The Susceptibility of Two Beauveria bassiana Strains on Rice Pests Nilaparvata lugens and Sogatella furcifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and B. bassiana Strains
2.2. Bioassay Analysis of B. bassiana to N. lugens and S. furcifera
2.3. Compatibility Analysis of B. bassiana with Insecticides
2.4. Effects of the Combined Use of B. bassiana with Insecticides on Rice Planthopper
2.5. Statistical Analysis
3. Results
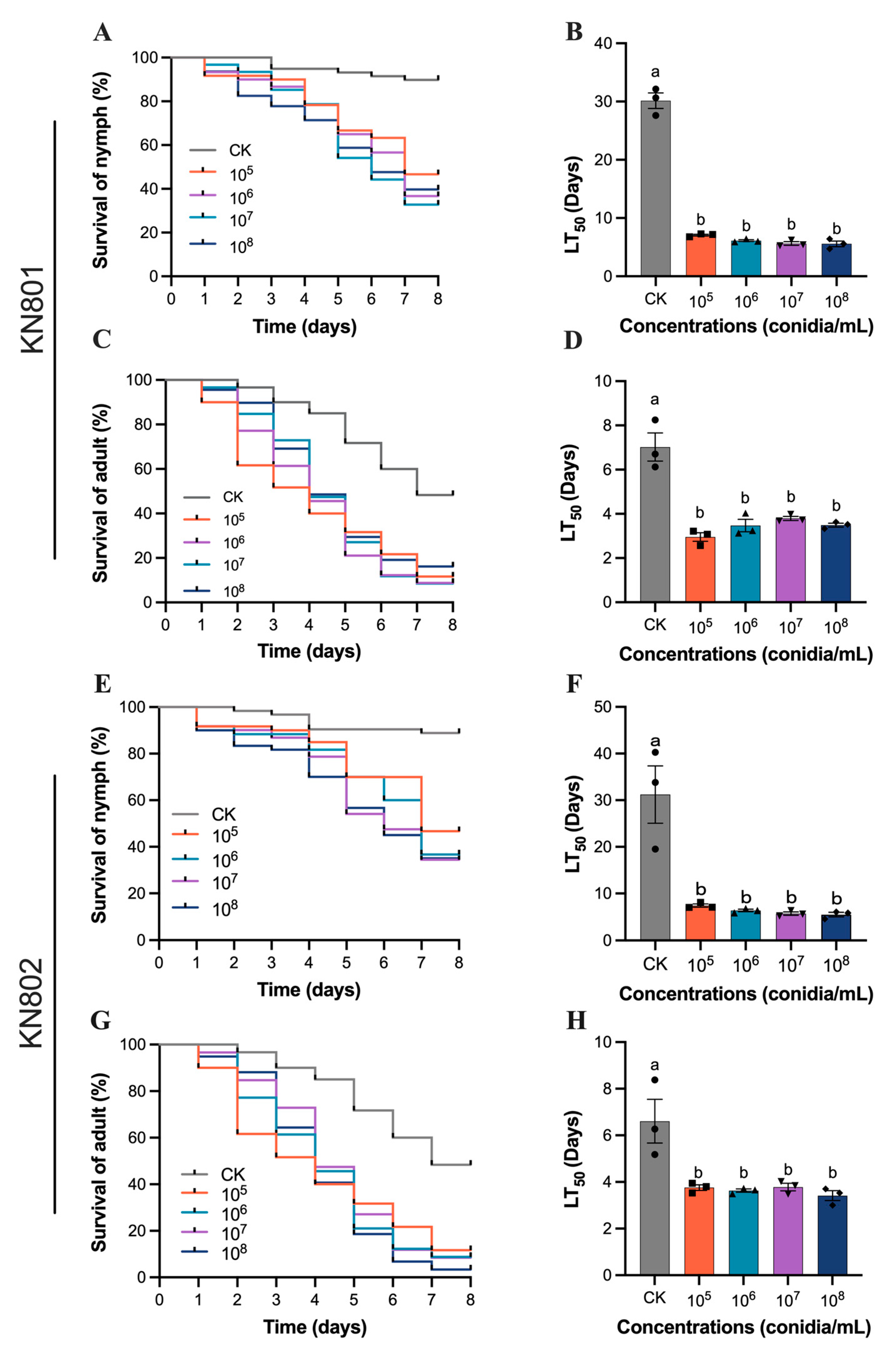
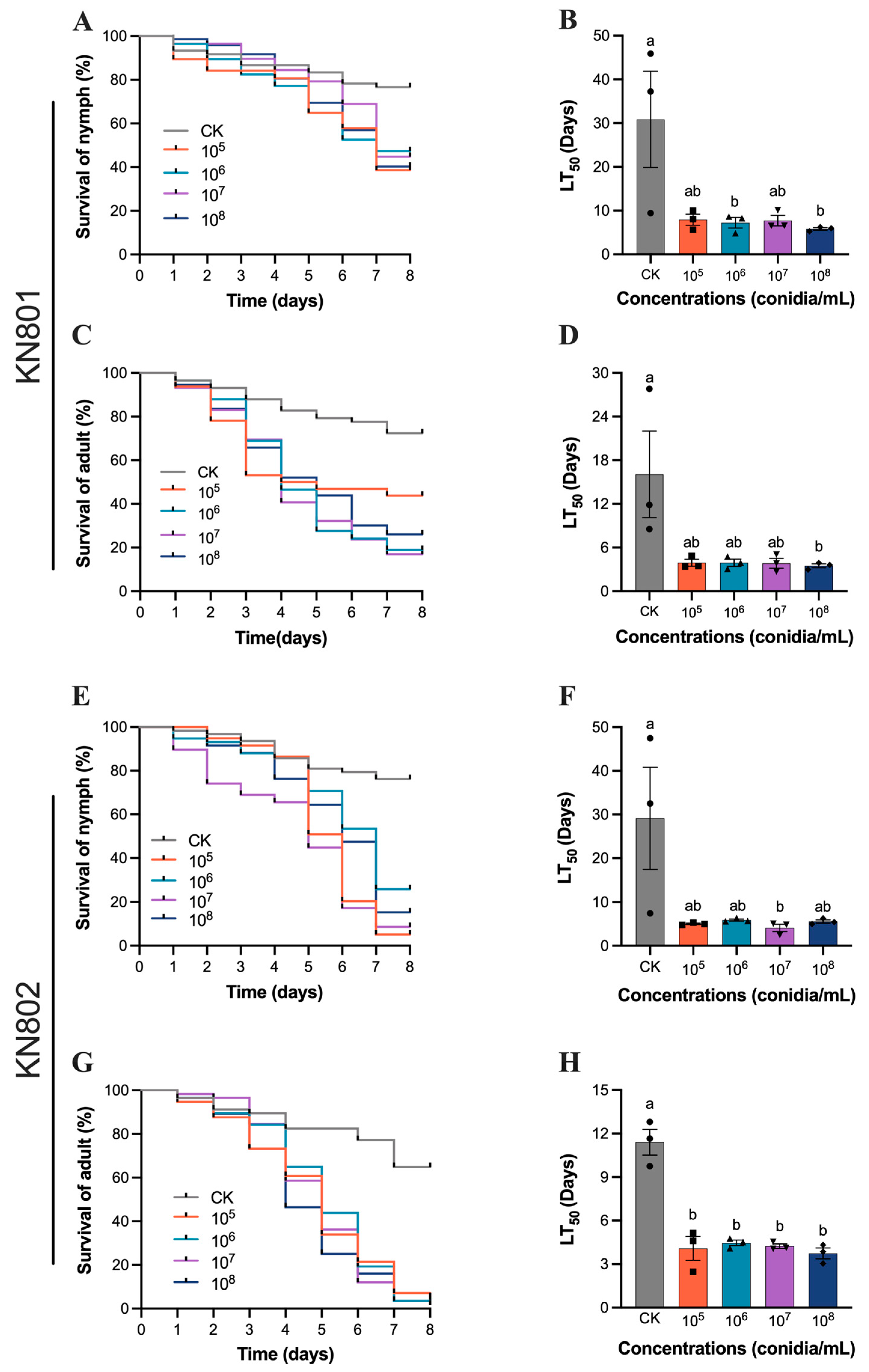
3.1. Susceptibility of B. bassiana to S. furcifera
3.2. Susceptibility of B. bassiana to N. lugens
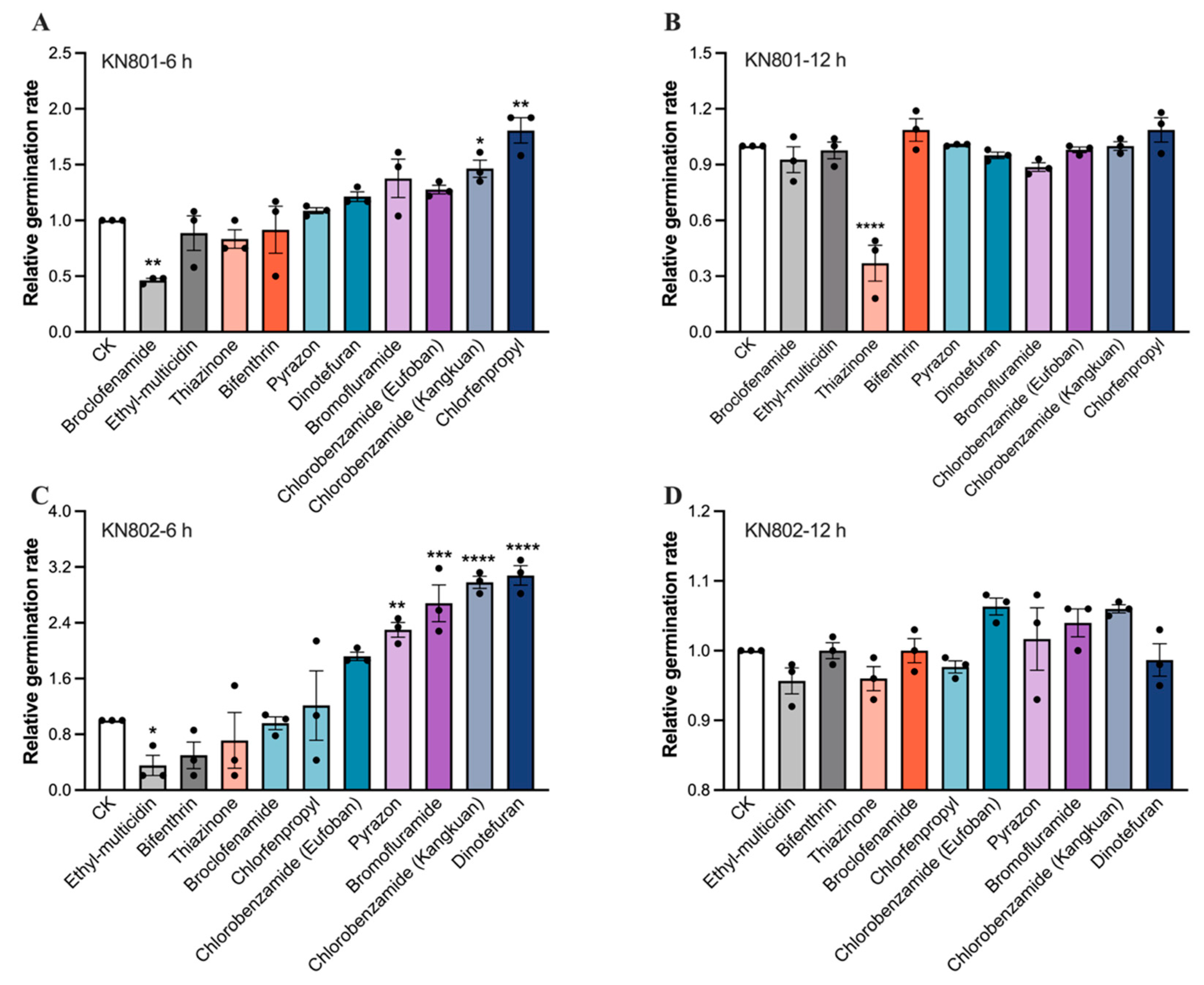
3.3. Compatibility of B. bassiana Strains with Insecticides
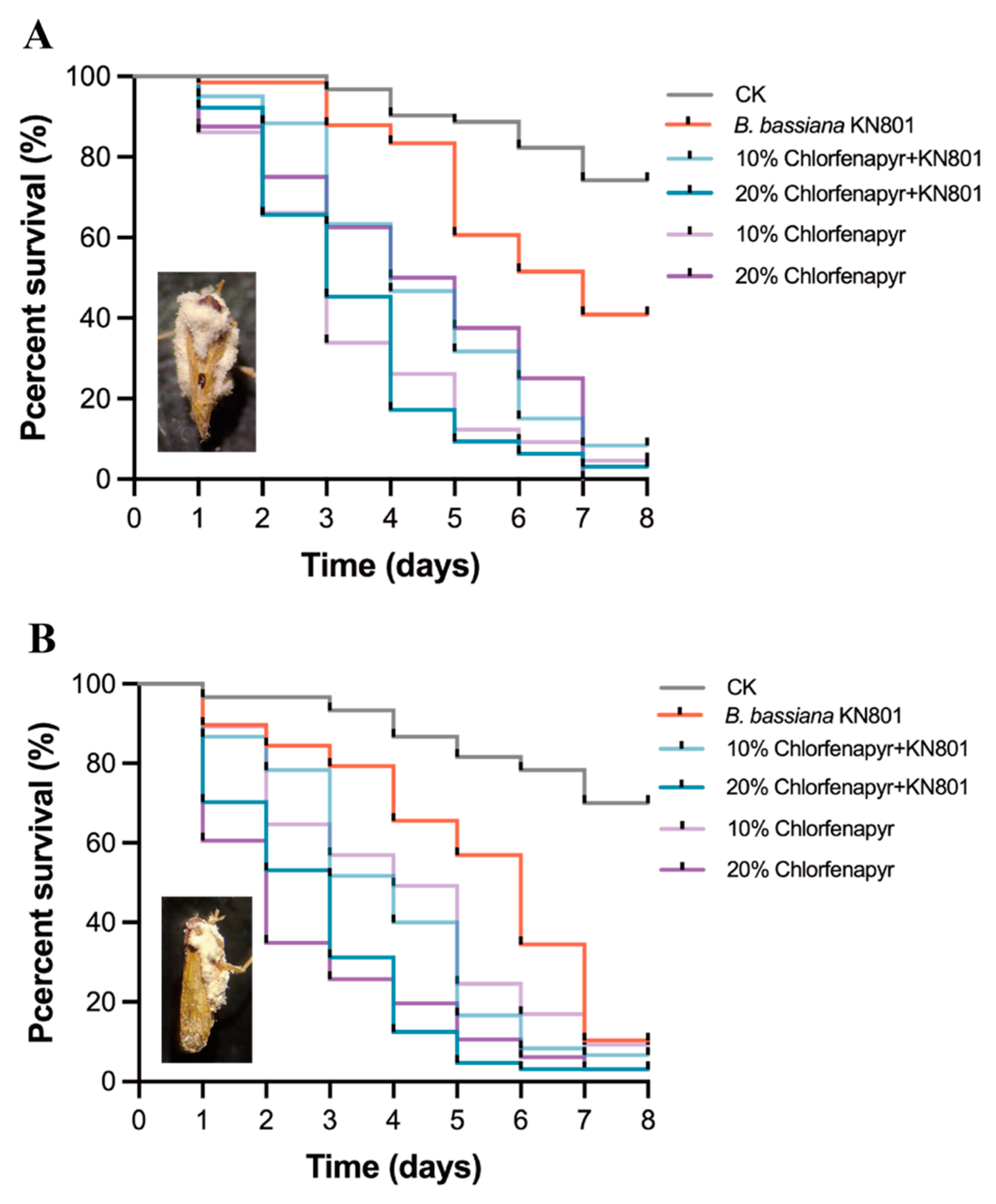
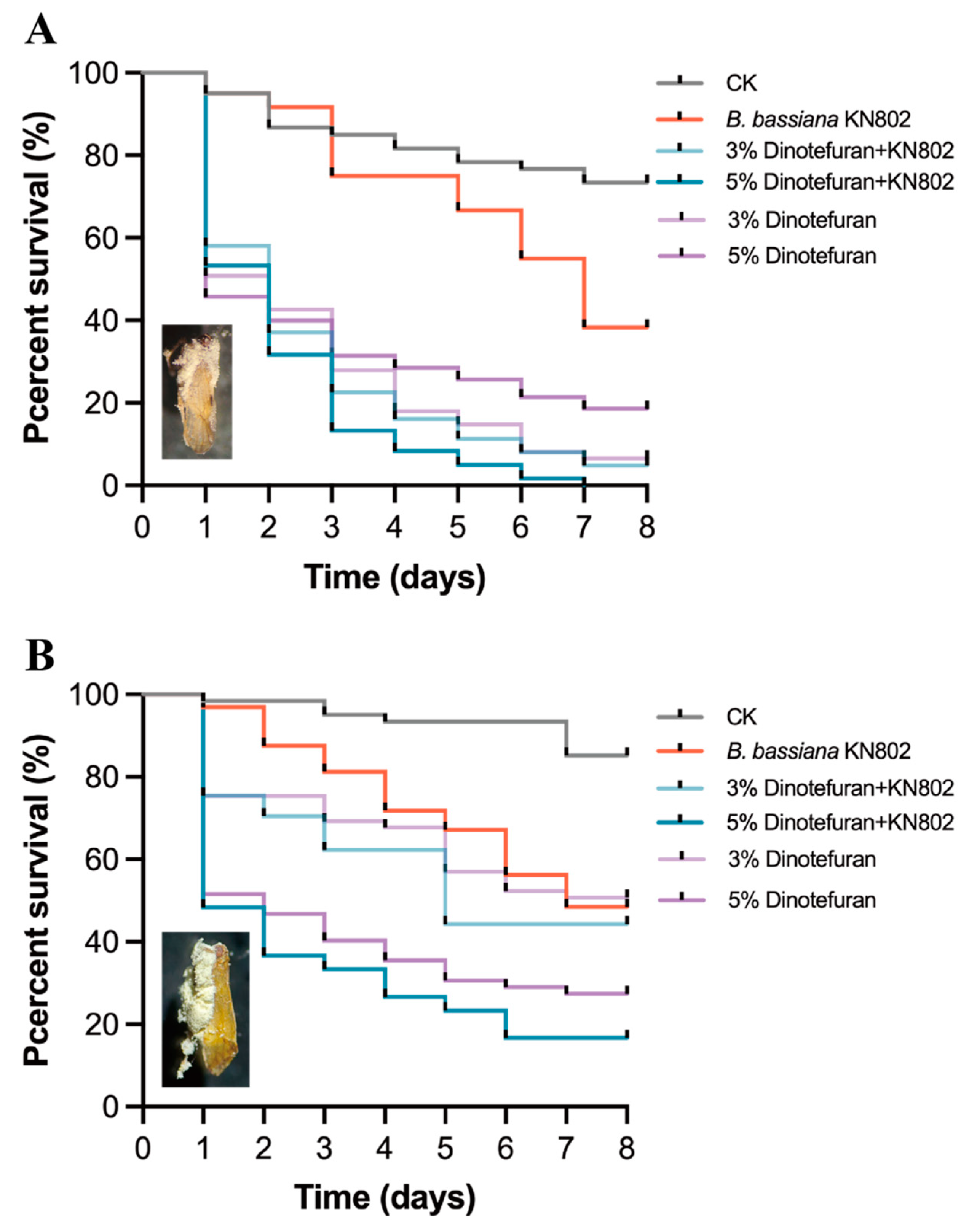
3.4. Effects of the Combined Used for N. lugens and S. furcifera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lou, Y.G.; Zhang, G.R.; Zhang, W.Q.; Hu, Y.; Zhang, J. Biological control of rice insect pests in China. Biol. Control 2013, 67, 8–20. [Google Scholar] [CrossRef]
- Tu, Z.; Ling, B.; Xu, D.; Zhang, M.; Zhou, G. Effects of southern rice black-streaked dwarf virus on the development and fecundity of its vector, Sogatella furcifera. Virol. J. 2013, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Li, Y. Rice reoviruses in insect vectors. Ann.Rev. Phytopathol. 2016, 54, 99–120. [Google Scholar] [CrossRef]
- Gandara, L.; Jacoby, R.; Laurent, F.; Spatuzzi, M.; Vlachopoulos, N.; Borst, N.O.; Ekmen, G.; Potel, C.M.; Garrido-Rodriguez, M.; Bohmert, A.L.; et al. Pervasive sublethal effects of agrochemicals on insects at environmentally relevant concentrations. Science 2024, 386, 446–453. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Gill, R.J.; Raine, N.E. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 2015, 28, 1459–1471. [Google Scholar] [CrossRef]
- Wu, S.F.; Zeng, B.; Zheng, C.; Mu, X.C.; Zhang, Y.; Hu, J.; Zhang, S.; Gao, C.F.; Shen, J.L. The evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) of China in the period 2012–2016. Sci. Rep. 2018, 8, 4586. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, J.; Wendel, J.; Jaronski, S.; Vitek, C.; Ciomperlik, M.; Flores, D. Assessment of two novel host-derived Beauveria bassiana (Hypocreales: Cordycipitaceae) isolates against the citrus pest, Diaphorina citri (Hemiptera: Liviidae). J. Econ. Entomol. 2022, 115, 56–64. [Google Scholar] [CrossRef]
- Senthil Kumar, C.M.; Jacob, T.K.; Devasahayam, S.; Rajeshkumar, K.C.; Lad, S.S.; D’Silva, S.; Geethu, C. Metarhizium indicum, a new species of entomopathogenic fungus infecting leafhopper, Busoniomimus manjunathi from India. J. Invertebr. Pathol. 2023, 198, 107919. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Keyhani, N.O.; Xia, Y.; Xie, J. The potential and limitations of entomopathogenic fungi as biocontrol agents for insect pest management. Entomol. Gen. 2024, 44, 797–811. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Garrido-Jurado, I.; Yousef-Yousef, M.; González-Mas, N. Multitrophic interactions of entomopathogenic fungi in BioControl. Biocontrol 2022, 67, 457–472. [Google Scholar] [CrossRef]
- Behie, S.W.; Bidochka, M.J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 2012, 336, 1576. [Google Scholar] [CrossRef] [PubMed]
- Behie, S.W.; Moreira, C.C.; Sementchoukova, I.; Barelli, L.; Zelisko, P.M.; Bidochka, M.J. Carbon translocation from a plant to an insect-pathogenic endophytic fungus. Nat. Commun. 2017, 8, 14245. [Google Scholar] [CrossRef] [PubMed]
- Panwar, N.; Szczepaniec, A. Endophytic entomopathogenic fungi as biological control agents of insect pests. Pest Manag. Sci. 2024, 80, 6033–6040. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Y.X. Host-Pathogen Interactions between spp. and Locusts. J. Fungi 2022, 8, 602. [Google Scholar] [CrossRef]
- Gao, T.N.; Wang, Z.L.; Huang, Y.; Keyhani, N.O.; Huang, Z. Lack of resistance development in to after multiple generations of selection. Sci. Rep. 2017, 7, 42727. [Google Scholar]
- Peng, G.; Xia, Y. Integration of an insecticidal scorpion toxin (BjαIT) gene into Metarhizium acridum enhances fungal virulence towards Locusta migratoria manilensis. Pest Manag. Sci. 2015, 71, 58–64. [Google Scholar] [CrossRef]
- Fan, Y.H.B.D.; Hawkings, C.; Ortiz-Urquiza, A.; Keyhani, N.O. Exploiting host molecules to augment mycoinsecticide virulence. Nat. Biotechnol. 2012, 30, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Garrido-Jurado, I.; González-Mas, N.; Yousef-Yousef, M. Ecosystem services of entomopathogenic ascomycetes. J. Invertebr. Pathol. 2023, 201, 108015. [Google Scholar] [CrossRef]
- García-Espinoza, F.; Yousef-Yousef, M.; del Rosal, M.J.G.; Cuenca-Medina, M.; Quesada-Moraga, E. Greenhouse melon crop protection and production through the compatible use of a parasitoid with endophytic entomopathogenic ascomycetes. J. Pest Sci. 2024, 97, 1899–1912. [Google Scholar] [CrossRef]
- Tang, J.; Liu, X.; Ding, Y.; Jiang, W.; Xie, J. Evaluation of Metarhizium anisopliae for rice planthopper control and its synergy with selected insecticides. Crop Prot. 2019, 121, 132–138. [Google Scholar] [CrossRef]
- Wakil, W.; Yasin, M.; Shapiro-Ilan, D. Effects of single and combined applications of entomopathogenic fungi and nematodes against Rhynchophorus ferrugineus Olivier. Sci. Rep. 2017, 7, 5971. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S. A review of biopesticides and their mode of action against insect pests. In Environmental Sustainability; KoganPage: London, UK, 2015; pp. 49–63. [Google Scholar]
- Peng, G.X.; Xie, J.Q.; Guo, R.; Keyhani, N.O.; Zeng, D.Y.; Yang, P.Y.; Xia, Y.X. Long-term field evaluation and large-scale application of a Metarhizium anisopliae strain for controlling major rice pests. J. Pest Sci. 2021, 94, 969–980. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, J.; Hong, M.; Xie, J. Suppression of rice planthopper populations by the entomopathogenic fungus Metarhizium anisopliae without affecting the rice microbiota. Appl. Environ. Microb. 2020, 86, e01337-20. [Google Scholar] [CrossRef]
- Su, J.; Wang, Z.; Zhang, K.; Tian, X.; Yin, Y.; Zhao, X.; Shen, A.; Gao, C.F. Status of insecticide resistance of the whitebacked planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Fla. Entomol. 2013, 96, 948–956. [Google Scholar] [CrossRef]
- Guo, J.; Xu, C.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef]
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Farrokhzadeh, H.; Sharifi, S.; Eroglu, G.B.; Karimi, J. A new fungal entomopathogen has potency as a biocontrol agent of longhorn beetle larva, Osphranteria coerulescencs. Int. J. Trop. Insect Sci. 2024, 44, 1185–1193. [Google Scholar] [CrossRef]
- Rice, S.J.; Baker, D.K.; Leemon, D.M. Development of mycoinsecticide formulations with Beauveria bassiana and Metarhizium anisopliae for the control of lesser mealworm, Alphitobius diaperinus, in chicken broiler houses. Biocontrol 2019, 64, 489–500. [Google Scholar] [CrossRef]
- Daza, F.F.F.; Roman, G.R.; Rodriguez, M.V.; Vargas, I.A.G.; Heano, H.C.; Cereda, M.P.; Mulet, R.A.C. Spores of Beauveria bassiana and Trichoderma lignorum as a bioinsecticide for the control of Atta cephalotes. Biol. Res. 2019, 52, 51. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.F.; Feng, M.G.; Ying, S.H.; Mu, W.J.; Chen, J.Q. Evaluation of alternative rice planthopper control by the combined action of oil-formulated Metarhizium anisopliae and low-rate buprofezin. Pest Manag. Sci. 2011, 67, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Avalos, A.M.; Bivián-Hernández, M.D.; Ibarra, J.E.; Del Rincón-Castro, M.C. High virulence of mexican entomopathogenic fungi against fall armyworm, (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 99–107. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S. Insect pathogenic fungi: Genomics, molecular interactions, and genetic improvements. Ann. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Molecular genetics of infection of insects. Adv. Genet. 2016, 94, 165–249. [Google Scholar]
- Tang, C.; Hu, X.; Tang, J.; Wang, L.; Liu, X.; Peng, Y.; Xia, Y.; Xie, J. The symbiont Acinetobacter baumannii enhances the insect host resistance to entomopathogenic fungus Metarhizium anisopliae. Commun. Biol. 2024, 7, 1184. [Google Scholar] [CrossRef] [PubMed]
- de Santana, A.S.; Cavalcante, U.M.T.; Sampaio, E.V.D.B.; Maia, L.C. Production, storage and costs of inoculum of arbuscular mycorrhizal fungi (AMF). Braz. J. Bot. 2014, 37, 159–165. [Google Scholar] [CrossRef]
- Ajvad, F.T.; Madadi, H.; Michaud, J.P.; Zafari, D.; Khanjani, M. Combined applications of an entomopathogenic fungus and a predatory mite to control fungus gnats (Diptera: Sciaridae) in mushroom production. Biol. Control 2020, 141, 104101. [Google Scholar]
- Mohammed, A.A.; Hatcher, P.E. Combining entomopathogenic fungi and parasitoids to control the green peach aphid. Biol. Control 2017, 110, 44–55. [Google Scholar] [CrossRef]
- Gu, M.Q.; Tian, J.X.; Lou, Y.L.; Ran, J.; Mohamed, A.; Keyhani, N.O.; Jaronski, S.; Wang, G.M.; Chen, X.Y.; Zang, L.S.; et al. Efficacy of granules for the control of and its synergistic effects with chemical pesticide, sex pheromone and parasitoid. Entomol. Gen. 2023, 43, 1211–1219. [Google Scholar] [CrossRef]
| Insecticides | Concentrations/Dosages | Producers |
|---|---|---|
| Dinotefuran | 865 μg/mL | Tongzhou Zhongda Agrochemical Co., Ltd., Nantong, China |
| Thiazinone | 0.337 5 μL/mL | Shenzhen Noposion Agrochemicals Co., Ltd., Shenzhen, China |
| Bifenthrin | 0.165 μL/mL | Yangnong Chemical, Yangzhou, China |
| Pyrazon | 5.325 mg/mL | Syngenta Nantong Crop Protection Co., Ltd., Nantong, China |
| Chlorobenzamide (Eufoban) | 1.1725 mg/mL | Bayer AG, Leverkusen, Germany |
| Ethyl-multicidin | 120 μg/mL | Dow AgroSciences China Limited, Nantong, China |
| Bromofluramide | 0.1 μL/mL | Mitsui Chemicals, Inc., Shanghai, China |
| Broclofenamide | 0.2 μL/mL | Hebei Longping Agricultural Science and Technology Co., Ltd., Shijiazhuang, China |
| Chlorobenzamide (Kangkuan) | 133.4 μg/mL | DuPont, Wilmington, DE, USA |
| Chlorfenpropyl | 0.265 μL/mL | BASF Plant Protection Limited Company, Nantong, China |
| Lethal Time | Targets | B. bassiana | 10% Chlorfenapyr + B. bassiana | 20% Chlorfenapyr + B. bassiana | 10% Chlorfenapyr | 20% Chlorfenapyr | CK |
|---|---|---|---|---|---|---|---|
| LT50 with 95% CIs (days) | N. lugens | 4.84 (4.46~5.22) | 3.4 (2.6~4.2) | 2.28 (2.11~2.45) | 3.99 (3.75~4.23) | 1.76 (1~2.52) | 8.87 (8.37~9.38) |
| S. furcifera | 5.87 (5.34~6.4) | 4.02 (3.97~4.06) | 2.89 (2.31~3.47) | 2.9 (2.79~3.01) | 2.63 (2.34~2.92) | 8.89 (7.56~10.21) | |
| LT90 with 95% CIs (days) | N. lugens | 7.88 (6.87~8.89) | 5.7 (3.94~7.45) | 4.48 (4.14~4.83) | 6.75 (5.84~7.65) | 3.9 (2.33~5.47) | 13.92 (12.9~14.93) |
| S. furcifera | 8.83 (8.47~9.19) | 6.44 (5.63~7.26) | 4.76 (3.54~5.97) | 5.38 (5.14~5.61) | 4.56 (4.19~4.94) | 12.73 (10.85~14.61) |
| Lethal Time | Targets | B. bassiana | 3% Dinotefuran + B. bassiana | 5% Dinotefuran + B. bassiana | 3% Dinotefuran | 5% Dinotefuran | CK |
|---|---|---|---|---|---|---|---|
| LT50 with 95% Cis (days) | N. lugens | 6.72 (7.66~5.78) | 5.23 (6.05~4.41) | 2.18 (2.91~1.44) | 6.42 (7.74~5.11) | 3.01 (4.3~1.73) | 10.83 (13~8.66) |
| S. furcifera | 6.15 (6.43~5.86) | 2.07 (2.96~1.18) | 1.61 (1.78~1.43) | 2.02 (2.1~1.95) | 1.61 (2.48~0.74) | 9.77 (10.35~9.2) | |
| LT90 with 95% Cis (days) | N. lugens | 11.4 (14.16~8.64) | 11.09 (11.78~10.41) | 6.74 (8.13~5.34) | 13.28 (15.09~11.47) | 8.71 (11.24~6.19) | 15.68 (20.58~10.78) |
| S. furcifera | 10.18 (11.02~9.34) | 4.63 (6.82~2.45) | 3.57 (4.27~2.88) | 5.46 (6.02~4.9) | 5.46 (6.19~4.72) | 17.07 (17.68~16.46) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Mu, H.; Peng, Y.; Huo, R.; Xie, J. The Susceptibility of Two Beauveria bassiana Strains on Rice Pests Nilaparvata lugens and Sogatella furcifera. J. Fungi 2025, 11, 128. https://doi.org/10.3390/jof11020128
Chen Z, Mu H, Peng Y, Huo R, Xie J. The Susceptibility of Two Beauveria bassiana Strains on Rice Pests Nilaparvata lugens and Sogatella furcifera. Journal of Fungi. 2025; 11(2):128. https://doi.org/10.3390/jof11020128
Chicago/Turabian StyleChen, Zhongwei, Hanqing Mu, Yifan Peng, Rui Huo, and Jiaqin Xie. 2025. "The Susceptibility of Two Beauveria bassiana Strains on Rice Pests Nilaparvata lugens and Sogatella furcifera" Journal of Fungi 11, no. 2: 128. https://doi.org/10.3390/jof11020128
APA StyleChen, Z., Mu, H., Peng, Y., Huo, R., & Xie, J. (2025). The Susceptibility of Two Beauveria bassiana Strains on Rice Pests Nilaparvata lugens and Sogatella furcifera. Journal of Fungi, 11(2), 128. https://doi.org/10.3390/jof11020128







