Inhibitory Effect and Mechanism of Hexanal on the Maturation of Peach-Shaped Phallus impudicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of Hexanal Concentration and Treatment Time
2.3. Transcriptome Analysis
2.3.1. RNA Extraction and Detection
2.3.2. Transcriptome Sequencing and Assembly
2.3.3. Screening and Bioinformatics Analysis of DEG
2.4. Lipidomic Analysis
2.4.1. Sample Preparation
2.4.2. LC-MS/MS Analysis
2.4.3. Quantitative Analysis of Lipids
2.5. Statistical Analysis
3. Results and Discussion
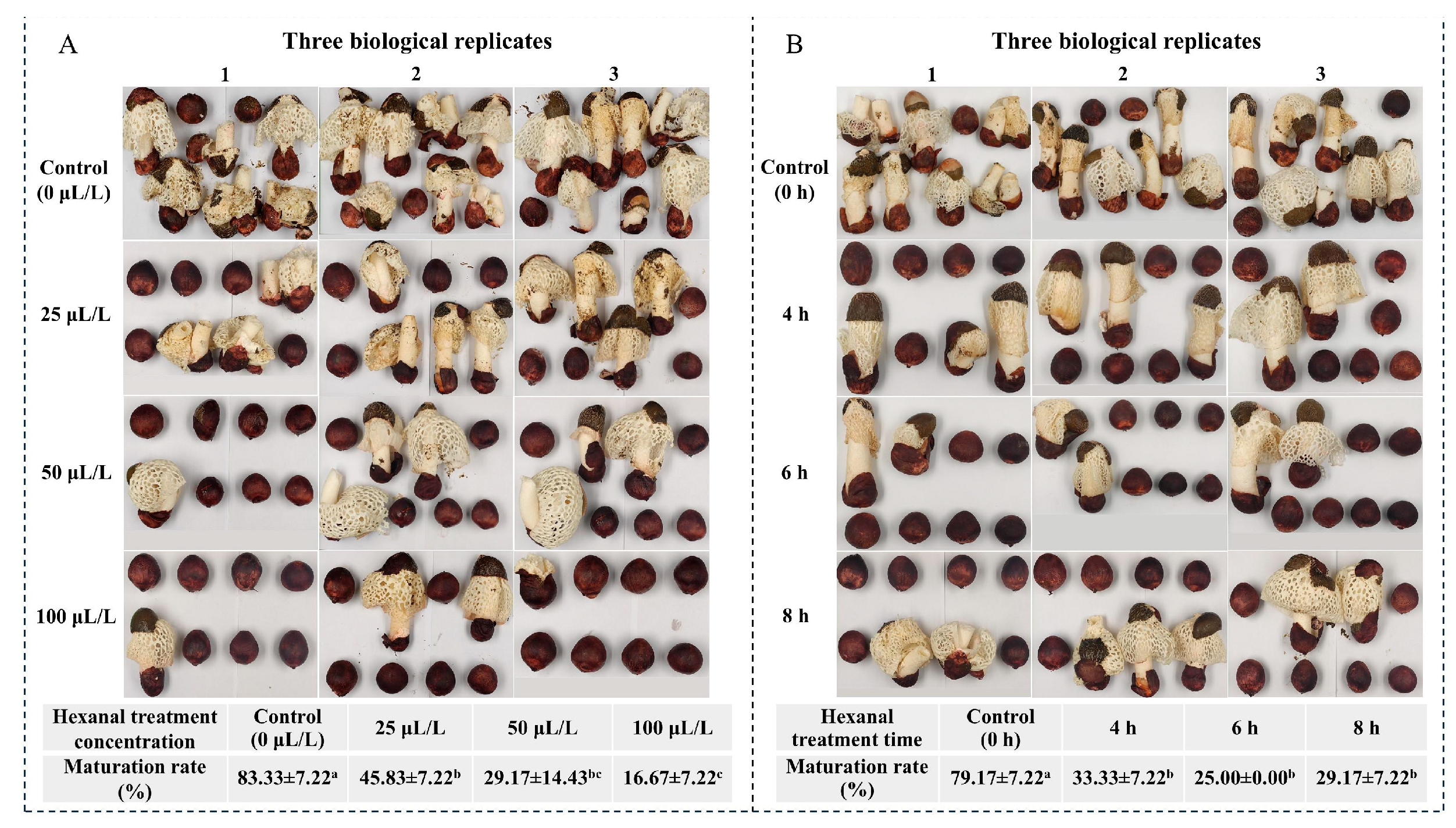
3.1. Effects of Hexanal Treatment on Maturation Rate of Peach-Shaped P. impudicus
3.2. Transcriptome Analysis of Hexanal Inhibiting Peach-Shaped P. impudicus Maturation
3.2.1. Sequencing Data and DEGs Analysis
3.2.2. KEGG Functional Enrichment Analysis of DEGs
3.2.3. Effects of Hexanal Treatment on Phosphatidylinositol Signaling System and MAPK Signaling Pathway
3.2.4. Effects of Hexanal Treatment on Cell Wall Remodeling
3.2.5. Effects of Hexanal Treatment on Antioxidant Enzymes
3.3. Lipidomic Analysis of Hexanal Inhibiting Peach-Shaped P. impudicus Maturation
3.3.1. Overview of the Lipidome
3.3.2. Abundance Changes of Lipid Categories and Differential Abundance of Lipids (DALs)
3.3.3. Effects of Hexanal Treatment on Phosphatidylinositol Signaling System at the Lipid Level
3.3.4. Effects of Hexanal Treatment on Cell Membranes of Stipes
- (1)
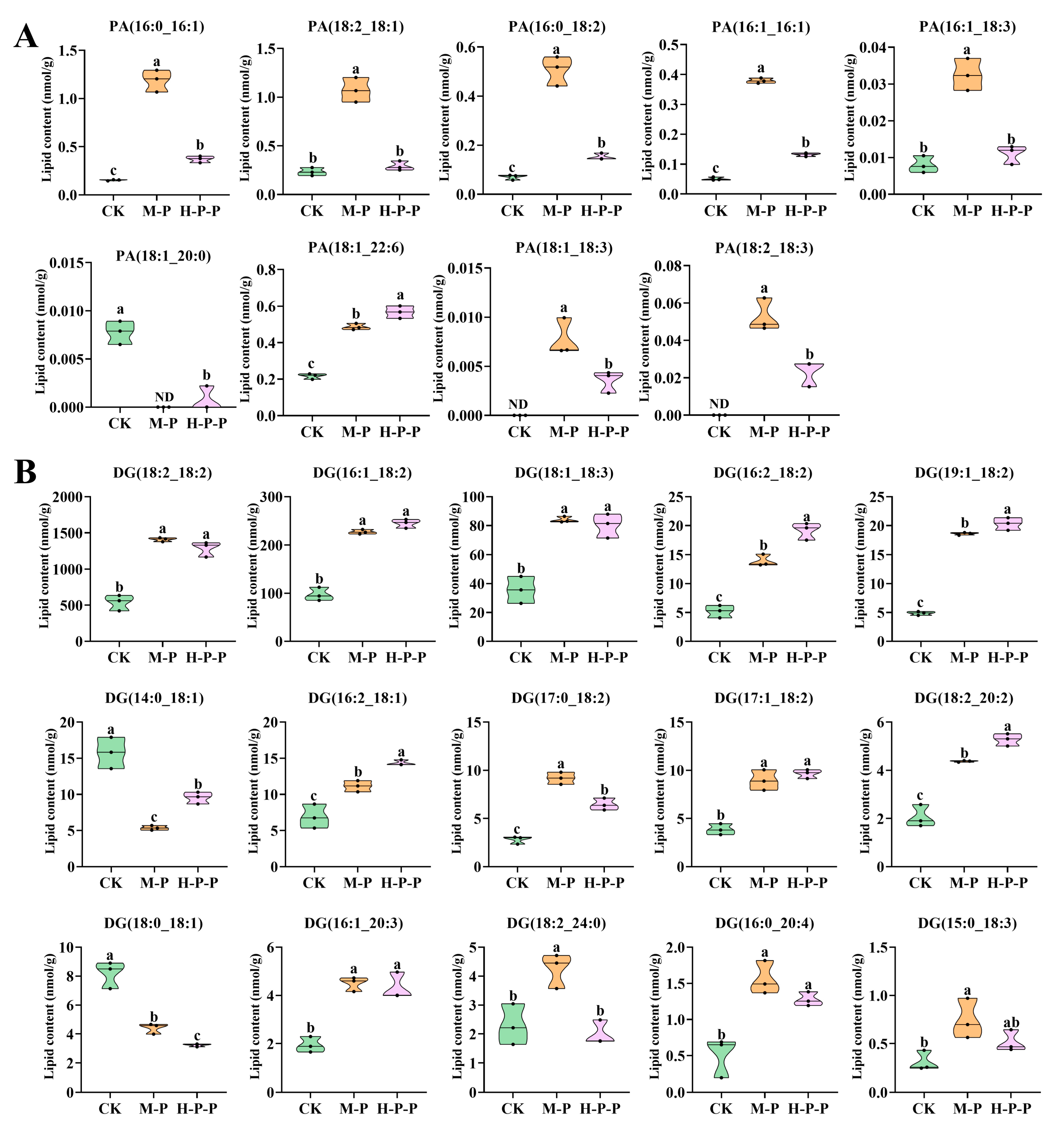
- Glycerolipids. Nine glycerolipids (DG, TG, MG, DGTS, DGDG, DGGA, SQDG, MGDG, and LDGTS) were detected, among which the total abundance of DG, MGDG, and LDGTS was significantly different among different groups (p < 0.05) (Figure 5A). A discussion on DG was presented in Section 3.3.3, but in this section, the focus is on MGDG and LDGTS. MGDG is a non-bilayer lipid, accounting for approximately 50% of the total lipid composition in the thylakoid membranes of higher plants and green algae [33,34]. MGDG abundance was significantly higher in CK than in M-P or H-P-P, with M-P that in particular being the lowest (p < 0.05). The representative molecules of MGDG also showed a similar change trend (Figure 7A). Consequently, the maturation process resulted in a reduction in MGDG abundance in P. impudicus. Notably, hexanal treatment impeded this effect. In particular, the abundance of LDGTS representative molecules, (LDGTS(18:2), LDGTS(16:0), LDGTS(18:1), LDGTS(18:3), LDGTS(16:1)), in H-P-P was significantly higher than that in CK or M-P (p < 0.05). Previous studies have demonstrated that LDGTS isolated from microalgae can improve blood high-density lipoprotein functions [35]. Therefore, further investigation into the functional activity of LDGTS in P. impudicus is required. Studies have reported that reducing the MGDG/DGDG ratio could confer upon plants the capacity for stress adaptation by enhancing the ability to maintain membrane structure in a bilayer conformation [36]. Similarly, this study found that the MGDG/DGDG ratios of M-P (76.2) and H-P-P (95.9) were lower than those of CK (224.4), which suggested that peach-shaped P. impudicus treated with hexanal could adapt to changes in external pressure.
- (2)
- Glycerophospholipids. In total, 13 glycerophospholipids (PE, PS, PC, PI, PG, PA, PMeOH, LPA, LPC, LPE, LPI, LPG, and LPS) were identified in CK, M-P and H-P-P. Among these, PE, PS, LPE, and PI exhibited the most pronounced differences between the various groups (Figure 5A). PEs, also known as phospholipids, are a crucial component of plant cell membranes. In comparison with those in M-P, the levels of PE(20:2_16:0), PE(22:6_18:2), and PE(17:0_18:1) were significantly lower in H-P-P (p < 0.05) (Figure 7B), indicating that three PE molecules may play a crucial role in cell membrane signaling, which explained why hexanal treatment lead to the downregulation of PLD gene expression in the stipes of P. impudicus. PS is usually located in the inner layer of the cell membrane and plays a role in various membrane-related functions [37]. The abundance of PS (18:0_18:3), PS (18:0_20:3) and PS (18:0_17:1) was significantly higher in M-P compared with that in CK (p < 0.05). The abundance of these PS molecules was significantly lower in H-P-P compared with that in M-P (p < 0.05), indicating that hexanal treatment significantly reduced the abundance of them, which played a pivotal role in stipe extension. PE reacted with serine to generate PS and ethanolamine under Ca2+ activation [38]. These results indicate that hexanal treatment might promote the process because H-P-P exhibited reduced PE abundance and increased PS abundance compared to M-P. LPE is a hydrolysis product of PE produced by phospholipase A [39]. The abundance of LPE(16:0), LPE(17:0), LPE(16:1), and LPE(20:2) in H-P-P was significantly lower than that in M-P (p < 0.05), which could be attributed to the hexanal treatment inhibiting PLA, which is similar to what was observed with PLD. PI plays a pivotal role in the phosphatidylinositol signaling system [40]. The abundance of PI (18:2_16:1) was significantly lower in H-P-P compared to that in M-P (p < 0.05). Thus, hexanal treatment inhibited P. impudicus maturation by inhibiting the phosphatidylinositol signaling system. Phospholipids are components of the lipid bilayer of membranes and are crucial in signal transduction [41].
- (3)
- Sphingolipids. Sphingolipids are bioactive lipids of cell membranes, and numerous studies have shown that sphingolipids control key cellular functions, including the cell cycle, cellular senescence, apoptosis, migration, etc. [49,50]. Four sphingolipids (Cer, Cert, HexCer, and SPH) were identified (Figure 5A). Zhu et al. [51] demonstrated that Ca2+ in Pleurotus eryngii may affect the development of stipes via membrane-localized sphingolipid molecules. The total abundance of sphingolipids in M-P and H-P-P was significantly higher than that in CK (p < 0.05), indicating that sphingolipids played a role in signal transduction during P. impudicus maturation. Additionally, the abundance of Cer(d18:1/17:0), Cer(t18:0/14:0), Cer(t18:0/16:0(2OH)), and HexCer(d18:1/16:1) in M-P was significantly higher than that in CK (p < 0.05) (Figure 7C). In contrast, there was no significant difference between H-P-P and CK (p > 0.05), which indicated that the sphingolipids above were closely related to P. impudicus maturation, and hexanal treatment inhibited P. impudicus maturation by inhibiting the sphingolipids.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, N.; Wang, D.; Li, X.; Li, H.; Luo, S.; Zhang, K.; Luo, P. The fungi of Dictyophora genus and its by-products: Recent progress owards the development of novel food formulations. Food Biosci. 2024, 60, 104126. [Google Scholar] [CrossRef]
- Duan, M.; Long, S.; Wu, X.; Feng, B.; Qin, S.; Li, Y.; Li, X.; Li, C.; Zhao, C.; Wang, L. Genome, transcriptome, and metabolome analyses provide new insights into the resource development in an edible fungus Dictyophora indusiata. Front. Microbiol. 2023, 14, 1137159. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X.; Zhang, Y.; Zou, P.; Cheng, L.; Gan, R.; Li, X.; Liu, D.; Geng, F. Quantitative proteomic and metabolomic analysis of Dictyophora indusiata fruiting bodies during post-harvest morphological development. Food Chem. 2021, 339, 127884. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, X.; Yang, B.; Liu, D.; Li, X.; Geng, F. De novo transcriptome and proteome analysis of Dictyophora indusiata fruiting bodies provides insights into the changes during morphological development. Int. J. Biol. Macromol. 2020, 146, 875–886. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fan, S.; Zeng, R.; Liu, D.; Wang, X.; Wang, J.; Geng, F. Effects of Refrigerated Storage on Restarted Morphological Development of Dictyophora indusiata Fruiting Bodies. Agronomy 2024, 14, 1539. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Chai, L.; Yin, D.; Lin, T.; Tao, Y.; Liu, S.; Qi, H.; Gao, X.; Jiang, J. Pre-harvest treatments: A different insight into preservation of strawberries. J. Berry Res. 2024, 14, 151–173. [Google Scholar] [CrossRef]
- Ashitha, G.; Sunny, A.C.; Nisha, R. Effect of pre-harvest and post-harvest hexanal treatments on fruits and vegetables: A review. Agric. Rev. 2020, 41, 124–131. [Google Scholar] [CrossRef]
- Preethi, P.; Soorianathasundaram, K.; Sadasakthi, A.; Subramanian, K.S.; Vijay Rakesh Reddy, S.; Paliyath, G.; Subramanian, J. Preharvest application of hexanal as a surface treatment improved the storage life and quality of mango fruits. Coatings 2021, 11, 1267. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Amer, A.; Parry, M.J.; Lim, L.-T.; Subramanian, J.; Paliyath, G. Postharvest hexanal vapor treatment delays ripening and enhances shelf life of greenhouse grown sweet bell pepper (Capsicum annum L.). Postharvest Biol. Technol. 2018, 136, 80–89. [Google Scholar] [CrossRef]
- Ranjan, S.; Paliyath, G.; Lim, L.-T.; Subramanian, J. Comparative study of hexanal dip and electrospun nanofiber mediated vapour treatments on enhancing the shelf life of pears. Can. J. Plant Sci. 2021, 101, 1029–1040. [Google Scholar] [CrossRef]
- Öz, A.T.; Ali, A. Retaining overall quality of fresh figs by postharvest hexanal vapor treatment during cold storage. Postharvest Biol. Technol. 2023, 205, 112539. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, J.; Kong, L.; Zhang, W.; Hou, X.; Cui, G. Genome-wide investigation of the PLD gene family in alfalfa (Medicago sativa L.): Identification, analysis and expression. BMC Genom. 2022, 23, 243. [Google Scholar]
- Silué, Y.; Nindjin, C.; Cissé, M.; Kouamé, K.A.; Amani, N.G.G.; Mbéguié-a-Mbéguié, D.; Lopez-Lauri, F.; Tano, K. Hexanal application reduces postharvest losses of mango (Mangifera indica L. variety “Kent”) over cold storage whilst maintaining fruit quality. Postharvest Biol. Technol. 2022, 189, 111930. [Google Scholar]
- El Kayal, W.; El-Sharkawy, I.; Dowling, C.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Effect of preharvest application of hexanal and growth regulators in enhancing shelf life and regulation of membrane-associated genes in strawberry. Can. J. Plant Sci. 2017, 97, 1109–1120. [Google Scholar]
- Padmanabhan, P.; Cheema, A.S.; Todd, J.F.; Lim, L.-T.; Paliyath, G. Ripening responses, fruit quality and phospholipase D gene expression in bell peppers exposed to hexanal vapor. Postharvest Biol. Technol. 2020, 170, 111317. [Google Scholar] [CrossRef]
- Tiwari, K.; Paliyath, G. Microarray analysis of ripening-regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiol. Biochem. 2011, 49, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Yumbya, P.; Ambuko, J.; Hutchinson, M.; Owino, W.; Juma, J.; Machuka, E.; Mutuku, J.M. Transcriptome analysis to elucidate hexanal’s mode of action in preserving the post-harvest shelf life and quality of banana fruits (Musa acuminata). J. Agric. Food Res. 2021, 3, 100114. [Google Scholar] [CrossRef]
- Ayala, J.C.; Wang, H.; Benitez, J.A.; Silva, A.J. RNA-Seq analysis and whole genome DNA-binding profile of the Vibrio cholerae histone-like nucleoid structuring protein (H-NS). Genom. Data 2015, 5, 147–150. [Google Scholar] [CrossRef]
- Kumar, S.K.; El Kayal, W.; Sullivan, J.A.; Paliyath, G.; Jayasankar, S. Pre-harvest application of hexanal formulation enhances shelf life and quality of ‘Fantasia’nectarines by regulating membrane and cell wall catabolism-associated genes. Sci. Hortic. 2018, 229, 117–124. [Google Scholar] [CrossRef]
- Mayr, J.A.; Kohlwein, S.D.; Paltauf, F. Identification of a novel, Ca2+-dependent phospholipase D with preference for phosphatidylserine and phosphatidylethanolamine in Saccharomyces cerevisiae. FEBS Lett. 1996, 393, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Gohain, D.; Bora, U.; Tamuli, R. Phospholipases play multiple cellular roles including growth, stress tolerance, sexual development, and virulence in fungi. Microbiol. Res. 2018, 209, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shao, Y.; Yang, Y.; Xu, C.; Jing, Z.; Li, H.; Xie, B.; Tao, Y. The Chromatin Modifier Protein FfJMHY Plays an Important Role in Regulating the Rate of Mycelial Growth and Stipe Elongation in Flammulina filiformis. J. Fungi 2022, 8, 477. [Google Scholar] [CrossRef]
- Li, M.; Bi, J.; Bai, Y.; Kang, L.; Duan, B.; Liu, Z.; Yuan, S. Accumulation and cross-linkage of β-1, 3/1, 6-glucan lead to loss of basal stipe cell wall extensibility in mushroom Coprinopsis cinerea. Carbohydr. Polym. 2021, 259, 117743. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Zhou, J.; Wang, R.; Zhang, X.; Liu, C.; Liu, Z.; Yuan, S. Glucanase-induced stipe wall extension shows distinct differences from chitinase-induced stipe wall extension of Coprinopsis cinerea. Appl. Environ. Microbiol. 2019, 85, 1–16. [Google Scholar] [CrossRef]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The role of antioxidants in the interplay between oxidative stress and senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Iakovou, E.; Kourti, M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Burgos, A.; Ma, L.; Zhang, Q.; Tang, D.; Ruan, J. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef]
- Cockcroft, S. Phosphatidic acid regulation of phosphatidylinositol 4-phosphate 5-kinases. Biochim. Biophys. Acta 2009, 1791, 905–912. [Google Scholar] [CrossRef]
- O’luanaigh, N.; Pardo, R.; Fensome, A.; Allen-Baume, V.; Jones, D.; Holt, M.R.; Cockcroft, S. Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 2002, 13, 3730–3746. [Google Scholar] [CrossRef]
- Stefan, C.J. Endoplasmic reticulum–plasma membrane contacts: Principals of phosphoinositide and calcium signaling. Curr. Opin. Cell Biol. 2020, 63, 125–134. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Abdul Hamid, Z.; Mohamad Anuar, N.N.; Jalil, J.; Mohd Nor, N.A.; Budin, S.B. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int. J. Mol. Sci. 2022, 23, 8582. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Goto, K. Diacylglycerol kinase ε in adipose tissues: A crosstalk between signal transduction and energy metabolism. Front. Physiol. 2022, 13, 815085. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.; Nerlich, J.; Lepetit, B.; Schaller, S.; Vieler, A.; Wilhelm, C. The lipid dependence of diadinoxanthin de-epoxidation presents new evidence for a macrodomain organization of the diatom thylakoid membrane. J. Plant Physiol. 2009, 166, 1839–1854. [Google Scholar] [CrossRef]
- Lepetit, B.; Goss, R.; Jakob, T.; Wilhelm, C. Molecular dynamics of the diatom thylakoid membrane under different light conditions. Photosynth. Res. 2012, 111, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Khattib, A.; Atrahimovich, D.; Dahli, L.; Vaya, J.; Khatib, S. Lyso-diacylglyceryltrimethylhomoserine (lyso-DGTS) isolated from Nannochloropsis microalgae improves high-density lipoprotein (HDL) functions. BioFactors 2020, 46, 146–157. [Google Scholar] [CrossRef]
- Yu, C.W.; Lin, Y.T.; Li, H.M. Increased ratio of galactolipid MGDG: DGDG induces jasmonic acid overproduction and changes chloroplast shape. New Phytol. 2020, 228, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, N.; Yang, J.; Sun, W.; Zhang, R.; Zheng, X.; Wang, Z.; Siebert, H.C.; Han, J. Preparation, Characterization, Evaluation of Neuroprotective Effect, and Related Mechanisms of Phosphatidylserine Emulsion in 5- and 12-Week Old Mice. J. Agric. Food Chem. 2022, 70, 1852–1864. [Google Scholar] [CrossRef]
- Vance, J.E. Historical perspective: Phosphatidylserine and phosphatidylethanolamine from the 1800s to the present. J. Lipid Res. 2018, 59, 923–944. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, Y.-P.; Bae, S.-W.; Ahn, G.-H.; Ryu, S.B. Lysophosphatidylethanolamine delays fruit softening of persimmon (Diospyros kaki). Hortic. Environ. Biotechnol. 2019, 60, 491–499. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Cockcroft, S. Phosphatidylinositol synthesis at the endoplasmic reticulum. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158471. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Takáč, T.; Novák, D.; Šamaj, J. Recent advances in the cellular and developmental biology of phospholipases in plants. Front. Plant Sci. 2019, 10, 432553. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, S.-C.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef]
- Pleskot, R.; Li, J.; Zarsky, V.; Potocky, M.; Staiger, C.J. Regulation of cytoskeletal dynamics by phospholipase D and phosphatidic acid. Trends Plant Sci. 2013, 18, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Takenawa, T. Phosphoinositide-binding interface proteins involved in shaping cell membranes. Proc. Jpn. Acad. Ser. B 2010, 86, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, F.; Mao, T.; Nie, J.; Yan, M.; Yuan, M.; Zhang, W. Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 2012, 24, 4555–4576. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Nguyen, V.C.; Chuang, L.; Kanehara, K. Membrane glycerolipid equilibrium under endoplasmic reticulum stress in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018, 500, 103–109. [Google Scholar] [CrossRef]
- Zuo, Z.F.; Li, Y.; Mi, X.F.; Li, Y.L.; Zhai, C.Y.; Yang, G.F.; Wang, Z.Y.; Zhang, K. Physiological and lipidomic response of exogenous choline chloride alleviating salt stress injury in Kentucky bluegrass (Poa pratensis). Front. Plant Sci. 2023, 14, 1269286. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1902–1921. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, J.; Li, Y.; Yang, B.; Guan, Y.; Xu, C.; Chen, F.; Chi, J.; Bao, Y. Comparative Proteomic Analysis of Pleurotus ostreatus Reveals Great Metabolic Differences in the Cap and Stipe Development and the Potential Role of Ca2+ in the Primordium Differentiation. Int. J. Mol. Sci. 2019, 20, 6317. [Google Scholar] [CrossRef] [PubMed]





| Type | Gene ID | Log2(Fold Change) | ||
|---|---|---|---|---|
| CK vs. M-P | CK vs. H-P-P | M-P vs. H-P-P | ||
| Chitinases | TRINITY_DN3514_c0_g1 | 0.61 | −1.46 | −1.98 |
| TRINITY_DN4688_c0_g1 | 0.90 | −0.85 | −1.65 | |
| TRINITY_DN3122_c0_g1 | 2.40 | 1.16 | −1.15 | |
| TRINITY_DN4313_c0_g1 | 8.13 | 6.71 | −1.32 | |
| Chitin synthases | TRINITY_DN4936_c0_g1 | 1.06 | 1.04 | 0.08 |
| TRINITY_DN31_c0_g1 | 1.15 | 0.36 | −0.70 | |
| TRINITY_DN244_c0_g1 | 1.33 | 0.26 | −0.98 | |
| TRINITY_DN1043_c0_g1 | 1.62 | 0.46 | −1.07 | |
| TRINITY_DN9021_c0_g1 | −0.57 | −1.86 | −1.20 | |
| TRINITY_DN4981_c0_g2 | 5.39 | 3.85 | −1.45 | |
| β-1,3-Glucosidase | TRINITY_DN14093_c0_g1 | 3.59 | 7.93 | 4.49 |
| TRINITY_DN9019_c0_g1 | 5.43 | 6.64 | 1.32 | |
| TRINITY_DN12787_c0_g1 | 6.59 | 7.80 | 1.31 | |
| TRINITY_DN684_c0_g1 | −1.53 | −0.74 | 0.89 | |
| TRINITY_DN1371_c0_g2 | −1.94 | −1.99 | 0.04 | |
| TRINITY_DN4148_c1_g1 | 7.70 | 6.58 | −1.03 | |
| TRINITY_DN3011_c0_g1 | 1.28 | 0.03 | −1.15 | |
| TRINITY_DN10744_c0_g1 | 5.27 | 3.97 | −1.20 | |
| TRINITY_DN13997_c0_g1 | 8.22 | 3.09 | −4.98 | |
| Endo-β-1,3(4)-glucanase | TRINITY_DN3286_c0_g1 | −0.37 | 0.76 | 1.23 |
| Endo-β-1,6-glucosidase | TRINITY_DN4364_c0_g2 | 8.02 | 4.20 | −3.75 |
| β-Glucosidase | TRINITY_DN8389_c1_g1 | 2.05 | 7.27 | 5.32 |
| TRINITY_DN738_c0_g1 | −2.18 | −1.32 | 0.95 | |
| TRINITY_DN6295_c1_g2 | 8.27 | 8.38 | 0.21 | |
| TRINITY_DN4234_c0_g3 | 6.90 | 6.54 | −0.27 | |
| TRINITY_DN576_c0_g1 | 1.06 | 0.18 | −0.78 | |
| TRINITY_DN12330_c0_g1 | 9.69 | 6.37 | −3.21 | |
| Endo-β-1, 3 (4)-glucanase | TRINITY_DN1764_c0_g2 | −1.88 | −0.15 | 1.83 |
| TRINITY_DN858_c0_g1 | −2.35 | −1.06 | 1.39 | |
| TRINITY_DN10744_c0_g1 | 5.27 | 3.97 | −1.20 | |
| Endo-β-1,4-glucanase | TRINITY_DN4078_c0_g2 | 1.24 | 0.86 | −0.29 |
| TRINITY_DN4535_c0_g1 | −1.47 | −1.86 | −0.31 | |
| Endoglucanase-7 | TRINITY_DN967_c0_g1 | 7.43 | 3.55 | −3.78 |
| Exoglucanase 3 | TRINITY_DN2746_c0_g1 | −1.24 | −0.75 | 0.58 |
| β-1,3-Glucan synthase | TRINITY_DN3479_c0_g3 | 1.67 | 0.85 | −0.72 |
| TRINITY_DN5554_c0_g1 | 6.26 | 5.54 | −0.63 | |
| TRINITY_DN5554_c0_g2 | 6.01 | 4.32 | −1.60 | |
| Type | Gene ID | Log2(Fold Change) | ||
|---|---|---|---|---|
| CK vs. M-P | CK vs. H-P-P | M-P vs. H-P-P | ||
| Catalase | TRINITY_DN3676_c1_g1 | −1.56 | 0.56 | 2.22 |
| TRINITY_DN9524_c0_g1 | 4.51 | 7.16 | 2.73 | |
| Superoxide dismutase | TRINITY_DN989_c0_g1 | −2.12 | −1.58 | 0.64 |
| TRINITY_DN2134_c0_g3 | 6.02 | 7.01 | 1.09 | |
| TRINITY_DN7788_c0_g2 | 2.90 | 4.30 | 1.48 | |
| TRINITY_DN2134_c0_g2 | 4.33 | 5.97 | 1.75 | |
| TRINITY_DN3251_c0_g1 | 3.52 | 5.31 | 1.88 | |
| TRINITY_DN10558_c0_g3 | 6.64 | 5.59 | −0.95 | |
| TRINITY_DN12621_c0_g1 | 7.00 | 3.77 | −3.10 | |
| TRINITY_DN11879_c0_g1 | 5.76 | 1.78 | −3.85 | |
| TRINITY_DN2134_c0_g4 | 5.87 | 1.77 | −3.97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Fan, S.; Hu, G.; Wang, B.; Liu, D.; Wang, X.; Wang, J.; Geng, F. Inhibitory Effect and Mechanism of Hexanal on the Maturation of Peach-Shaped Phallus impudicus. J. Fungi 2025, 11, 127. https://doi.org/10.3390/jof11020127
He H, Fan S, Hu G, Wang B, Liu D, Wang X, Wang J, Geng F. Inhibitory Effect and Mechanism of Hexanal on the Maturation of Peach-Shaped Phallus impudicus. Journal of Fungi. 2025; 11(2):127. https://doi.org/10.3390/jof11020127
Chicago/Turabian StyleHe, Hong, Shuya Fan, Gan Hu, Beibei Wang, Dayu Liu, Xinhui Wang, Jinqiu Wang, and Fang Geng. 2025. "Inhibitory Effect and Mechanism of Hexanal on the Maturation of Peach-Shaped Phallus impudicus" Journal of Fungi 11, no. 2: 127. https://doi.org/10.3390/jof11020127
APA StyleHe, H., Fan, S., Hu, G., Wang, B., Liu, D., Wang, X., Wang, J., & Geng, F. (2025). Inhibitory Effect and Mechanism of Hexanal on the Maturation of Peach-Shaped Phallus impudicus. Journal of Fungi, 11(2), 127. https://doi.org/10.3390/jof11020127







