200 × 500-mL flask containing 100 g rice and 150 mL H2O at 26 °C for 11 days. The rice cultures were extracted with 4 L ethyl acetate (EtOAc) three times and concentrated under reduced pressure to give a crude extract (29.3 g), which was dissolved in 300 mL acetonitrile (ACN)/H2O (9:1, v/v) and extracted with an equal volume of n-hexane to remove oily constituents to afford the sub-crude extract (16.5 g). The sub-crude extract was subjected to silica gel column chromatography (CC) (200–300 mesh) and eluted with a stepwise gradient of petroleum ether (PE)/EtOAc (1:0, 6:1, 5:1, 4:1, 2:1, 1:1, 1:2, and 0:1, v/v) to yield 44 fractions (1–44). Fractions 37–41 were then eluted with 1.8 L PE/EtOAc (1:2, v/v). Further separation and purification of fraction 38 was carried out by semipreparative HPLC with ACN/H2O (45:55, v/v) at a flow rate of 2.5 mL/min to yield the mixture of 4 and 4′ (2.0 mg).
To isolate 5, ΔtdaQΔtri5 mutant TYPL43 was cultivated in a 200 × 500-mL flask containing 50 g rice and 75 mL H2O at 26 °C for 11 days. The rice cultures were extracted with 4 L EtOAc three times and concentrated under reduced pressure to give a crude extract (30.0 g), which was dissolved in 400 mL ACN/H2O (9:1, v/v) and extracted with an equal volume of n-hexane to remove oily constituents to afford the sub-crude extract (17.2 g). The sub-crude extract was subjected to silica gel column chromatography (CC) (200–300 mesh) and eluted with a stepwise gradient of PE/EtOAc (1:0, 3:1, 2:1, 1:1, 1:2, 1:3, and 0:1, v/v) to yield 55 fractions (1–55). Fractions 20–36 were then eluted with 3.5 L PE/EtOAc (1:1, v/v). Further separation and purification of fraction 31 was carried out by semipreparative HPLC with ACN/H2O (25:75, v/v) at a flow rate of 2.5 mL/min to yield 5 (1.7 mg).
To isolate 6 and 7, ΔtdaQΔtdaIΔtri5Δthlig4 mutant TYPL52 was cultivated in a 200 × 500-mL flask containing 50 g rice and 75 mL H2O at 26 °C for 9 days. The rice cultures were extracted with 4 L EtOAc three times and concentrated under reduced pressure to give a crude extract (25.5 g), which was dissolved in 200 mL ACN/H2O (9:1, v/v) and extracted with an equal volume of n-hexane to remove oily constituents to afford the sub-crude extract (13.0 g). The sub-crude extract was subjected to silica gel column chromatography (CC) (200–300 mesh) and eluted with a stepwise gradient of PE/EtOAc (1:0, 5:1, 3:1, 2:1, 1:1, 1:2, and 0:1, v/v) to yield 32 fractions (1–32). Fractions 20–25 were then eluted with 2 L PE/EtOAc (1:1, v/v). Further separation and purification of fraction 21 was carried out by semipreparative HPLC with ACN/H2O (40:60, v/v) at a flow rate of 2.5 mL/min to yield 6 (1.0 mg). Similarly, fraction 22 was processed through semipreparative HPLC under identical conditions, resulting in the isolation of compound 7 (1.5 mg).
2.3. HPLC and LC-MS Analysis
Semipreparative purification on HPLC was performed on an SSI HPLC system (Teledyne SSI Lab Alliance Series III pump system and Series 1500 Photodiode Array Detector, Torrance, CA, USA) with an Eclipse XDB-C18 column (250 × 9.4 mm, 5 μm, Aligent, Santa Clara, CA, USA) at a flow rate of 2.5 mL/min. UV absorptions at 230 nm, 254 nm, and 340 nm are illustrated.
LC-MS analysis was performed on an Agilent HPLC 1200 series system equipped with a single quadrupole mass selective detector and an Agilent 1100LC MSD model G1946D mass spectrometer by using a Venusil XBP C18 column (3.0 by 50 mm, 3 μm, Bonna-Agela Technologies, Tianjin, China). Water (A) with 0.1% (v/v) formic acid and ACN (B) were used as the solvents at a flow rate of 0.5 mL/min. The substances were eluted with a linear gradient from 5 to 100% B in 30 min, then washed with 100% (v/v) solvent B for 5 min, and equilibrated with 5% (v/v) solvent B for 10 min. The mass spectrometer was set in electrospray positive ion mode for ionization. HR-ESI-MS data were recorded on an Agilent Technologies 6520 Accurate-Mass Q-TOF LC/MS spectrometer equipped with an electrospray ionization (ESI) source. The HR-ESI-MS data of compounds analyzed in this study are available at the GNPS website (https://massive.ucsd.edu, accessed on 7 March 2025) under MassIVE ID number MSV000091394.
2.4. NMR Analysis
NMR spectra were recorded on a Bruker Avance-500 MHz spectrometer at room temperature (Bruker Corporation, Karlsruhe, Germany). All spectra were processed with MestReNova 12.0.3 (Metrelab). Chemical shifts are referenced to those of the solvent signals.
3. Results
3.1. Analysis of Differential Compounds in the Gene Deletion Mutants of T. hypoxylon
In previous studies, we have constructed single and double gene deletion mutants of tda genes to identify the biosynthetic intermediates and elucidate the biosynthesis of α, β′-disulfide bridged ETPs (Figure 1B) [16,26,27]. Single deletions of four tda genes, which encode for CYP TdaP, acyltransferase TdaF, and flavin-containing enzymes TdaR and TdaE, respectively, led to the identification of 20 novel ETP derivatives formed before the formation of the α, β′-disulfide [27]. Deletion of the CYP-encoding gene tdaP completely abolished the formation of 1–3 and led to the accumulation of P1–P5, P1′, P2′, and P5′ (Figure 2). P1 and P1′ are a pair of isomers characterized by thiomethyl groups at both α and α′ positions, as well as a β′-acetoxy group. Upon demethylthiolation, P1 and P1′ are transformed into another pair of isomers, namely P2 and P2′. P3 was deduced as the precursor of TdaP in the biosynthetic pathway of 1–3 carrying α, α′-disulfide and β′-acetoxy group. P4 was identified in ΔtdaR mutant and can be converted to P5 and P5′ via the spontaneous retro-aldol reaction. At the late stage of α, β′-disulfide formation, two CYPs TdaQ and TdaB collaboratively catalyze the construction of 1,2-oxazine. Deletion of tdaQ eliminated the hydroxylation at N10, thereby disrupting the construction of 1, 2-oxazine [16]. Three shunt products Q1–Q3 were isolated from ΔtdaQ mutant and identified as α, β′-disulfide-containing derivatives. Among these compounds, Q2 is distinguished from Q1 by the presence of a methoxy group at the C7′ position, while Q3 differs from Q2 through hydroxylation at the C9 position. Furthermore, double gene deletion of tdaQ and tdaI led to the accumulation and subsequent identification of QI1, which serves as the biosynthetic precursor for Q1–Q3 (Figure 2).
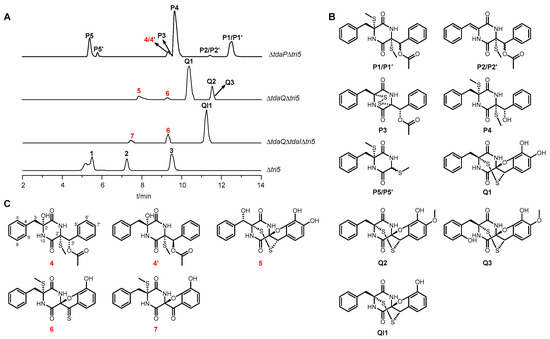
Figure 2.
Differential compounds identified from single gene deletion mutants of tdaP and tdaQ and double gene deletion mutants of tdaQ and tdaI in the background of Trichoderma hypoxylon Δtri5, which was previously constructed for the elimination of the predominant secondary metabolites (SMs), trichothecenes of wild type (WT) [28]. (A) LC-MS analysis of the crude extracts of ΔtdaPΔtri5, ΔtdaQΔtri5, ΔtdaQΔtdaIΔtri5, and Δtri5 mutants. (B) Twelve ETP derivatives identified from the three mutants in our previous studies [16,27]. (C) Five novel ETP derivatives, including 4–7 and 4′, identified from the three mutants in this study. 1–3 are the final ETP products of tda gene cluster. EICs refer to [M+H]+ ions of 1–3, together with [M+Na]+ ions of 4–7, 4′, P1–P5, P1′, P2′, P5′, Q1–Q3, and QI1 with tolerance ranges of ±0.25. The new compounds identified in this study are indicated in red.
To identify more differential SMs in tda gene deletion mutants, we optimized the cultivation conditions of ΔtdaP, ΔtdaQ, and ΔtdaQΔtdaI in this study. These three strains were cultivated on rice media at 26 °C for 11, 11, and 9 days, respectively. Subsequently, we extracted the cultures with EtOAc and concentrated them under reduced pressure to give the crude extracts. LC-MS analysis of the culture supernatants revealed not only the previously identified compounds but also several novel differential product peaks (Figure 2A). With the exception of P1–P5 and P1′–P3′, cultivation of ΔtdaP mutant resulted in the accumulation of a novel product peak 4 eluting at 9.1 min with an [M+Na]+ ion at m/z 437.1 ± 0.25. Cultivation of ΔtdaQ mutant resulted in the accumulation of the previously identified Q1–Q3 and two novel product peaks, i.e., 5 eluting at 7.9 min with an [M+Na]+ ion at m/z 441.0 ± 0.25 and 6 eluting at 9.2 min with an [M+Na]+ ion at m/z 423.0 ± 0.25. Double gene deletion mutant of tdaQ and tdaI also resulted in the accumulation of the product peak of 6, together with another product peak 7 eluting at 7.5 min with an [M+Na]+ ion at m/z 407.1 ± 0.25. Then, we performed targeted isolation using a combination of normal-phase silica gel chromatography, and semi-preparative HPLC, leading to the identification of five novel ETP derivatives, i.e., 4–7 and 4′.
3.2. Characterization of Five Novel ETP Derivatives from Gene Deletion Mutants of T. hypoxylon
The white powder compound was isolated from the gene deletion mutant of tdaP, and its molecular formula, C21H22N2O5S, was deduced from HR-ESI-MS analysis showing an [M+Na]+ ion at m/z 437.1149 (calcd. 437.1142), indicating twelve unsaturation degrees (Table S2). The 1H and 13C NMR spectroscopic analysis revealed that the sample contained a mixture of compounds, suggesting the presence of a pair of isomers (4 and 4′) (Table 1 and Figure S3). Three carbonyl carbons (δC 167.1, 165.3, and 168.7), two imines (δH 9.14 and 7.81), and two phenyl moieties were detected in compound 4, suggesting the structural framework as a cyclo-l-Phe-l-Phe (cFF). The attachment of a benzyl moiety to C2 was determined by the HMBC correlations from H2-3 to C1, C2, C4, C5, and C9, and 1H-1H COSY correlations of H-6 with H-5 and H-7, and H-8 with H-7 and H-9, as well as the chemical shifts of C4 (δC 135.4), C5 (δC 130.8), C6 (δC 128.3), C7 (δC 128.7), C8 (δC 128.3), and C9 (δC 130.8) (Figure 3). The attachment of phenyl moiety to C3′ was determined by the HMBC correlations from H-3′ to C4′, C5′, and C9′, and 1H-1H COSY correlations of H-6′ with H-5′ and H-7′, and H-8′ with H-7′ and H-9′, as well as the chemical shifts of C4′ (δC 134.1), C5′ (δC 128.0), C6′ (δC 127.9), C7′ (δC 126.7), C8′ (δC 127.9), and C-9′ (δC 128.0). Furthermore, the HMBC correlations from H-3′ and H3-12′ to C11′, respectively, together with the downfield chemical shift of C3′ (δC 77.2) assigned the carbonyl group at C11 and indicated the connection between C3′ and C12 via an ester bond. The thiomethyl group attached to C2′ was confirmed by the HMBC correlations from H3-SCH3 (δH 0.86) to C2′ (δC 71.2). These results indicated that compound 4 carries one thiomethyl group at C2′ and an acetoxy group at C3′, which are identical with those in the previously identified compounds P1 and P1′ [27]. However, compound 4 differs from P1/P1′ by the presence of a hydroxy group (δH 6.74) at C2 (δC 82.6). As a result, comprehensive NMR analysis indicated compound 4 as a novel derivative from cFF, which carries a hydroxy group at C2, a thiomethyl group at C2′, and an acetoxy group at C3′. Like P1 and P1′, compounds 4 and 4′ are a pair of isomers bearing different configurations of β′-acetoxy groups.

Table 1.
NMR data of 4, 4′, and 5 in DMSO-d6 (500 MHz for 1H NMR and 125 MHz for 13C NMR).
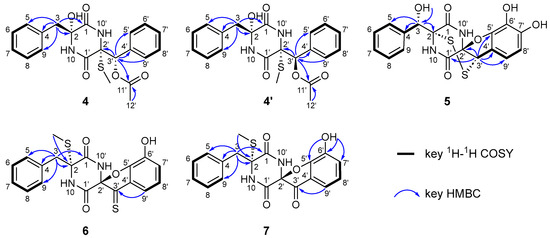
Figure 3.
Key 1H-1H COSY and HMBC correlations of compounds 4–7 and 4′.
Compound 5, obtained as a white powder, was isolated from a gene deletion mutant of tdaQ and assigned the molecular formula C18H14N2O6S2 based on HR-ESI-MS analysis showing an [M+Na]+ ion at m/z 441.0188 (calcd. 441.0185), corresponding to 13 unsaturation degrees (Table S2). Comprehensive NMR analysis revealed that compound 5 was an ETP derivative from cFF, displaying characteristic DKP signals such as two carbonyl carbons (δC 167.3 and 165.4) and two imine protons (δH 9.48 and 9.34) (Table 1 and Figure S4). Similar to the previously identified Q1–Q3, compound 5 bears transannular disulfide bridge attached to C2 (δC 74.6) and C3′ (δC 54.4) positions, together with the spirofuran, which can be determined by the carbon signals of C2′ (δC 96.3) and C5′ (δC 146.3) [16]. However, only one singlet proton signal (δH 5.11) was observed being attached to the C3 position (δC 71.6) and showed clear correlations to C2 (δC 74.6), C4 (139.6), and C9 (δC 128.9) in 2D NMR spectra (Figure 3). The hydroxy group at C3 was then confirmed by the HMBC correlation from δH 6.19 to δC 71.6. In addition, comparative analysis with the NMR data of Q1 suggested the presence of two additional hydroxy groups at C6′ (δC 129.7) and C7′ (δC 148.3). Based on these findings, compound 5 was identified as a novel ETP derivative featuring an α, β′-disulfide bridge, a spirofuran moiety, and dihydroxy substitutions at C3 and C7′.
Compounds 6 and 7 were isolated from the double gene deletion mutant of tdaQ and tdaI. Compound 6, obtained as a yellow powder, was assigned the molecular formula C19H16N2O4S2 based on HR-ESI-MS analysis showing an [M+Na]+ ion at m/z 423.0453 (calcd. 423.0444), corresponding to 13 unsaturation degrees (Table S2). Similar to 4, 4′, and 5, compound 6 is also derived from the cFF skeleton showing the characteristic DKP signals such as two carbonyl carbons (δC 165.8 and 160.2) and two imine protons (δH 9.54 and 9.49) (Table 2 and Figure S5). Comparative NMR analysis between compound 6 and the previously identified QI1 revealed distinct structural features: (1) the absence of an α, β′-disulfide bridge, (2) the presence of a spirofuran ring, and (3) a thiomethyl group at C2, as evidenced by HMBC correlations from δH 2.3 to δC 67.6 and 1H-1H COSY correlations of δH 2.3 with δH 13.5 (Figure 3) [27]. The C3-methylene protons (δH 3.04 and 3.50) showed HMBC correlations to C1 (δC 165.8), C2 (δC 67.6), C4 (δC 134.3), C5 (δC 130.4), and C9 (δC 130.4). Interestingly, the 13C NMR spectrum of compound 6 displayed a characteristic thiocarbonyl signal at δC 227.5. Careful analysis of the HMBC spectrum allowed us to assign the C3′-thiocarbonyl group through a clear correlation from H-9′ (δH 7.08) of the phenyl moiety to C3′ (δC 227.5). Based on these findings, compound 6 was identified as a novel cFF derivative featuring a C2-thiomethyl group, a C3′-thiocarbonyl group, and a spirofuran ring system.

Table 2.
NMR data of 6 and 7 in DMSO-d6 (500 MHz for 1H NMR and 125 MHz for 13C NMR).
Compound 7, obtained as a yellow powder, was assigned the molecular formula C19H16N2O5S based on HR-ESI-MS analysis showing an [M+Na]+ ion at m/z 407.0674 (calcd. 407.0672), corresponding to 13 unsaturation degrees (Table S2). The 1H and 13C NMR data of 7 were very similar to those of 6, with the exception of the presence of a carbonyl group at C3′ (δC 194.9) instead of the thiocarbonyl group in 6 (δC 227.5) (Table 2 and Figure S6). HMBC analysis showed a clear correlation from H-9′ (δH 7.03) of the phenyl moiety to C3′-carbonyl group (δC 194.9) (Figure 3). Thus, compound 7 was identified as a novel cFF derivative featuring a C2-thiomethyl group, a C3′-carbonyl group, and a spirofuran ring system.
3.3. Plausible Biosynthetic Pathway of Compounds 4–7 and 4′
As previously reported, we have elucidated the biosynthetic pathway of 1–3 for understanding the formation of α, β′-disulfide bridged ETPs [16,27]. The nonribosomal peptide synthetase (NRPS) TdaA catalyzes the coupling of two units of l-Phe to afford cFF, which is further oxidized at α, α′, and β′ positions by the CYP TdaS (Scheme 1). In analogy to the biosynthesis of gliotoxin, a four-enzyme cascade by TdaL, TdaV, TdaJ, and TdaT is responsible for GSH addition and the subsequent degradation [2,29,30,31,32,33]. Then, TdaR, TdaF, and TdaP catalyze the installation of α, α′-disulfide, β′-acetoxy, and C5′, C6′-dihydroxy groups, respectively. TdaE harboring the noncanonical CMYQ motif further catalyzes the migration of α, α′-disulfide to give QI1 carrying both α, β′-disulfide and spirofuran. At the late stage of α, β′-disulfide formation, two CYPs TdaB and TdaQ are responsible for C8, C9-epoxidation and N-hydroxylation, respectively, facilitating spontaneous cyclization to yield 1,2-oxazine. Subsequently, the CYP TdaI and methyltransferases TdaO and TdaH catalyze the formation of C6′, C7′-methoxy groups at the phenyl moiety. Further oxidation by the CYP TdaG and reduction by TdaD complete the biosynthesis of 1.
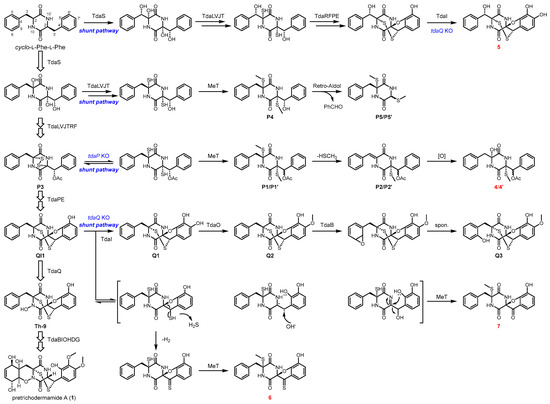
Scheme 1.
Plausible formation of compounds 4–7 and 4′ in Trichoderma hypoxylon. The new compounds identified in this study are indicated in red.
Considering the characterized functions of Tda enzymes, we proposed the formation of 4–7 and 4′ in this study (Scheme 1). Compound P3 that accumulates in the ΔtdaP mutant can be converted into the α, α‘-dithiol adduct through reduction. This adduct would be methylated to produce P1/P1′, which is further reduced to yield P2/P2′. Then, another oxygenase catalyzes the hydroxylation at the C2 position to afford 4/4′. Compound 5, which was identified in ΔtdaQ mutant, differs from Q1 at the C3-hydroxy group. We speculate that the CYP TdaS would also catalyze the high oxidation of cFF at α, α′, β, and β′ positions, which is similar to the catalysis of AclC in aspirochlorine biosynthesis [5,34,35]. In the single gene deletion mutant of tdaQ and double gene deletion mutant of tdaQ and tdaI, QI1 can be spontaneously transformed into the α, α′-dithiol intermediate, facilitating the electron transfer and hydrogen sulfide elimination, ultimately leading to the generation of a carbonyl group in compound 7. The α, α′-dithiol intermediate can also be alternatively reduced to give the thiocarbonyl group in compound 6.
4. Conclusions
Taken together, through comprehensive analysis and systematic isolation of three tda gene deletion mutants, including ΔtdaP, ΔtdaQ, and ΔtdaQΔtdaI, we successfully identified five novel ETP derivatives, designated as 4–7 and 4′. Compounds 4 and 4′ carrying α-hydroxyl, α′-thiomethyl, and β′-acetoxy groups are a pair of isomers with different configurations at β′ position. The structures of 4 and 4′ are similar with the previously reported pseuboydone C, which harbors α-hydroxyl and α′-thiomethyl groups and displays significant cytotoxicity against the Sf9 cells from the fall armyworm Spodoptera frugiperda [36,37]. Compound 5 possesses the typical α, β′-disulfide fused with the spirofuran ring and dihydroxy substitutions at C3 and C7′. Compounds 6 and 7 are cFF derivatives featuring a C2-thiomethyl group, a C3′-thiocarbonyl or carbonyl group, and a spirofuran ring system. Based on detailed structural elucidation and biosynthetic pathway of 1–3 in T. hypoxylon, we also proposed the formation of 4–7 and 4′. Our findings significantly expand the known structural diversity of ETPs, particularly emphasizing new modifications at the C3 and C3′ positions of the cFF core. Furthermore, our results prove again the remarkable catalytic promiscuity of Tda enzymes and enhance our understanding of ETP biosynthesis in fungi. Combined with the previous studies, a vast number of ETPs and their derivatives have been identified in the agriculturally beneficial fungi Trichoderma spp. [9,20,26,38,39]. We speculate that such diverse compounds could be important for the antagonism of Trichoderma spp. towards other species. Further studies could focus on the specific roles of diverse ETPs on Trichoderma spp. and provide new insights for bioactive SMs in biocontrol applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof11040241/s1, Table S1: Strains used in this study; Table S2: HR-ESI-MS data of the compounds 4–7 and 4′; Figure S1: Tda gene cluster from Trichoderma hypoxylon; Figure S2: ETP derivatives previously isolated from T. hypoxylon strains; and Figures S3–S6: NMR spectra of 4–7 and 4′ in DMSO-d6. References [16,27,40] are cited in the supplementary materials.
Author Contributions
Conceptualization, W.-B.Y. and J.F.; methodology, W.-B.Y. and J.F.; validation, Z.R., Y.L., P.-L.W. and J.F.; formal analysis, Z.R., Y.L. and P.-L.W.; investigation, Z.R., Y.L. and S.Z.; data curation, Z.R., Y.L. and J.F.; writing—original draft preparation, Z.R. and Y.L.; writing—review and editing, D.W., J.F. and W.-B.Y.; visualization, J.F. and Z.R.; supervision, W.-B.Y.; project administration, J.F. and W.-B.Y.; funding acquisition, J.F. and W.-B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the National Natural Science Foundation of China [grant no. 32200046], Key Research Program of Frontier Sciences, CAS [grant no. ZDBS-LY-SM016], and the Chinese Academy of Sciences Project for Young Scientists in Basic Research [grant no. YSBR-111].
Institutional Review Board Statement
This study does not contain any studies with human or animal subjects.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Orlova, T.I.; Bulgakova, V.G.; Polin, A.N. Secondary metabolites from marine microorganisms. II. Marine fungi and their habitats. Antibiot. Khimioter. 2016, 61, 52–63. [Google Scholar] [PubMed]
- Fan, J.; Wei, P.-L.; Yin, W.-B. Formation of bridged disulfide in epidithiodioxopiperazines. ChemBioChem 2024, 25, e202300770. [Google Scholar] [CrossRef]
- Huber, E.M. Epipolythiodioxopiperazine-based natural products: Building blocks, biosynthesis and biological activities. ChemBioChem 2022, 23, e202200341. [Google Scholar] [CrossRef]
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic carbon–sulfur bond formation in natural product biosynthesis. Chem. Rev. 2017, 117, 5521–5577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, X.; Huang, X.; Wang, H.; Anjum, K.; Gu, Q.; Zhu, T.; Zhang, G.; Li, D. Irregularly bridged epipolythiodioxopiperazines and related analogues: Sources, structures, and biological activities. J. Nat. Prod. 2020, 83, 2045–2053. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Q.; Chen, S.; Wang, S.; Lu, J.; Gao, X.; Zhang, D.-D.; Jin, X. Natural epidithiodiketopiperazine alkaloids as potential anticancer agents: Recent mechanisms of action, structural modification, and synthetic strategies. Bioorg. Chem. 2023, 137, 106642. [Google Scholar] [CrossRef]
- Esteban, P.; Redrado, S.; Comas, L.; Domingo, M.P.; Millán-Lou, M.I.; Seral, C.; Algarate, S.; Lopez, C.; Rezusta, A.; Pardo, J.; et al. In vitro and in vivo antibacterial activity of gliotoxin alone and in combination with antibiotics against Staphylococcus aureus. Toxins 2021, 13, 85. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Zhang, X.; Yang, L.; Liu, J.; Wightman, S.M.; Lv, L.; Liu, Z.; Wang, C.Y.; Zhao, C. Identification of marine natural product Pretrichodermamide B as a STAT3 inhibitor for efficient anticancer therapy. Mar. Life Sci. Technol. 2023, 5, 94–101. [Google Scholar] [CrossRef]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrugge, M.; Muller, W.E.G.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2021, 35, 257–265. [Google Scholar] [CrossRef]
- Fox, E.M.; Howlett, B.J. Biosynthetic gene clusters for epipolythiodioxopiperazines in filamentous fungi. Mycol. Res. 2008, 112, 162–169. [Google Scholar] [CrossRef]
- Guo, C.-J.; Yeh, H.-H.; Chiang, Y.-M.; Sanchez, J.F.; Chang, S.-L.; Bruno, K.S.; Wang, C.C.C. Biosynthetic pathway for the epipolythiodioxopiperazine acetylaranotin in Aspergillus terreus revealed by genome-based deletion analysis. J. Am. Chem. Soc. 2013, 135, 7205–7213. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, R.; Ge, M.; Sun, Y.; Li, Y.; Shan, L. Gliotoxin, a natural product with ferroptosis inducing properties. Bioorg. Chem. 2023, 133, 106415. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.; Xiang, X.; Tang, Z.; Liu, K.; Su, Q.; Zhang, X.; Li, L. Chaetocin: A review of its anticancer potentials and mechanisms. Eur. J. Pharmacol. 2021, 910, 174459. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.A.; Bingol, E.; Qi, A.; Baker, D.; Ritchie, F.; Karandeni Dewage, C.S.; Fitt, B.D.L.; Huang, Y.J. Leptosphaeria biglobosa inhibits the production of sirodesmin PL by L. maculans. Pest Manag. Sci. 2024, 80, 2416–2425. [Google Scholar] [CrossRef]
- Xu, G.B.; He, G.; Bai, H.H.; Yang, T.; Zhang, G.L.; Wu, L.W.; Li, G.Y. Indole akaloids from Chaetomium globosum. J. Nat. Prod. 2015, 78, 1479–1485. [Google Scholar] [CrossRef]
- Fan, J.; Ran, H.; Wei, P.-L.; Li, Y.; Liu, H.; Li, S.-M.; Hu, Y.; Yin, W.-B. Pretrichodermamide A biosynthesis reveals the hidden diversity of epidithiodiketopiperazines. Angew. Chem. Int. Ed. 2023, 62, e202217212. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, N.; Mei, Y.N.; Chu, Y.L.; Ge, H.M.; Song, Y.C.; Ng, S.W.; Tan, R.X. An antibacterial metabolite from Lasiodiplodia pseudotheobromae F2. Phytochemistry 2014, 100, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mándi, A.; Li, X.-M.; Meng, L.-H.; Kurtán, T.; Wang, B.-G. Peniciadametizine A, a dithiodiketopiperazine with a unique spiro [furan-2, 7′-pyrazino [1, 2-b][1, 2] oxazine] skeleton, and a related analogue, peniciadametizine B, from the marine sponge-derived fungus Penicillium adametzioides. Mar. Drugs 2015, 13, 3640–3652. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; Huo, G.-M.; Zhang, Q.; Li, D.; Wang, D.-C.; Qi, J.-Z.; Han, W.-B.; Gao, J.-M. Phaeosphaones: Tyrosinase inhibitory thiodiketopiperazines from an endophytic Phaeosphaeria fuckelii. J. Nat. Prod. 2020, 83, 1592–1597. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takahashi, O.; Kirikoshi, R.; Yagi, A.; Ogasawara, T.; Bunya, Y.; Rotinsulu, H.; Uchida, R.; Namikoshi, M. Epipolythiodiketopiperazine and trichothecene derivatives from the NaI-containing fermentation of marine-derived Trichoderma cf. brevicompactum. J. Antibiot. 2020, 73, 559–567. [Google Scholar] [CrossRef]
- Howell, C.R.; Stipanovic, R.D. Gliovirin, a new antibiotic from Gliocladium virens, and its role in the biological control of Pythium ultimum. Can. J. Microbiol. 1983, 29, 321–324. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Sun, J.; Pei, Y.; Li, E.; Li, W.; Hyde, K.D.; Yin, W.-B.; Liu, X. A new species of Trichoderma hypoxylon harbours abundant secondary metabolites. Sci. Rep. 2016, 6, 37369. [Google Scholar] [CrossRef]
- Liu, H.; Fan, J.; Zhang, P.; Hu, Y.; Liu, X.; Li, S.-M.; Yin, W.-B. New insights into the disulfide bond formation enzymes in epidithiodiketopiperazine alkaloids. Chem. Sci. 2021, 12, 4132–4138. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ran, H.; Wei, P.-L.; Li, Y.; Liu, H.; Li, S.-M.; Yin, W.-B. An ortho-quinone methide mediates disulfide migration in the biosynthesis of epidithiodiketopiperazines. Angew. Chem. Int. Ed. 2023, 62, e202304252. [Google Scholar] [CrossRef]
- Liu, H.; Pu, Y.-H.; Ren, J.-W.; Li, E.-W.; Guo, L.-X.; Yin, W.-B. Genetic dereplication driven discovery of a tricinoloniol acid biosynthetic pathway in Trichoderma hypoxylon. Org. Biomol. Chem. 2020, 18, 5344–5348. [Google Scholar] [CrossRef]
- Scharf, D.H.; Chankhamjon, P.; Scherlach, K.; Heinekamp, T.; Roth, M.; Brakhage, A.A.; Hertweck, C. Epidithiol formation by an unprecedented twin carbon-sulfur lyase in the gliotoxin pathway. Angew. Chem. Int. Ed. 2012, 51, 10064–10068. [Google Scholar] [CrossRef]
- Scharf, D.H.; Chankhamjon, P.; Scherlach, K.; Heinekamp, T.; Willing, K.; Brakhage, A.A.; Hertweck, C. Epidithiodiketopiperazine biosynthesis: A four-enzyme cascade converts glutathione conjugates into transannular disulfide bridges. Angew. Chem. Int. Ed. 2013, 52, 11092–11095. [Google Scholar] [CrossRef]
- Scharf, D.H.; Remme, N.; Habel, A.; Chankhamjon, P.; Scherlach, K.; Heinekamp, T.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. A dedicated glutathione S-transferase mediates carbon-sulfur bond formation in gliotoxin biosynthesis. J. Am. Chem. Soc. 2011, 133, 12322–12325. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Kuttenlochner, W.; Scharf, D.H.; Brakhage, A.A.; Hertweck, C.; Groll, M.; Huber, E.M. Structural and mechanistic insights into C-S bond formation in gliotoxin. Angew. Chem. Int. Ed. 2021, 60, 14188–14194. [Google Scholar] [CrossRef]
- Marion, A.; Groll, M.; Scharf, D.H.; Scherlach, K.; Glaser, M.; Sievers, H.; Schuster, M.; Hertweck, C.; Brakhage, A.A.; Antes, I.; et al. Gliotoxin biosynthesis: Structure, mechanism, and metal promiscuity of carboxypeptidase GliJ. ACS Chem. Biol. 2017, 12, 1874–1882. [Google Scholar] [CrossRef]
- Tsunematsu, Y.; Maeda, N.; Sato, M.; Hara, K.; Hashimoto, H.; Watanabe, K.; Hertweck, C. Specialized flavoprotein promotes sulfur migration and spiroaminal formation in aspirochlorine biosynthesis. J. Am. Chem. Soc. 2021, 143, 206–213. [Google Scholar] [CrossRef]
- Tsunematsu, Y.; Maeda, N.; Yokoyama, M.; Chankhamjon, P.; Watanabe, K.; Scherlach, K.; Hertweck, C. Enzymatic amide tailoring expedites unusual Retro-Aldol-type amino acid conversion to form antifungal cyclopeptide. Angew. Chem. Int. Ed. 2018, 57, 14051–14054. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.-J.; Wang, K.-T.; Xu, M.-Y.; Zhang, J.-J.; Lam, C.-K.; Zhong, G.-H.; Xu, J.; Yang, D.-P.; Li, H.-J.; Wang, L.-Y. Secondary metabolites with chemical diversity from the marine-derived fungus Pseudallescheria boydii F19-1 and their cytotoxic activity. RSC Adv. 2016, 6, 76206–76213. [Google Scholar] [CrossRef]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and biological activities of diketopiperazines from marine organisms: A review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef]
- Sherkhane, P.D.; Bansal, R.; Banerjee, K.; Chatterjee, S.; Oulkar, D.; Jain, P.; Rosenfelder, L.; Elgavish, S.; Horwitz, B.A.; Mukherjee, P.K. Genomics-driven discovery of the gliovirin biosynthesis gene cluster in the plant beneficial fungus Trichoderma virens. ChemistrySelect 2017, 2, 3347–3352. [Google Scholar] [CrossRef]
- Tang, R.; Kimishima, A.; Ishida, R.; Setiawan, A.; Arai, M. Selective cytotoxicity of epidithiodiketopiperazine DC1149B, produced by marine-derived Trichoderma lixii on the cancer cells adapted to glucose starvation. J. Nat. Med. 2020, 74, 153–158. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Li, W.; Liu, X.; Li, E.; Yin, W.-B. A highly efficient genetic system for the identification of a harzianum B biosynthetic gene cluster in Trichoderma hypoxylon. Microbiology 2018, 164, 769–778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).