Effect of Heat Stress on the Biosynthesis of Exopolysaccharides from Rhodotorula glutinis YM25079 and Its Underlying Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Cultural Conditions
2.2. Growth and Metabolic Characterization of Strain YM25079
2.2.1. One-Way Experiments with Culture Media
2.2.2. Growth and Metabolic Profiling
2.2.3. Kinetic Modeling of Strain Growth [26]
2.3. Extraction and Purification of YM25079 EPS
2.4. Molecular Weight and Monosaccharide Composition Analysis
2.5. UV and FT-IR Spectrometric Analysis
2.6. Analysis of Thermal Properties
2.7. Scanning Electron Microscopy (SEM) and Atomic Force Micrograph (AFM) Analysis of EPS
2.8. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis of EPS
2.9. Rheological Analysis
2.10. Determination of α-Amylase Inhibitory Activity
2.11. Transcriptome Sequencing
2.11.1. Culture Conditions and Cell Sample Preparation
2.11.2. Total RNA Extraction and Library Construction
2.11.3. Differential Expression Analysis and Gene Function Enrichment
2.11.4. Real-Time Quantitative PCR Analysis
2.12. Statistical Analysis
3. Results
3.1. Effect of Carbon Source and Its Additive Amount on the Yield of EPS
3.2. Growth Curves of R. glutinis YM25079 and Metabolism of EPS
3.3. Kinetic Modeling Fitting of Strain Growth
3.4. Purification of Crude EPSs
3.5. Molecular Mass and Monosaccharide Composition of EPS
3.6. UV and FT-IR Spectrum Observation
3.7. Thermal Analysis
3.8. SEM Analysis of EPS
3.9. AFM Analysis of EPS
3.10. NMR Spectroscopy Analysis
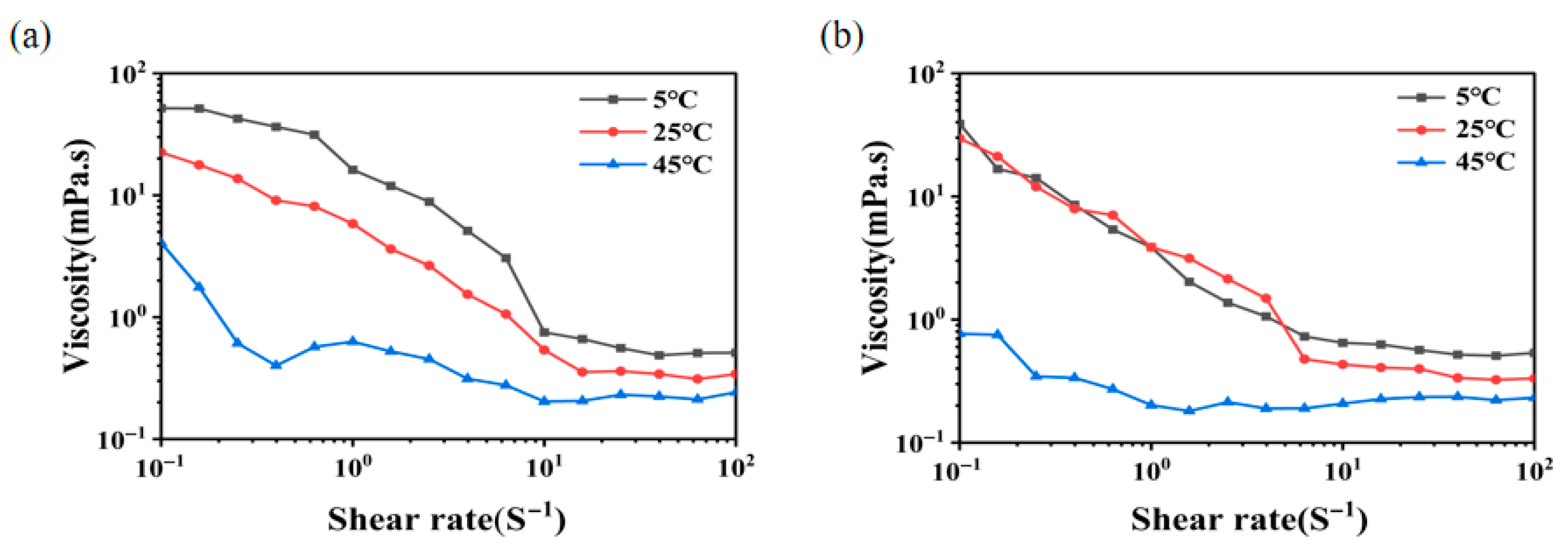
3.11. Rheological Measurements
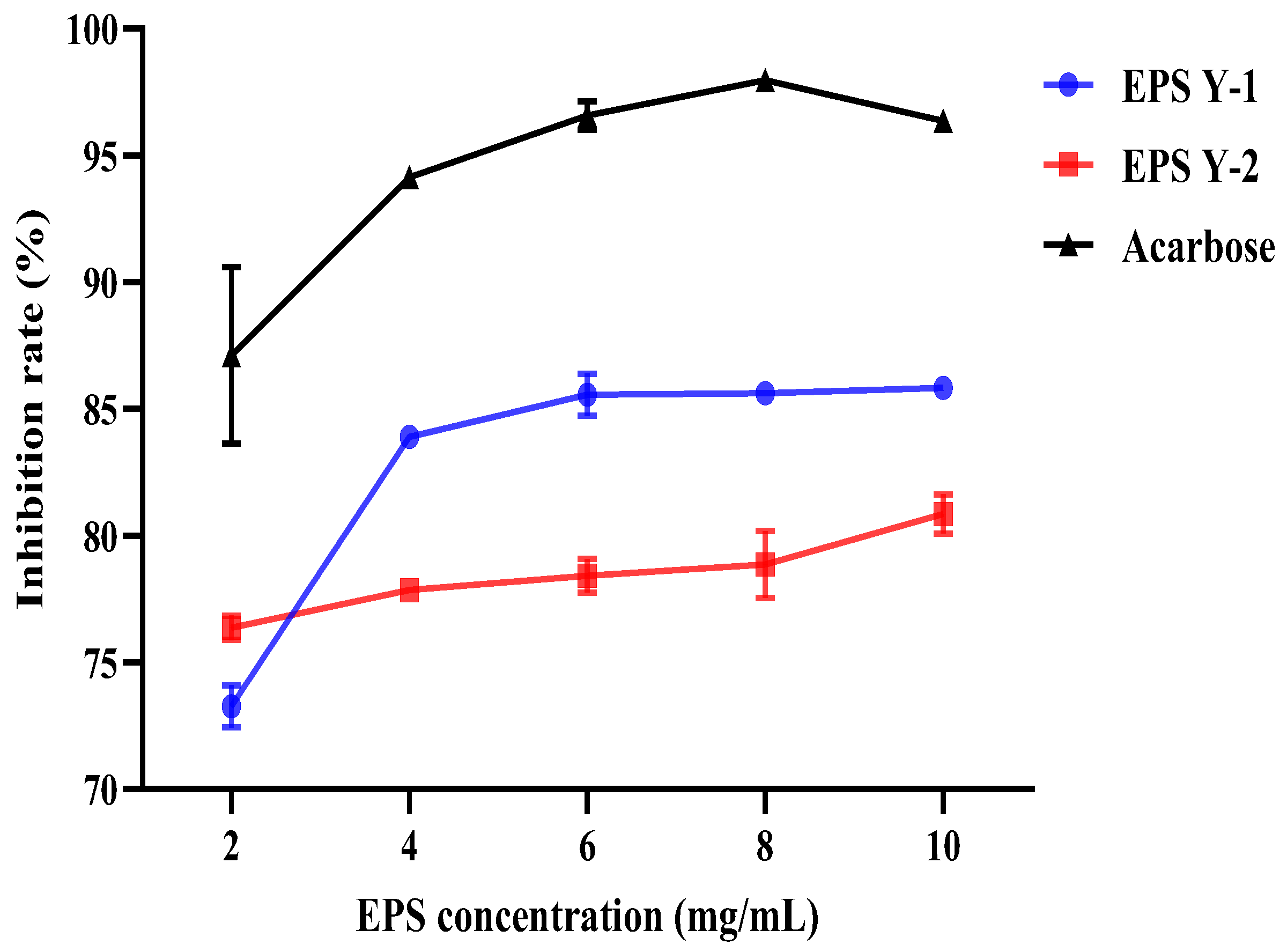
3.12. α-Amylase Inhibitory Activities
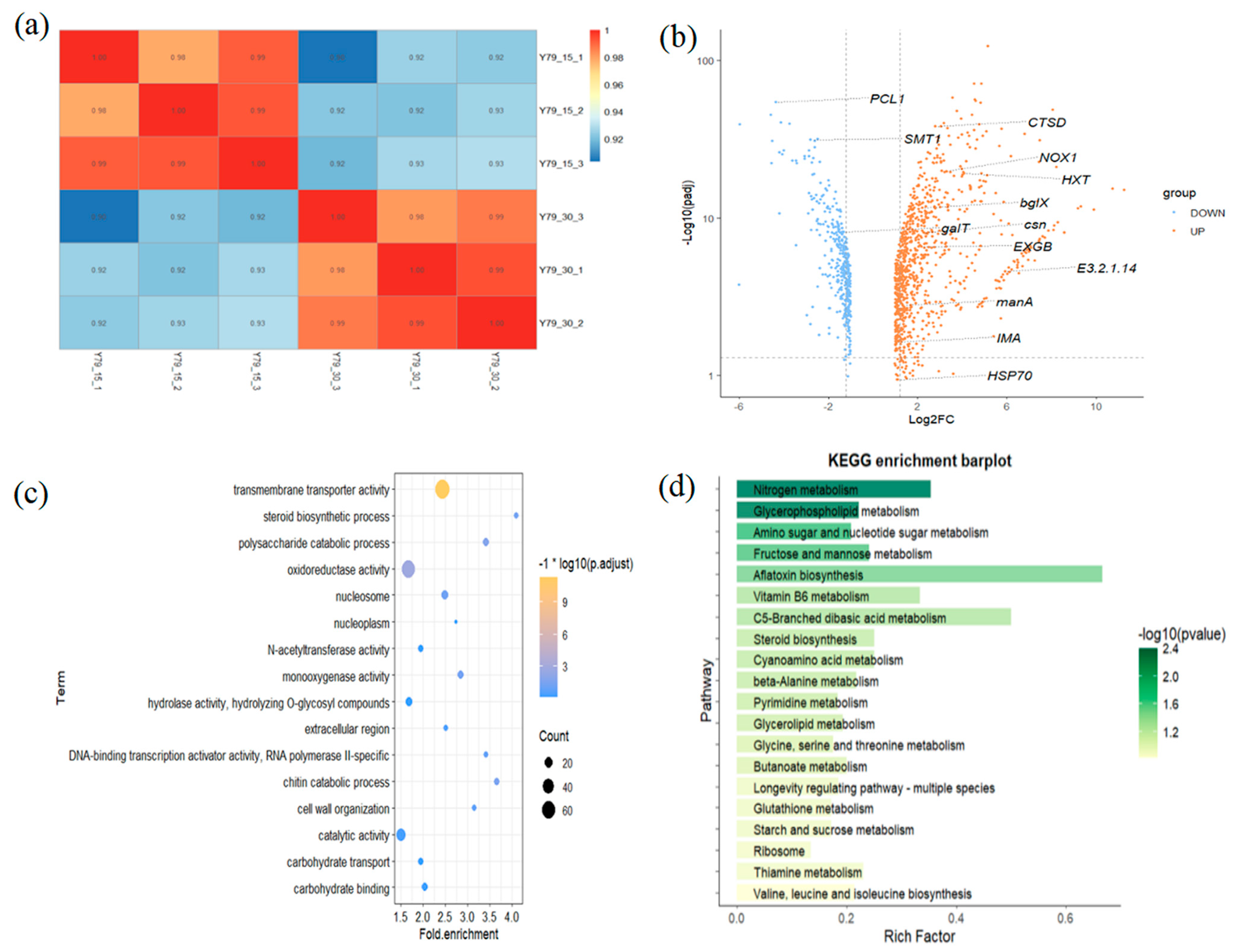
3.13. Transcriptome Analysis of the YM25079 Strain in Response to Heat Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szopinska, A.; Morsomme, P. Quantitative Proteomic Approaches and Their Application in the Study of Yeast Stress Responses. OMICS J. Integr. Biol. 2010, 14, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pousada, C.; Nevitt, T.; Menezes, R. The yeast stress response: Role of the Yap family of b-ZIP transcription factors The PABMB Lecture delivered on 30 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005, 272, 2639–2647. [Google Scholar] [CrossRef]
- Mancuso Nichols, C.A.; Garon, S.; Bowman, J.P.; Raguenes, G.; Guezennec, J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 2004, 96, 1057–1066. [Google Scholar] [CrossRef]
- Nichols, C.A.M.; Guezennec, J.; Bowman, J.P. Bacterial Exopolysaccharides from Extreme Marine Environments with Special Consideration of the Southern Ocean, Sea Ice, and Deep-Sea Hydrothermal Vents: A Review. Mar. Biotechnol. 2005, 7, 253–271. [Google Scholar] [CrossRef]
- Roberson, E.B.; Firestone, M.K. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Evaluation of the production of exopolysaccharide by plant growth promoting yeast Rhodotorula sp. strain CAH2 under abiotic stress conditions. Int. J. Biol. Macromol. 2019, 121, 55–62. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Na, R.; Du, R.; Ping, W.; Ge, J.; Zhao, D. Purification, characterization and partial biological activities of exopolysaccharide produced by Saccharomyces cerevisiae Y3. Int. J. Biol. Macromol. 2022, 206, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, X.; Wang, B.; Lou, W.; Chen, X.; Hua, J.; Sun, Y.J.; Zhao, Y.; Peng, T. Characterization, antioxidativity, and anti-carcinoma activity of exopolysaccharide extract from Rhodotorula mucilaginosa CICC 33013. Carbohydr. Polym. 2018, 181, 768–777. [Google Scholar] [CrossRef]
- Matsuo, K.; Isogai, E.; Araki, Y. Utilization of Exocellular Mannan from Rhodotorula glutinis as an Immunoreactive Antigen in Diagnosis of Leptospirosis. J. Clin. Microbiol. 2000, 38, 3750–3754. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Urooj, Y.; Qamar, S.A.; Khalid, N. Improved exopolysaccharide production from Bacillus licheniformis MS3: Optimization and structural/functional characterization. Int. J. Biol. Macromol. 2020, 151, 984–992. [Google Scholar] [CrossRef]
- Cania, B.; Vestergaard, G.; Krauss, M.; Fliessbach, A.; Schloter, M.; Schulz, S. A long-term field experiment demonstrates the influence of tillage on the bacterial potential to produce soil structure-stabilizing agents such as exopolysaccharides and lipopolysaccharides. Environ. Microbiome 2019, 14, 1. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Ge, J.; Song, G.; Du, R. Application of Microbial Exopolysaccharides in Environment. Chin. Agric. Sci. Bull. 2024, 40, 66–74. [Google Scholar]
- Nguyen, P.-T.; Nguyen, T.-T.; Vo, T.-N.-T.; Nguyen, T.-T.-X.; Hoang, Q.-K.; Nguyen, H.-T. Response of Lactobacillus plantarum VAL6 to challenges of pH and sodium chloride stresses. Sci. Rep. 2021, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, J.; Ye, S.; Liu, Z.; Ding, Y. Characterization of antioxidant activity of exopolysaccharides from endophytic Lysinibacillus sphaericus Ya6 under osmotic stress conditions. Process Biochem. 2022, 113, 87–96. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Yan, Z.; Wan, J.; Lu, S.; Cheng, X. Effect of Cd stress on physiological metabolism and exopolysaccharide synthesis of Enterococcus faecalis CX2-6. Acta Sci. Circumstantiae 2024, 44, 441–452. [Google Scholar] [CrossRef]
- Song, H.; He, M.; Wu, C.; Gu, C.; Wang, C. Global transcriptomic analysis of an Arctic Chlorella-Arc reveals its eurythermal adaptivity mechanisms. Algal Res. 2020, 46, 101792. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, X.; Gao, M.; Shen, A.; Li, H.; Ji, Z. Exopolysaccharide Yield and Environmental Adaptation of Rhizobia Regulated by Gene envZ, iscS and asnC. Biotechnol. Bull. 2024, 40, 277–284. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef]
- Xue, F.; Miao, J.; Zhang, X.; Luo, H.; Tan, T. Studies on lipid production by Rhodotorula glutinis fermentation using monosodium glutamate wastewater as culture medium. Bioresour. Technol. 2008, 99, 5923–5927. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, E.Y.; Chu, K.H. Biopolymer Production by the Yeast Rhodotorula glutinis. Adv. Mater. Res. 2012, 550–553, 1048–1051. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, A.; Li, M.-J.; Cao, P.-F.; Chen, T.-X.; Zhang, G.; Shi, L.; Jiang, A.-L.; Zhao, M.-W. Heat Stress Modulates Mycelium Growth, Heat Shock Protein Expression, Ganoderic Acid Biosynthesis, and Hyphal Branching of Ganoderma lucidum via Cytosolic Ca2+. Appl. Environ. Microbiol. 2016, 82, 4112–4125. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Hu, B.; Ji, X.; Wei, Y.; Lin, L.; Zhang, Q. Correlation of polyunsaturated fatty acids with the cold adaptation of Rhodotorula glutinis. Yeast 2015, 32, 683–690. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Du, C.; Mou, H.; Cui, J.; Guan, H.; Hwang, H.; Wang, P. Characterization of high yield exopolysaccharide produced by Phyllobacterium sp. 921F exhibiting moisture preserving properties. Int. J. Biol. Macromol. 2017, 101, 562–568. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, X. Effects of Carbon Sources on Fermentation Kinetics and Structures of Exopolysaccharide From Papiliotrema aureus. J. Nucl. Agric. Sci. 2021, 35, 384–395. [Google Scholar] [CrossRef]
- Hu, S.-M.; Zhou, J.-M.; Zhou, Q.-Q.; Li, P.; Xie, Y.-Y.; Zhou, T.; Gu, Q. Purification, characterization and biological activities of exopolysaccharides from Lactobacillus rhamnosus ZFM231 isolated from milk. LWT 2021, 147, 111561. [Google Scholar] [CrossRef]
- Chen, G.; Fang, C.; Chen, X.; Wang, Z.; Liu, M.; Kan, J. High-pressure ultrasonic-assisted extraction of polysaccharides from Mentha haplocalyx: Structure, functional and biological activities. Ind. Crops Prod. 2019, 130, 273–284. [Google Scholar] [CrossRef]
- Cao, Y.; Kou, R.; Huang, X.; Wang, N.; Di, D.; Wang, H.; Liu, J. Separation of polysaccharides from Lycium barbarum L. by high-speed countercurrent chromatography with aqueous two-phase system. Int. J. Biol. Macromol. 2024, 256, 128282. [Google Scholar] [CrossRef]
- Kou, R.; Zuo, G.; Liu, J.; Di, D.; Guo, M. Structural properties and hypoglycaemic activity of polysaccharides extracted from the fruits of Lycium barbarum L. using various extraction media. Ind. Crops Prod. 2022, 188, 115725. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lv, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Zhao, F.; Xiao, H.; Zhou, Z.; Han, Y. Purification and characterization of dextran produced by Leuconostoc pseudomesenteroides PC as a potential exopolysaccharide suitable for food applications. Process Biochem. 2019, 87, 187–195. [Google Scholar] [CrossRef]
- Du, R.; Pei, F.; Kang, J.; Zhang, W.; Wang, S.; Ping, W.; Ling, H.; Ge, J. Analysis of the structure and properties of dextran produced by Weissella confusa. Int. J. Biol. Macromol. 2022, 204, 677–684. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Shi, J. Rheological Properties of Exopolysaccharide Produced by Tibetan Kefir. Food Sci. 2016, 37, 1–5. [Google Scholar] [CrossRef]
- Luo, Q. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef]
- Huang, F.; Hong, R.; Zhang, R.; Yi, Y.; Dong, L.; Liu, L.; Jia, X.; Ma, Y.; Zhang, M. Physicochemical and biological properties of longan pulp polysaccharides modified by Lactobacillus fermentum fermentation. Int. J. Biol. Macromol. 2019, 125, 232–237. [Google Scholar] [CrossRef]
- Chen, J.; Pang, W.; Kan, Y.; Zhao, L.; He, Z.; Shi, W.; Yan, B.; Chen, H.; Hu, J. Structure of a pectic polysaccharide from Pseudostellaria heterophylla and stimulating insulin secretion of INS-1 cell and distributing in rats by oral. Int. J. Biol. Macromol. 2018, 106, 456–463. [Google Scholar] [CrossRef]
- Zeng, F. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef]
- Souissi, N.; Boughriba, S.; Abdelhedi, O.; Hamdi, M.; Jridi, M.; Li, S.; Nasri, M. Extraction, structural characterization, and thermal and biomedical properties of sulfated polysaccharides from razor clam Solen marginatus. RSC Adv. 2019, 9, 11538–11551. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Liu, X.; Shen, M.-Y.; Nie, S.-P.; Zhang, H.; Li, C.; Gong, D.-M.; Xie, M.-Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef]
- Silva, D.A.; De Paula, R.C.M.; Feitosa, J.P.A.; De Brito, A.C.F.; Maciel, J.S.; Paula, H.C.B. Carboxymethylation of cashew tree exudate polysaccharide. Carbohydr. Polym. 2004, 58, 163–171. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Chen, C. Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 2014, 105, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, M.I.; Rinaudo, M.; Vancso, G.J. Force Spectroscopy of Hyaluronan by Atomic Force Microscopy: From Hydrogen-Bonded Networks toward Single-Chain Behavior. Biomacromolecules 2007, 8, 2648–2652. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bansal, P.; Singh, J.; Dhanda, S. Purification, partial structural characterization and health benefits of exopolysaccharides from potential probiotic Pediococcus acidilactici NCDC 252. Process Biochem. 2020, 99, 79–86. [Google Scholar] [CrossRef]
- Du, R.; Xing, H.; Yang, Y.; Jiang, H.; Zhou, Z.; Han, Y. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydr. Polym. 2017, 174, 409–416. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, S. Extraction, structure characterization and biological activity of polysaccharides from Malus prunifolia. China Food Addit. 2023, 34, 81–89. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wang, Y.; Anjum, N.; Ahmad, A.; Khan, S.T. Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir—Part II. Food Hydrocoll. 2013, 30, 343–350. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Wu, Y.; Ren, Y.; Liu, Q.; Wang, Q.; Zhou, X.; Cai, M.; Zhang, Y. De novo transcriptome sequencing of marine-derived Aspergillus glaucus and comparative analysis of metabolic and developmental variations in response to salt stress. Genes. Genomics 2017, 39, 317–329. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, L.; Liu, H. Optimization of Heat Stress Condition for Increased Polysaccharide Biosynthesis in Lentinula edodes. Acta Edulis Fungi 2018, 25, 42–46. [Google Scholar] [CrossRef]
- Mezhoud, N.; Zili, F.; Bouzidi, N.; Helaoui, F.; Ammar, J.; Ouada, H.B. The effects of temperature and light intensity on growth, reproduction and EPS synthesis of a thermophilic strain related to the genus Graesiella. Bioprocess Biosyst. Eng. 2014, 37, 2271–2280. [Google Scholar] [CrossRef]
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of the Heat Shock Response and Protein Chaperones: Budding Yeast (Saccharomyces cerevisiae) as a Model System. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef]
- Espinoza, F.H.; Ogas, J. Cell Cycle Control by a Complex of the Cyclin HCS26 (PCL1) and the Kinase PH085. Science 1994, 266, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Campanella, J.E.M.; Candido, T.D.S.; Barbosa, L.C.B.; Gomes, A.A.S.; Leite, C.A.; Higashi, E.S.; Barbugli, P.A.; Fontes, M.R.D.M.; Bertolini, M.C. The neurospora crassa PCL-1 cyclin is a PHO85-1 (PGOV) kinase partner that directs the complex to glycogen metabolism and is involved in calcium metabolism regulation. Front. Microbiol. 2022, 13, 1078972. [Google Scholar] [CrossRef] [PubMed]
- Horák, J. Regulations of sugar transporters: Insights from yeast. Curr. Genet. 2013, 59, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Mahasenan, K.V.; Batuecas, M.T.; De Benedetti, S.; Kim, C.; Rana, N.; Lee, M.; Hesek, D.; Fisher, J.F.; Sanz-Aparicio, J.; Hermoso, J.A.; et al. Catalytic Cycle of Glycoside Hydrolase BglX from Pseudomonas aeruginosa and Its Implications for Biofilm Formation. ACS Chem. Biol. 2020, 15, 189–196. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Lu, M.; Guo, C.; Wang, T.; Fan, J.; Zhang, C.; Qiu, J.; Chen, Y.; Zhang, Q. Effect of Heat Stress on the Biosynthesis of Exopolysaccharides from Rhodotorula glutinis YM25079 and Its Underlying Mechanisms. J. Fungi 2025, 11, 883. https://doi.org/10.3390/jof11120883
Huang R, Lu M, Guo C, Wang T, Fan J, Zhang C, Qiu J, Chen Y, Zhang Q. Effect of Heat Stress on the Biosynthesis of Exopolysaccharides from Rhodotorula glutinis YM25079 and Its Underlying Mechanisms. Journal of Fungi. 2025; 11(12):883. https://doi.org/10.3390/jof11120883
Chicago/Turabian StyleHuang, Rong, Minrao Lu, Caina Guo, Taishen Wang, Jingdie Fan, Chengmei Zhang, Jingwen Qiu, Yuan Chen, and Qi Zhang. 2025. "Effect of Heat Stress on the Biosynthesis of Exopolysaccharides from Rhodotorula glutinis YM25079 and Its Underlying Mechanisms" Journal of Fungi 11, no. 12: 883. https://doi.org/10.3390/jof11120883
APA StyleHuang, R., Lu, M., Guo, C., Wang, T., Fan, J., Zhang, C., Qiu, J., Chen, Y., & Zhang, Q. (2025). Effect of Heat Stress on the Biosynthesis of Exopolysaccharides from Rhodotorula glutinis YM25079 and Its Underlying Mechanisms. Journal of Fungi, 11(12), 883. https://doi.org/10.3390/jof11120883





