Diversity of Sordariales Fungi: Identification of Seven New Species of Naviculisporaceae Through Morphological Analyses and Genome Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Fungal Isolation and Availability
2.2. DNA Isolation for Genome Sequencing, Next-Generation Sequencing and Genome Assembly
2.3. Phylogenetic Analyses
2.4. Phylogenomic Analyses
2.5. Mating-Type Locus Annotation
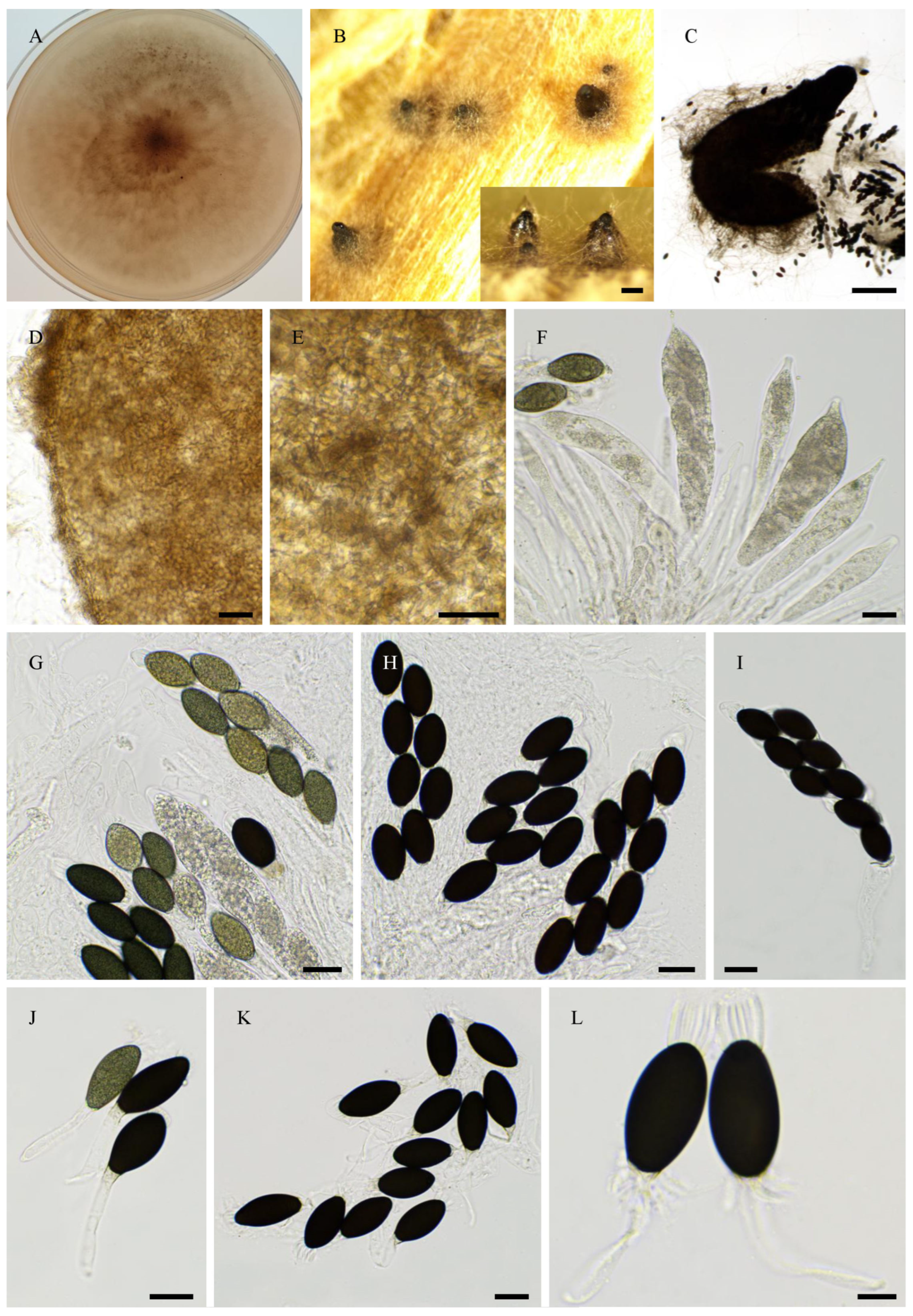
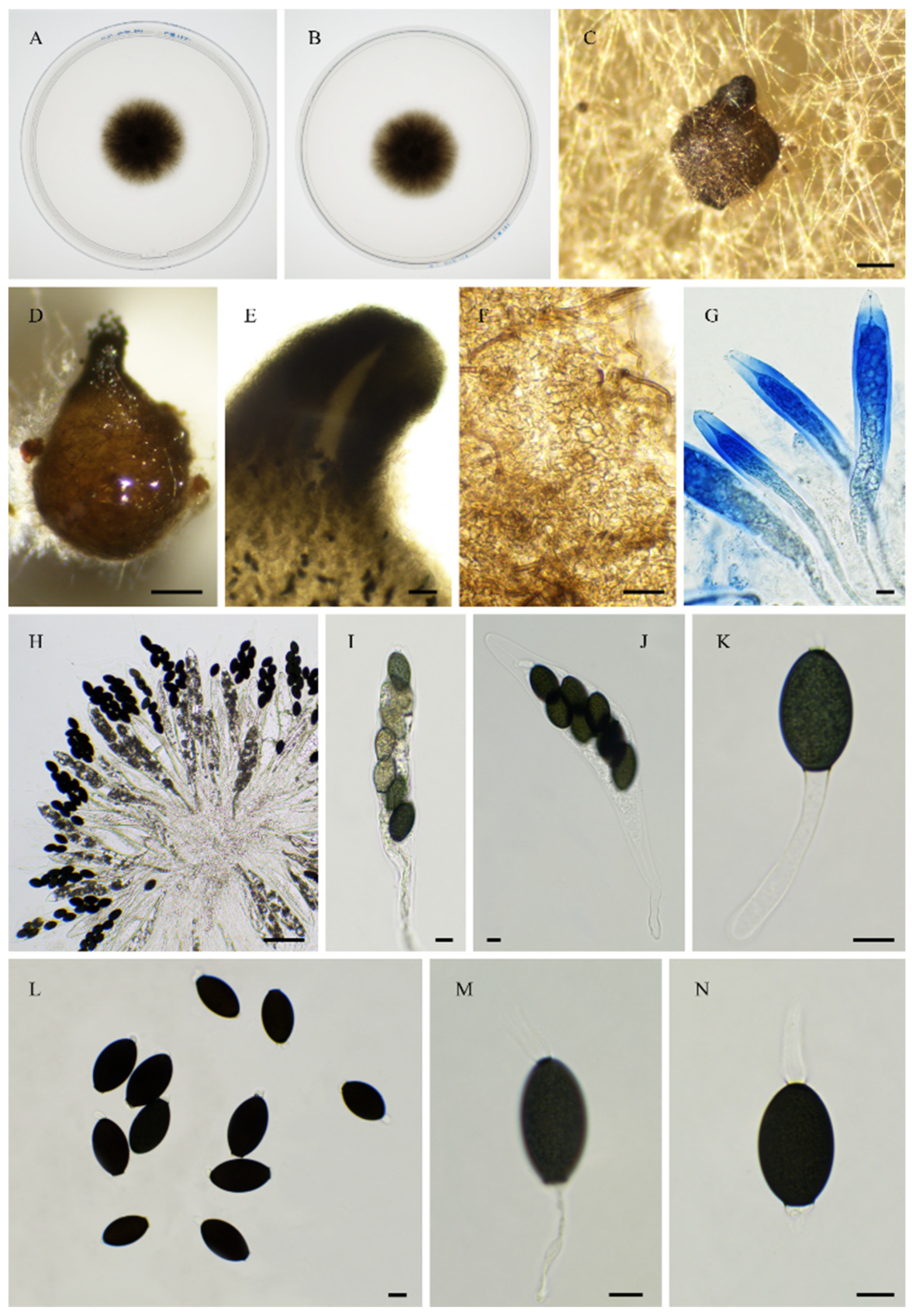
2.6. Morphological Analyses
3. Results
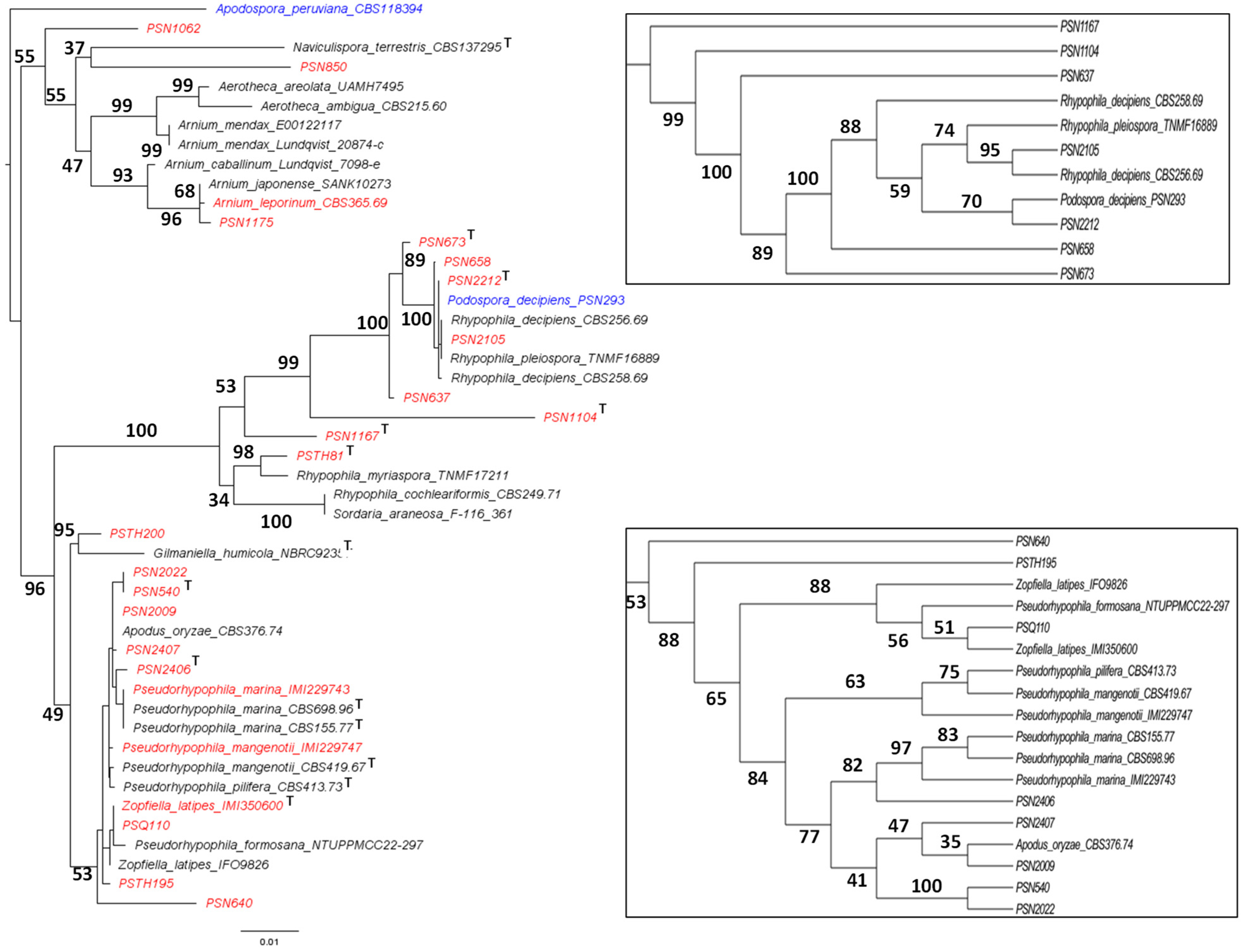
3.1. Fungal Isolates and Species Delimitation
- (1)
- Six previously known species, two of which are newly placed in the Naviculisporaceae: R. myriaspora (PSN2105 and likely CBS 256.69), R. pleiospora (PSN658), P. latipes (formerly Z. latipes; IMI350600 and PSQ110), P. mangenotii (IMI229747), P. marina (IMI229743) and P. oryzae (formerly A. oryzae; PSN2009), adding to the previously sequenced R. decipiens (PSN293);
- (2)
- Seven new species, including five Rhypophila species: R. thailandica sp. nov. (PSTH81), R. reunionensis sp. nov. (PSN1167), R. brasiliensis sp. nov. (PSN1104), R. camarguensis sp. nov. (PSN673) and R. alpibus sp. nov. (PSN2212), as well as two Pseudorhypophila ones: P. guyanensis sp. nov (PSN2406), and P. gallica sp. nov. (PSN540 and PSN2022);
- (3)
- Eight newly isolated strains awaiting further characterization, some of which may belong to species new to science: P. aff. araneosa PSN1062, Naviculispora sp. PSN850, A. aff. japonense PSN1175, Rhypophila sp. PSN637, Gilmaniella sp. PSTH200, Pseudorhypophila sp. PSN640, Pseudorhypophila sp. PSN2407 and Pseudorhypophila sp. PSTH195;
- (4)
- One collection strain: A. leporinum: CBS 365.69.
3.2. Phylogenomic Analysis
3.3. Mating-Type Locus Analysis
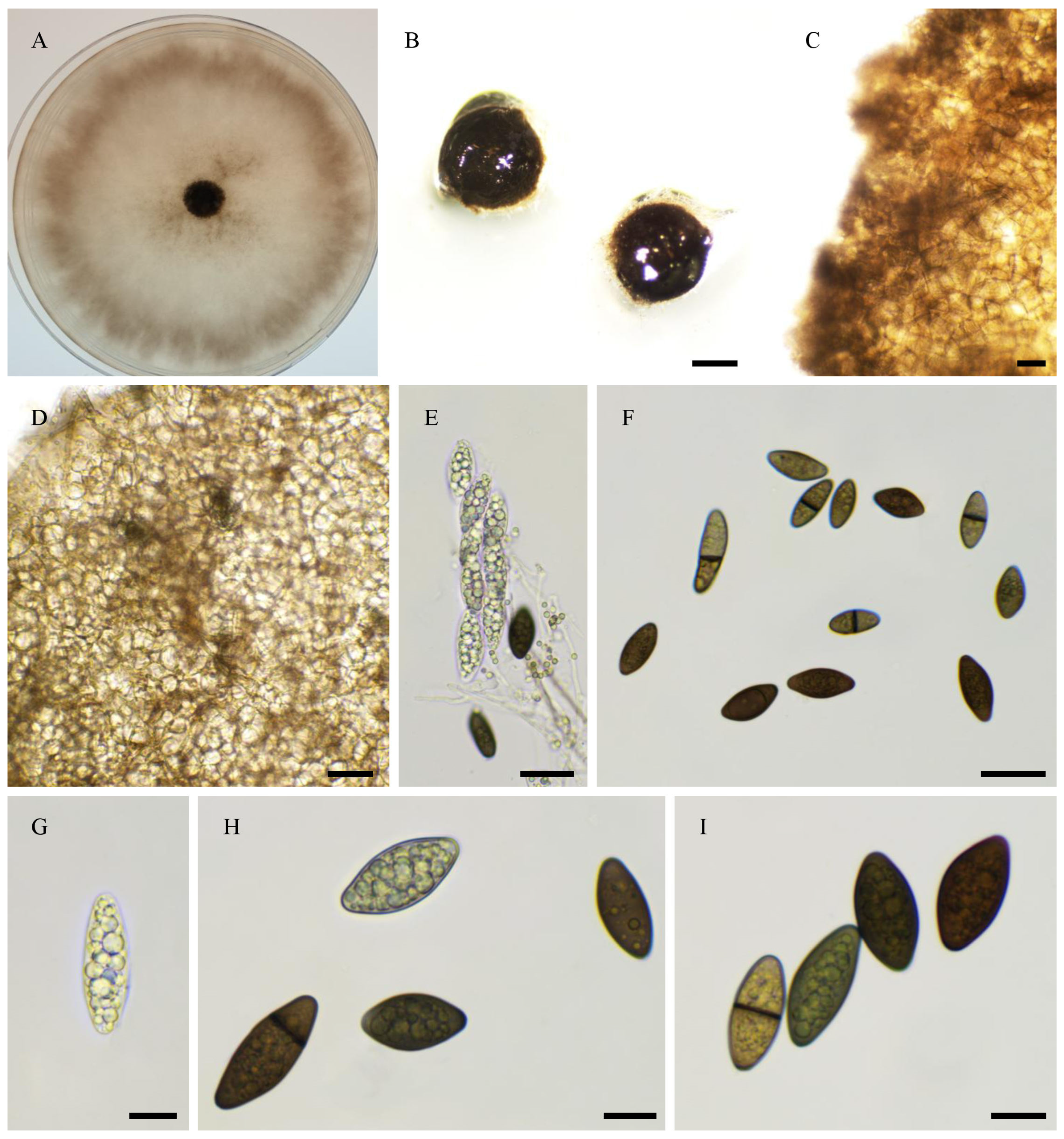
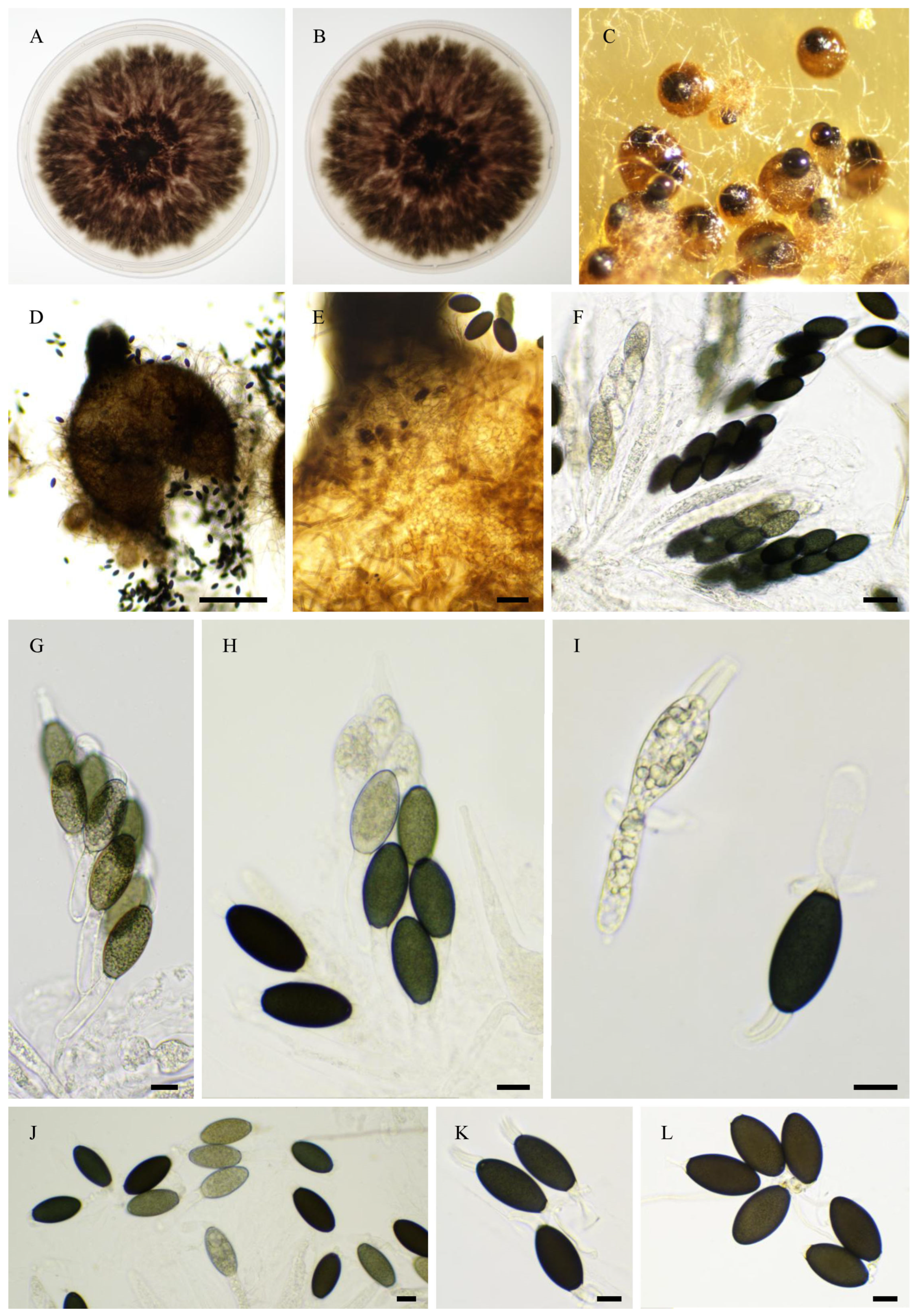
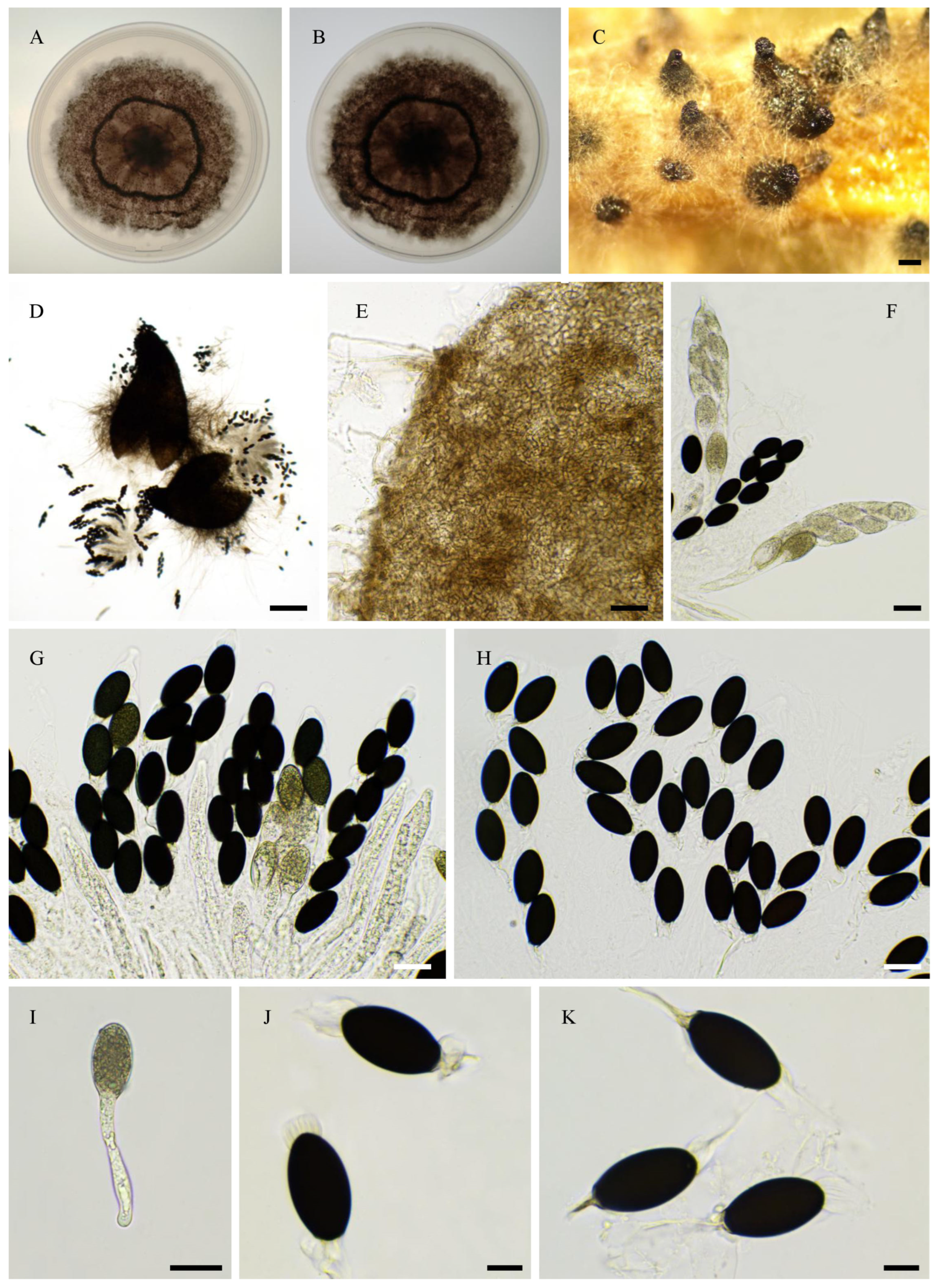
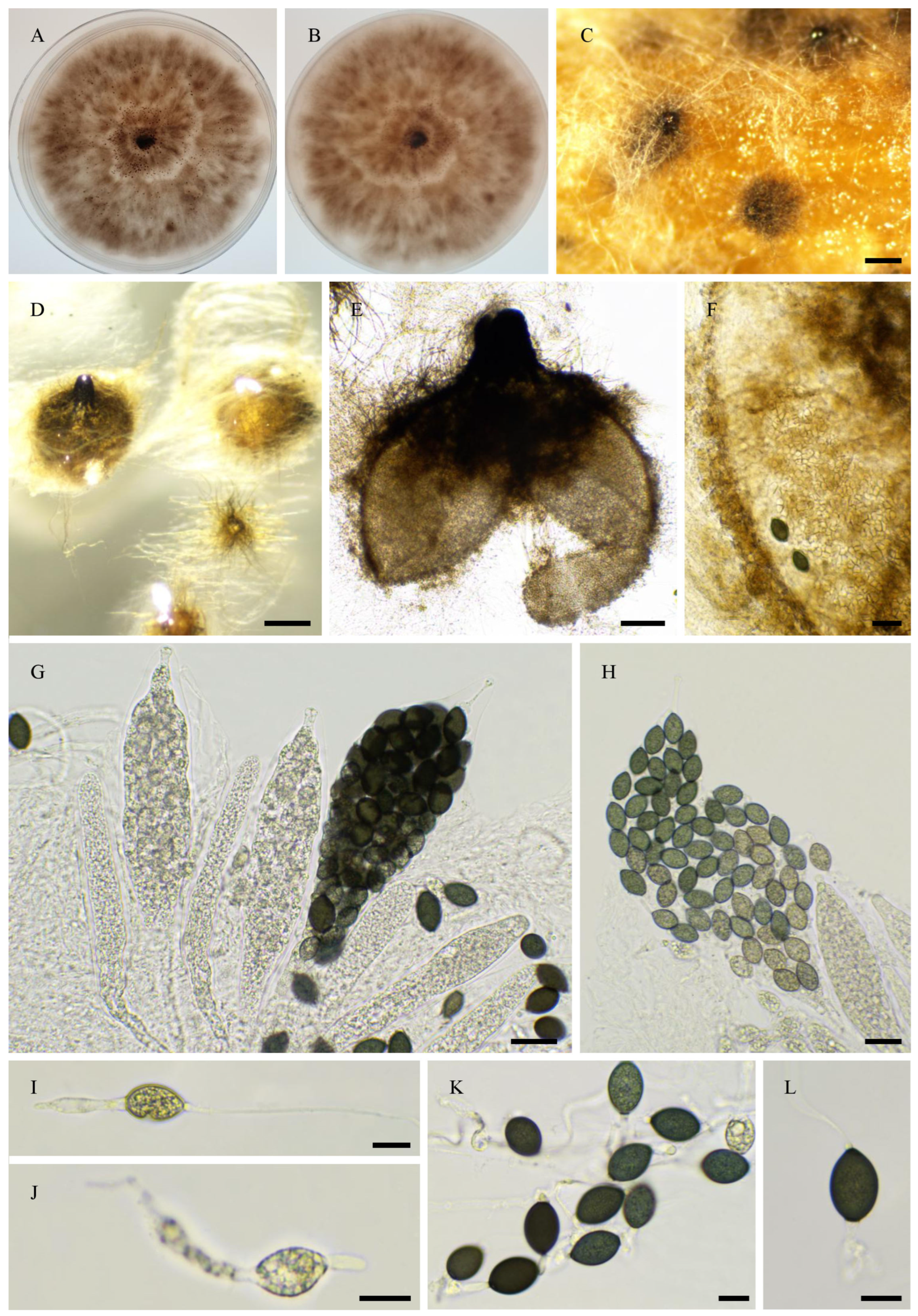
3.4. Taxonomy
3.4.1. New Species
3.4.2. New Combinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANI | Average nucleotide identity |
| ITS | Internal transcribed spacer |
| kbp | Kilobase-pair |
| LSU | rDNA large subunit |
| RPB2 | RNA polymerase II subunit 2 |
| TUB2 | β-tubulin |
References
- Gostinčar, C. Towards Genomic Criteria for Delineating Fungal Species. J. Fungi 2020, 6, 246. [Google Scholar] [CrossRef]
- Lalanne, C.; Silar, P. FungANI, a BLAST-based program for analyzing average nucleotide identity (ANI) between two fungal genomes, enables easy fungal species delimitation. Fungal Genet. Biol. 2025, 177, 103969. [Google Scholar] [CrossRef]
- Lin, P.; Kook, M.; Yi, T.H.; Yan, Z.F. Current Fungal Taxonomy and Developments in the Identification System. Curr. Microbiol. 2023, 80, 375. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, E.; Gautier, V.; Lalanne, C.; Levert, E.; Chahine, E.; Hartmann, F.E.; Giraud, T.; Silar, P. Huge genetic diversity of Schizothecium tetrasporum (Wint.). N. Lundq.: Delimitation of 18 species distributed into three complexes through genome sequencing. Mycosphere 2025, 16, 2936–2974. Available online: https://www.mycosphere.org/pdf/MYCOSPHERE_16_1_19.pdf (accessed on 11 October 2025). [CrossRef]
- Dettman, J.R.; Jacobson, D.J.; Taylor, J.W. a multilocus genealogical approach to phylogenetic species recognition in the model eukaryote NEUROSPORA. Evolution 2003, 57, 2703–2720. [Google Scholar] [CrossRef] [PubMed]
- Dettman, J.R.; Jacobson, D.J.; Turner, E.; Pringle, A.; Taylor, J.W. reproductive isolation and phylogenetic divergence in neurospora: Comparing methods of species recognition in a model eukaryote. Evolution 2003, 57, 2721–2741. [Google Scholar] [CrossRef]
- Dettman, J.R.; Jacobson, D.J.; Taylor, J.W. Multilocus sequence data reveal extensive phylogenetic species diversity within the Neurospora discreta complex. Mycologia 2006, 98, 436–446. [Google Scholar] [CrossRef]
- Svedberg, J.; Vogan, A.A.; Rhoades, N.A.; Sarmarajeewa, D.; Jacobson, D.J.; Lascoux, M.; Hammond, T.M.; Johannesson, H. An introgressed gene causes meiotic drive in Neurospora sitophila. Proc. Natl. Acad. Sci. USA 2021, 118, e2026605118. [Google Scholar] [CrossRef]
- Menkis, A.; Bastiaans, E.; Jacobson, D.J.; Johannesson, H. Phylogenetic and biological species diversity within the Neurospora tetrasperma complex. J. Evol. Biol. 2009, 22, 1923–1936. [Google Scholar] [CrossRef]
- Boucher, C.; Nguyen, T.-S.; Silar, P. Species delimitation in the Podospora anserina/P. pauciseta/P. comata species complex (Sordariales). Crypt. Mycol. 2017, 38, 485–506. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Miller, A.N.; Cano-Lira, J.F.; Guarro, J.; García, D.; Stadler, M.; Huhndorf, S.M.; Stchigel, A.M. Re-Evaluation of the Order Sordariales: Delimitation of Lasiosphaeriaceae s. str., and Introduction of the New Families Diplogelasinosporaceae, Naviculisporaceae, and Schizotheciaceae. Microorganisms 2020, 8, 1430. [Google Scholar] [CrossRef]
- Harms, K.; Milic, A.; Stchigel, A.M.; Stadler, M.; Surup, F.; Marin-Felix, Y. Three New Derivatives of Zopfinol from Pseudorhypophila mangenotii gen. et comb. nov. J. Fungi 2021, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.W.; Yang, J.I.; Srimongkol, P.; Stadler, M.; Karnchanatat, A.; Ariyawansa, H.A. Fungal frontiers in toxic terrain: Revealing culturable fungal communities in Serpentine paddy fields of Taiwan. IMA Fungus 2025, 16, e155308. [Google Scholar] [CrossRef] [PubMed]
- Hensen, N.; Bonometti, L.; Westerberg, I.; Brännström, I.O.; Guillou, S.; Cros-Aarteil, S.; Calhoun, S.; Haridas, S.; Kuo, A.; Mondo, S.; et al. Genome-scale phylogeny and comparative genomics of the fungal order Sordariales. Mol. Phylogenetics Evol. 2023, 189, 107938. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Lundqvist, N. Nordic Sordariaceae S. Lat.; Acta Universitatis Upsaliensis: Uppsala, Sweden, 1972; pp. 147–152. [Google Scholar]
- Thiyagaraja, V.; Hyde, K.; Piepenbring, M.; Davydov, E.; Dai, D.; Abdollahzadeh, J.; Bundhun, D.; Chethana, K.; Crous, P.; Gajanayake, A. Orders of Ascomycota. Mycosphere 2025, 16, 536–1411. [Google Scholar] [CrossRef]
- Silar, P.; Gautier, V.; Lalanne, C.; Tangthirasunun, N.; Arthur, M.; Hartmann, F.E.; Giraud, T. The Podospora anserina species complex in metropolitan and overseas France with description of a new species, Podospora reunionensis sp. nov. Cryptogam. Mycol. 2025, 46, 87–101. [Google Scholar] [CrossRef]
- Silar, P. Podospora anserina; HAL: Lyon, France, 2020; Available online: https://hal.archives-ouvertes.fr/hal-02475488 (accessed on 11 October 2025).
- Vittorelli, N.; Rodríguez de la Vega, R.C.; Snirc, A.; Levert, E.; Gautier, V.; Lalanne, C.; De Filippo, E.; Gladieux, P.; Guillou, S.; Zhang, Y.; et al. Stepwise recombination suppression around the mating-type locus in an ascomycete fungus with self-fertile spores. PLoS Genet. 2023, 19, e1010347. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; dePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2014; Available online: https://escholarship.org/uc/item/1h3515gn (accessed on 11 October 2025).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Crouan, P.L.; Crouan, H.M. Sordaria DNtrs. In Florule du Finistère; Klincksieck, F., Ed.; J.B. & A. Lefournier: Paris, France; Brest, France, 1867; Available online: https://www.biodiversitylibrary.org/item/43942#page/9/mode/1up (accessed on 11 October 2025).
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, W7–W13. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Cain, R.F. Studies of Coprophilous Sphaeriales in Ontario; University of Toronto: Toronto, ON, Canada, 1934. [Google Scholar]
- Mirza, J.H.; Cain, R.F. Revision of the genus Podospora. Can. J. Bot. 1969, 47, 1999–2048. [Google Scholar] [CrossRef]
- Doveri, F. Fungi Fimicoli Italici; Associazione Micologia Bresadola: Trento, Italy, 2004. [Google Scholar]
- Cai, L.; Jeewon, R.; Hyde, K.D. Molecular systematics of Zopfiella and allied genera: Evidence from multi-gene sequence analyses. Mycol. Res. 2006, 110, 359–368. [Google Scholar] [CrossRef]
- Lundqvist, N. Tripterospora (Sordariaceae s. lat., Pyrenomycetes). Bot. Not. 1969, 122, 589–603. Available online: https://journals.lub.lu.se/bn/article/view/11343 (accessed on 11 October 2025).
- Huang, S.-K.; Hyde, K.D.; Mapook, A.; Maharachchikumbura, S.S.N.; Bhat, J.D.; McKenzie, E.H.C.; Jeewon, R.; Wen, T.-C. Taxonomic studies of some often over-looked Diaporthomycetidae and Sordariomycetidae. Fungal Divers. 2021, 111, 443–572. [Google Scholar] [CrossRef]
- von Arx, J.A. On Thielavia and some similar genera of ascomycetes. Stud. Mycol. 1975, 8, 1–29+22pl. Available online: https://www.studiesinmycology.org/sim/Sim08/fulltext.htm (accessed on 11 October 2025).
- Von Arx, J.; Hennebert, G. Triangularia mangenotii nov. sp. Bull. Soc. Mycol. Fr. 1968, 84, 423–426. Available online: https://gallica.bnf.fr/ark:/12148/bd6t53441122/f439.item (accessed on 11 October 2025).
- Furuya, K.; Udagawa, S. Two new species of cleistothecial Ascomycetes. J. Jpn. Bot. 1975, 50, 249–254. [Google Scholar]
- Pöggeler, S.; Risch, S.; Kück, U.; Osiewacz, H.D. Mating-type genes from the homothallic fungus Sordaria macrospora are functionally expressed in a heterothallic ascomycete. Genetics 1997, 147, 567–580. [Google Scholar] [CrossRef]
- Bennett Richard, J.; Turgeon, B.G. Fungal Sex: The Ascomycota. Microbiol. Spectr. 2016, 4, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Debuchy, R.; Berteaux-Lecellier, V.; Silar, P. Mating Systems and Sexual Morphogenesis in Ascomycetes. In Cellular and Molecular Biology of Filamentous Fungi; Borkovich, K., Ebbole, D., Eds.; ASM Press: Washington, DC, USA, 2010; pp. 501–535. [Google Scholar] [CrossRef]
- Cailleux, R. Champignons stercoraux de République Centrafricaine, III Podospora nouveaux. Cah. Maboké 1969, 7, 87–102. Available online: https://biostor.org/reference/255548 (accessed on 11 October 2025).
- Berka, R.M.; Grigoriev, I.V.; Otillar, R.; Salamov, A.; Grimwood, J.; Reid, I.; Ishmael, N.; John, T.; Darmond, C.; Moisan, M.C.; et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 2011, 29, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Han, P.J.; Bai, F.Y.; Luo, A.; Bensch, K.; Meijer, M.; Kraak, B.; Han, D.Y.; Sun, B.D.; Crous, P.W.; et al. Taxonomy, phylogeny and identification of Chaetomiaceae with emphasis on thermophilic species. Stud. Mycol. 2022, 101, 121–243. [Google Scholar] [CrossRef] [PubMed]
- Steindorff, A.S.; Aguilar-Pontes, M.V.; Robinson, A.J.; Andreopoulos, B.; LaButti, K.; Kuo, A.; Mondo, S.; Riley, R.; Otillar, R.; Haridas, S.; et al. Comparative genomic analysis of thermophilic fungi reveals convergent evolutionary adaptations and gene losses. Commun. Biol. 2024, 7, 1124. [Google Scholar] [CrossRef]
- Aime, M.C.; Miller, A.N.; Aoki, T.; Bensch, K.; Cai, L.; Crous, P.W.; Hawksworth, D.L.; Hyde, K.D.; Kirk, P.M.; Lücking, R.; et al. How to publish a new fungal species, or name, version 3.0. IMA Fungus 2021, 12, 11. [Google Scholar] [CrossRef]
- Hutchinson, M.I.; Powell, A.J.; Tsang, A.; O’Toole, N.; Berka, R.M.; Barry, K.; Grigoriev, I.V.; Natvig, D.O. Genetics of mating in members of the Chaetomiaceae as revealed by experimental and genomic characterization of reproduction in Myceliophthora heterothallica. Fungal Genet. Biol. 2016, 86, 9–19. [Google Scholar] [CrossRef]



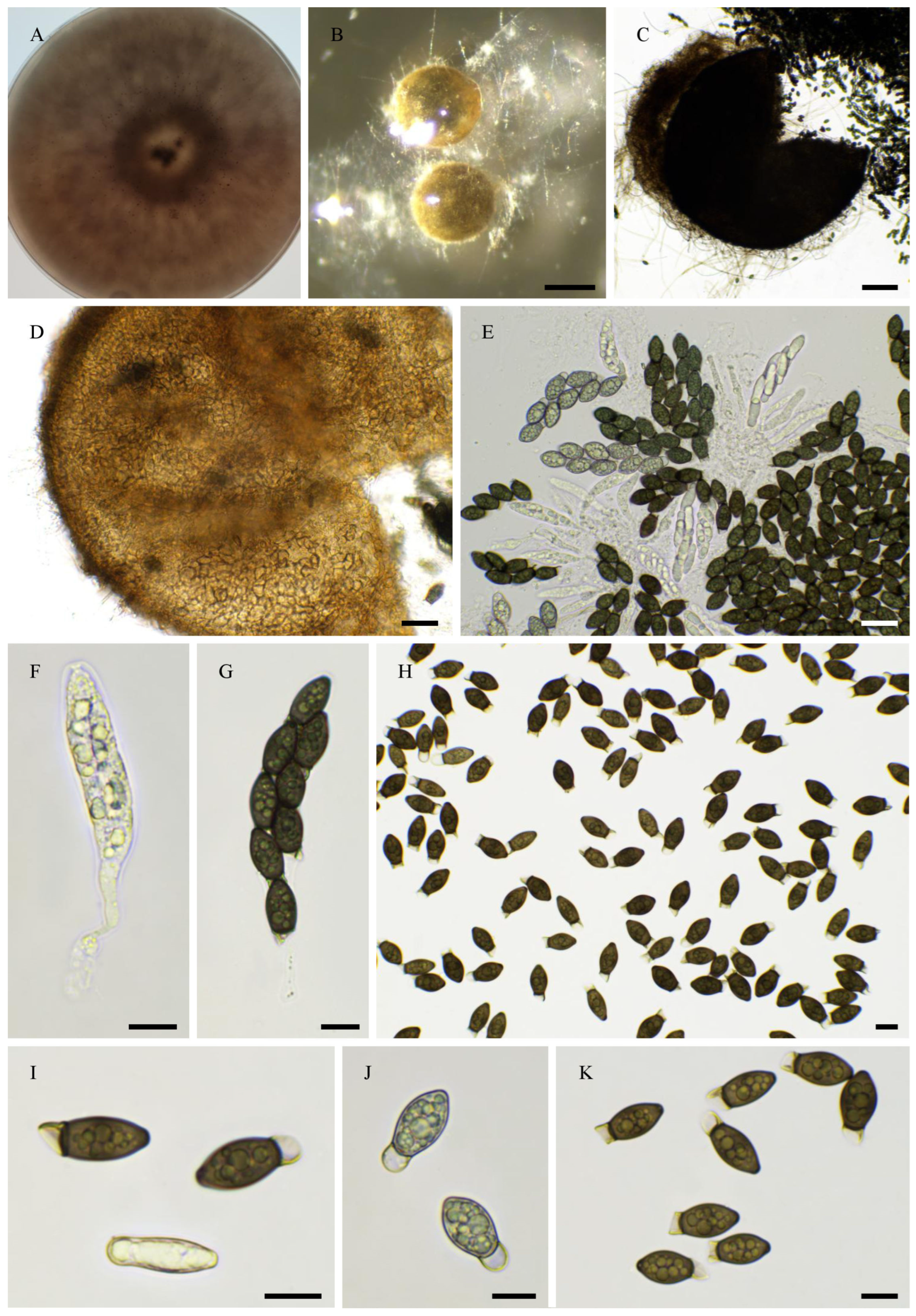
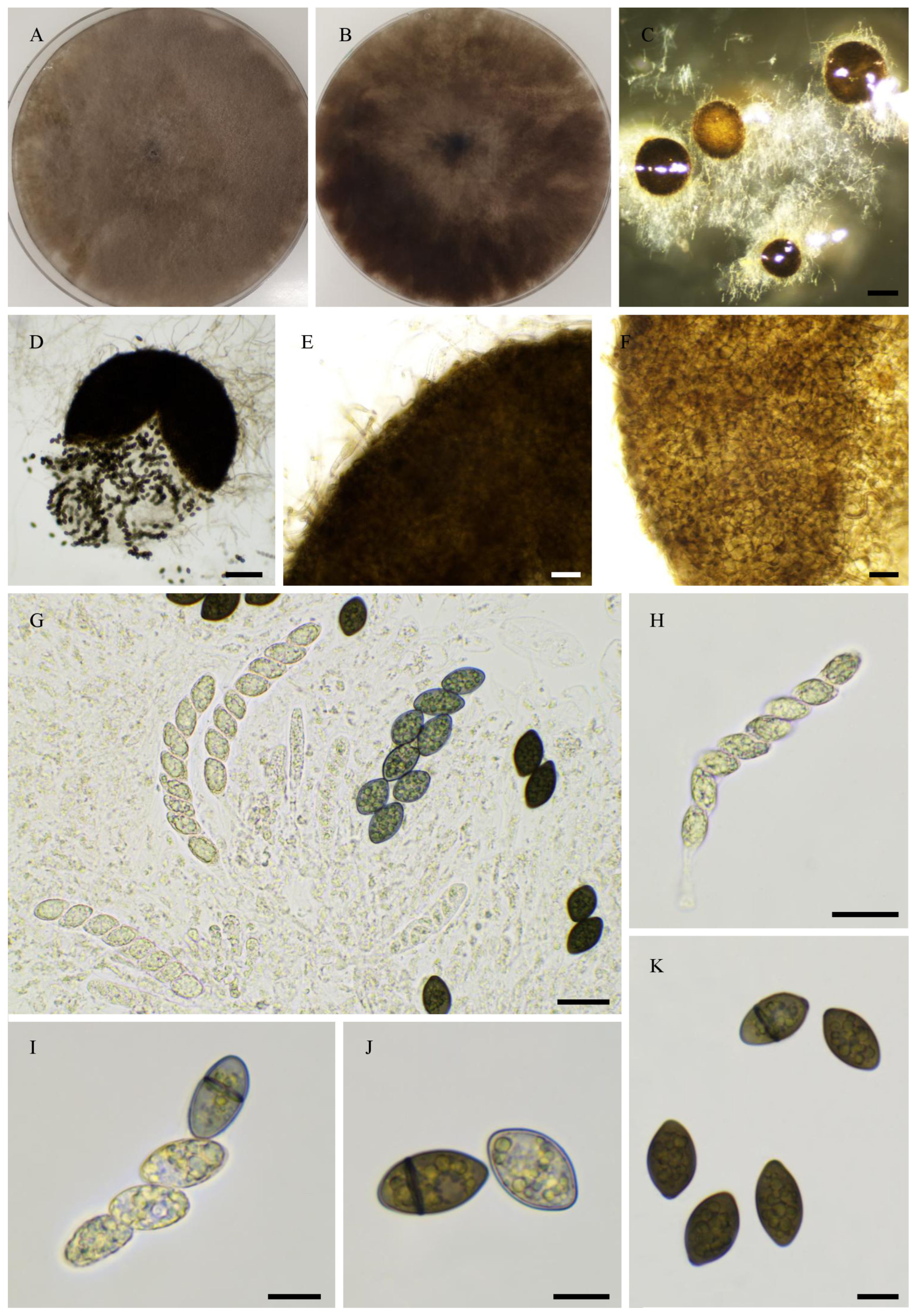

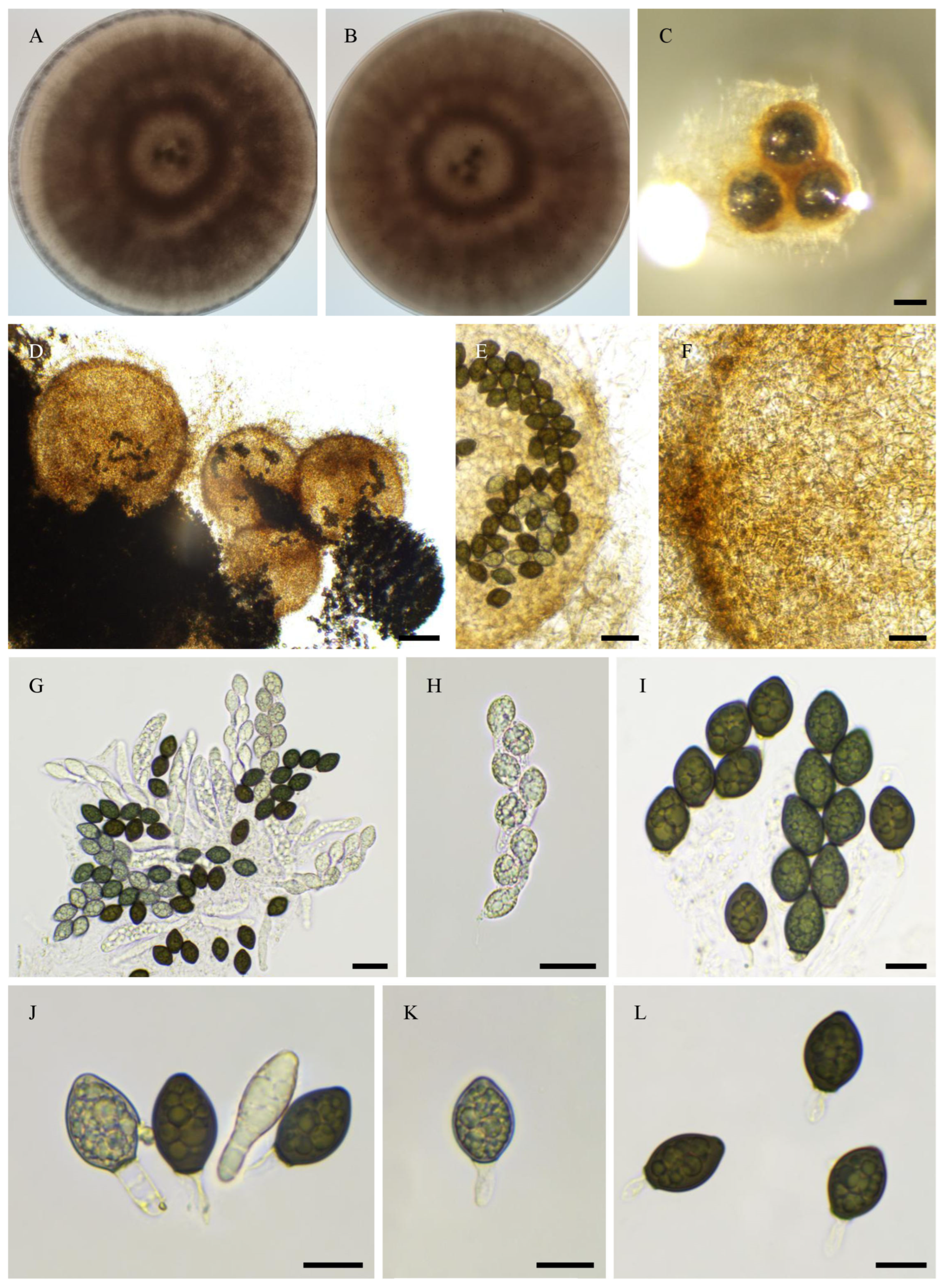
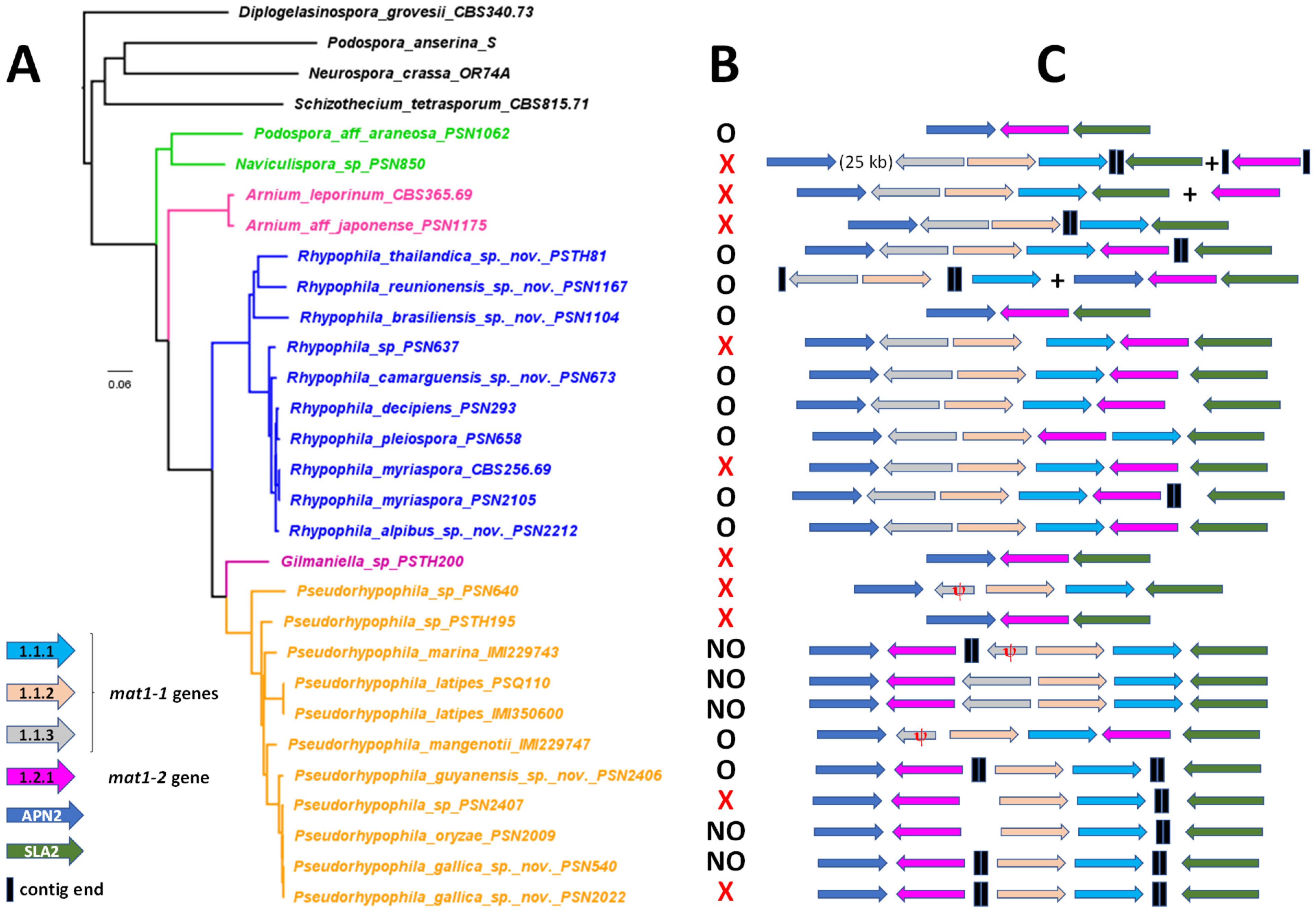


| Strain | Species (Other Names *) | Isolation Year | Origin | Substrate | Sequencing Palteform | Genome Size (Mb) | Probable Breeding System |
|---|---|---|---|---|---|---|---|
| PSN1062 | Podospora aff. araneosa | 2021 | Alps, France | Hare dung | Novogene | 40.3 | heterothallic |
| PSN850 | Naviculispora sp. | 2021 | Camargue, France | Horse dung | Novogene | 41.8 | heterothallic |
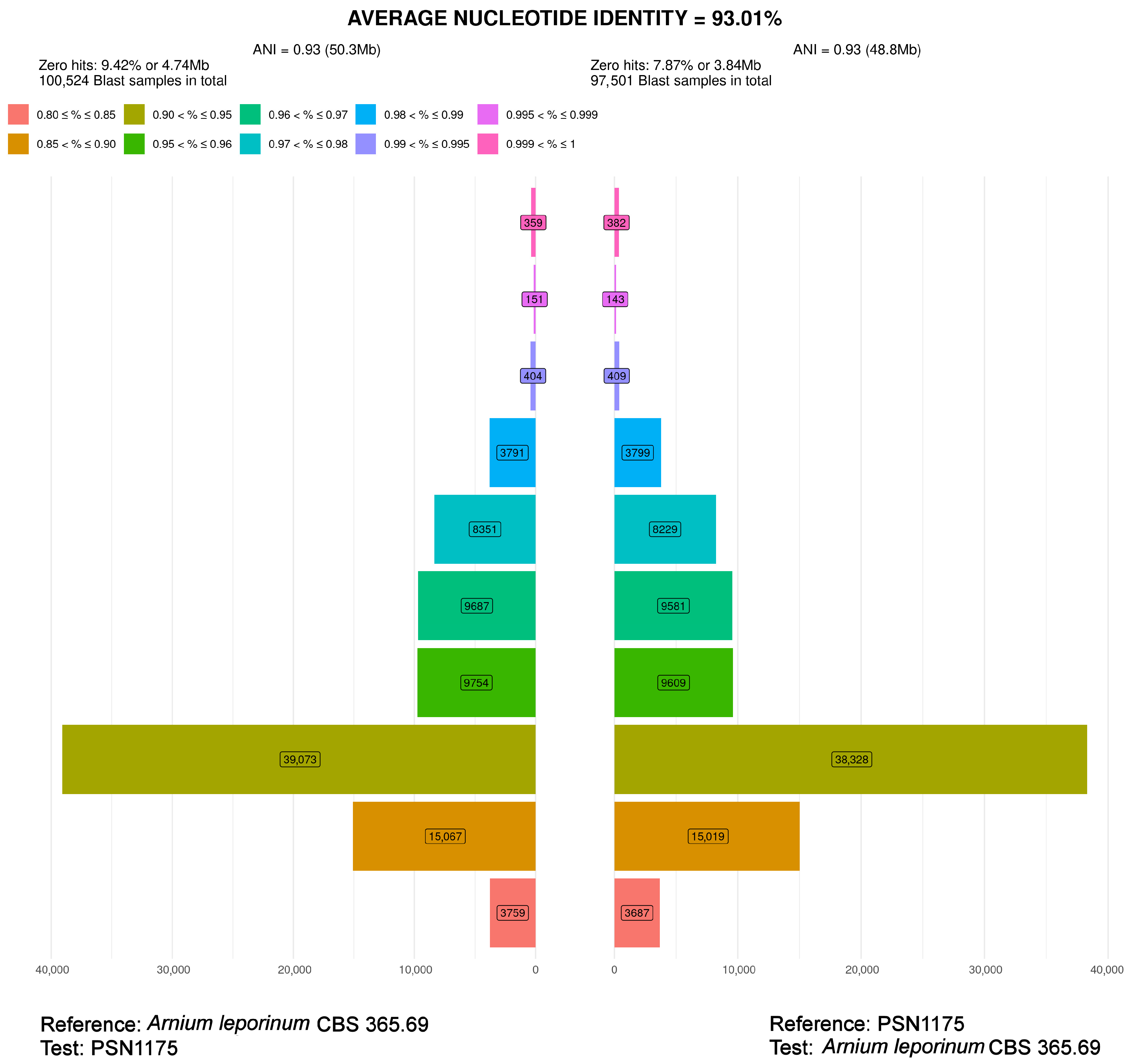
| CBS 365.69 | Arnium leporinum (Podospora leporina) | 1969 | The Netherlands | Rabbit dung | JGI | 48.5 | heterothallic |
| PSN1175 | Arnium aff. japonense | 2021 | Kobarld, Slovenia | Deer dung | Biomics | 49.6 | heterothallic |
| PSTH81 | Rhypophila thailandica sp. nov. | 2023 | Ayuthaya, Thailand | Elephant dung | Novogene | 50.3 | homothallic |
| PSN1167 | Rhypophila reunionensis sp. nov. | 2022 | La Réunion Island, France | Cow dung | Novogene | 46.3 | heterothallic |
| PSN1104 | Rhypophila brasiliensis sp. nov. | 2021 | Campinas, Brazil | Capybara dung | Biomics | 45.9 | heterothallic |
| PSN637 | Rhypophila sp. | 2021 | Ontario, Canada | White rabbit dung | JGI | 46.9 | homothallic |
| PSN673 | Rhypophila camarguensis sp. nov. | 2021 | Camargue, France | Cow dung | Novogene | 45.9 | homothallic |
| PSN293 $ | Rhypophila decipiens (Podospora decipiens) | 2017 | Auvergne, France | Donkey dung | JGI | 47.1 | homothallic |
| PSN2105 | Rhypophila myriaspora | 2024 | Picardie, France | Horse dung | Novogene | 48.2 | homothallic |
| CBS 256.69 | Rhypophila myriaspora (Rhypophila decipiens) | 1969 | The Netherlands | Rabbit dung | JGI | 48.3 | homothallic |
| PSN658 | Rhypophila pleiospora | 2021 | Ontario, Canada | Sylvillagus floridanus dung | JGI | 46.7 | homothallic |
| PSN2212 | Rhypophila alpibus sp. nov. | 2024 | Alps, Italy | Cow dung | Novogene | 47.9 | homothallic |
| PSTH200 | Gilmaniella sp. | 2023 | Chonburi, Thailand | Elephant dung | Novogene | 51.8 | heterothallic |
| PSN640 | Pseudorhypophila sp. | 2021 | Chad | Camel dung | JGI | 47.6 | heterothallic |
| PSTH195 | Pseudorhypophila sp. | 2023 | Chiang Rai, Thailand | Soil | Novogene | 50.6 | heterothallic |
| IMI229743 | Pseudorhypophila marina (Zopfiella marina) | 1978 | Japan | Marine mud | JGI | 53.7 | homothallic |
| IMI350600 | Pseudorhypophila latipes comb. nov. (Zopfiella latipes) | 1991 | U.K. | Not stated by the provider | Novogene | 52.3 | homothallic |
| PSQ110 | Pseudorhypophila latipes comb. nov. | 2019 | Québec, Canada | Soil | Novogene | 52.6 | homothallic |
| IMI229747 | Pseudorhypophila mangenotii (Triangularia mangenotii) | 1978 | Japan | Soil | JGI | 54.3 | homothallic |
| PSN2406 | Pseudorhypophila guyanensis sp. nov. | 2025 | Guyane | Soil | Novogene | 52.6 | homothallic |
| PSN2407 | Pseudorhypophila sp. | 2025 | Guyane | Soil | Novogene | 52.8 | homothallic |
| PSN2009 | Pseudorhypophila oryzae comb. nov. | 2024 | Centre-Val de Loire, France | Soil | Novogene | 56.2 | homothallic |
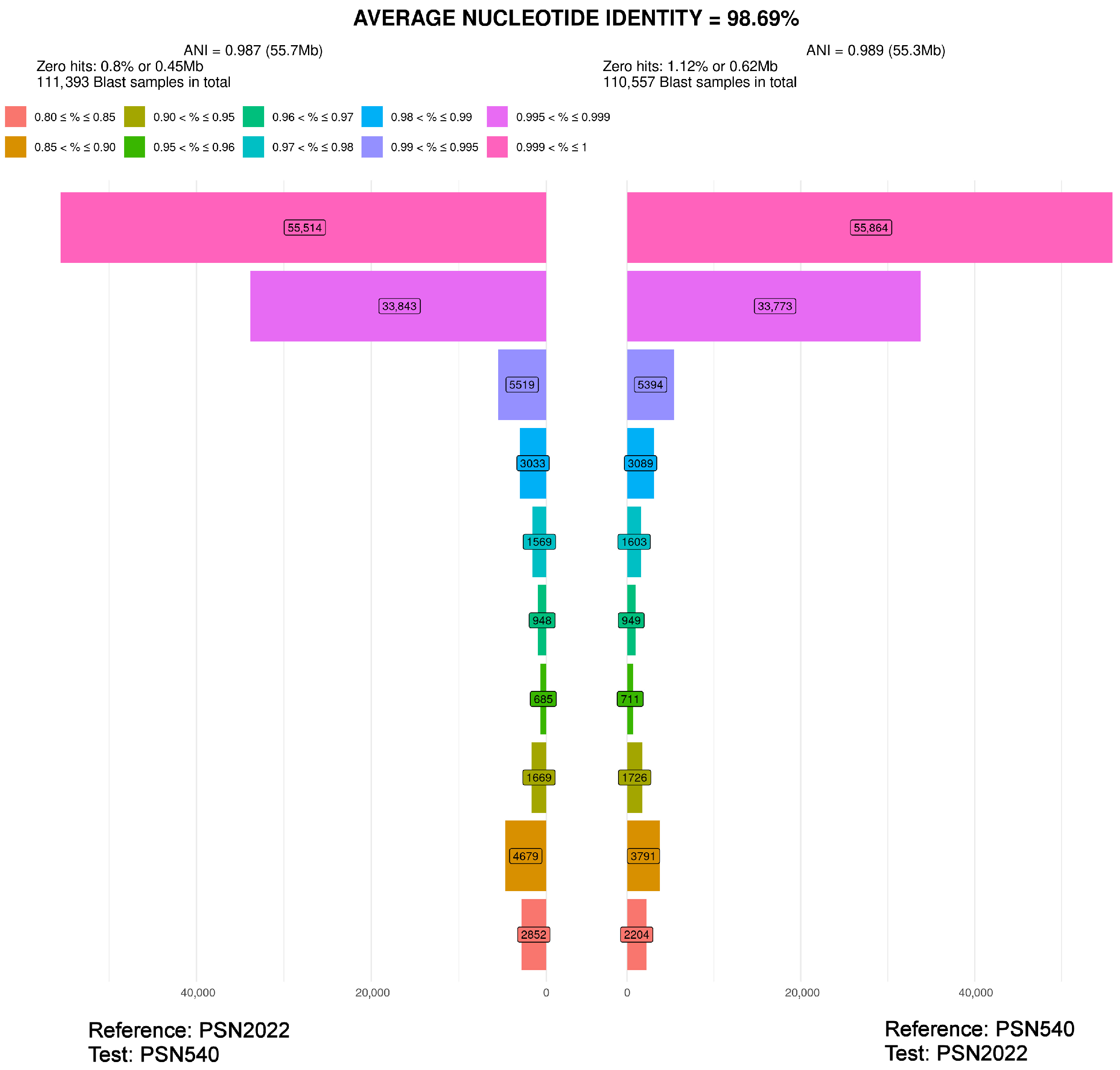
| PSN540 | Pseudorhypophila gallica sp. nov. | 2020 | Picardie, France | Soil | JGI | 55.4 | homothallic |
| PSN2022 | Pseudorhypophila gallica sp. nov. | 2024 | Centre-Val de Loire, France | Soil | Novogene | 54.6 | homothallic |
| Strain | Taxa | ITS | LSU |
|---|---|---|---|
| CBS 118,394 | Apodospora peruviana | EU573703 | KF557665 |
| CBS 506.70 T | Apodus deciduus | NR_145141.1 | NG_056953 |
| CBS 376.74 | Apodus oryzae | AY68120 | AY681166 |
| CBS 215.60 | Areotheca ambigua | AY999137 | AY999114 |
| UAMH 7495 | Areotheca areolata | AY587911 | AY587936 |
| Lundqvist 7098-e | Arnium caballinum | NA | KF557672 |
| SANK 10,273 | Arnium japonense | NA | KF557680 |
| Lundqvist20874-c | Arnium mendax | NA | KF557687 |
| E00122117 | Arnium mendax | NA | KF557688 |
| NBRC 9235 T | Gilmaniella humicola | available at https://www.nite.go.jp/nbrc/catalogue/ (accessed on 11 October 2025) | |
| CBS 137,295 T | Naviculispora terrestris | MT784136 | KP981439 |
| NTUPPMCC 22-297 T | Pseudorhypophila formosana | PV476805 | PV476867 |
| CBS 155.77 T | Pseudorhypophila marina | MK926851 | MK926851 |
| CBS 698.96 | Pseudorhypophila marina | MK926853 | MK926853 |
| CBS 413.73 T | Pseudorhypophila pilifera | MK926852 | MK926852 |
| CBS 419.67 T | Pseudorhypophila mangenotii | MT784143 | KP981444 |
| CBS 249.71 | Rhypophila cochleariformis | AY999123 | AY999098 |
| CBS 258.69 | Rhypophila decipiens | KX171946 | AY780073 |
| TNM F17211 | Rhypophila myriaspora $ | EF197083 | NA |
| TNM F16889 | Rhypophila pleiospora | EF197084 | NA |
| F-116,361 | Sordaria araneosa | FJ175160 | NA |
| IFO9826 | Zopfiella latipes | AY999129 | AY999107 |
| PSN2212 | PSN2105 | CBS 256.69 | PSN293 | |
|---|---|---|---|---|
| PSN658 | 94.99% | 95.07% | 95.08% | 95.17% |
| PSN2212 | 95.08% | 95.09% | 95.21% | |
| PSN2105 | 99.74% | 95.20% | ||
| CBS 256.69 | 95.20% |
| PSQ110 | IMI350600 | IMI229747 | PSN2406 | IMI229743 | PSN540 | PSN2022 | PSN2407 | PSN2009 | |
|---|---|---|---|---|---|---|---|---|---|
| PSTH195 | 87.90% | 88.47% | 88.49% | 87.75% | 89.14% | 87.86% | 87.86% | 87.85% | 87.87% |
| PSQ110 | 99.26% | 87.91% | 87.25% | 88.36% | 87.34% | 87.36% | 87.28% | 87.42% | |
| IMI350600 | 87.93% | 87.25% | 88.37% | 87.38% | 87.38% | 87.32% | 87.46% | ||
| IMI229747 | 88.25% | 89.02% | 88.35% | 88.36% | 88.35% | 88.35% | |||
| PSN2406 | 87.78% | 94.18% | 94.18 | 94.10% | 94.00% | ||||
| IMI229743 | 87.90% | 87.91% | 87.84% | 87.95% | |||||
| PSN540 | 98.69% * | 96.28% | 97.26% | ||||||
| PSN2022 | 96.43% | 97.30% | |||||||
| PSN2407 | 96.44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tangthirasunun, N.; Gautier, V.; Lalanne, C.; Bonometti, L.; Cros-Arteil, S.; Hayes, R.D.; Calhoun, S.; Riley, R.; Pangilinan, J.; Lipzen, A.; et al. Diversity of Sordariales Fungi: Identification of Seven New Species of Naviculisporaceae Through Morphological Analyses and Genome Sequencing. J. Fungi 2025, 11, 880. https://doi.org/10.3390/jof11120880
Tangthirasunun N, Gautier V, Lalanne C, Bonometti L, Cros-Arteil S, Hayes RD, Calhoun S, Riley R, Pangilinan J, Lipzen A, et al. Diversity of Sordariales Fungi: Identification of Seven New Species of Naviculisporaceae Through Morphological Analyses and Genome Sequencing. Journal of Fungi. 2025; 11(12):880. https://doi.org/10.3390/jof11120880
Chicago/Turabian StyleTangthirasunun, Narumon, Valérie Gautier, Christophe Lalanne, Lucas Bonometti, Sandrine Cros-Arteil, Richard D. Hayes, Sarah Calhoun, Robert Riley, Jasmyn Pangilinan, Anna Lipzen, and et al. 2025. "Diversity of Sordariales Fungi: Identification of Seven New Species of Naviculisporaceae Through Morphological Analyses and Genome Sequencing" Journal of Fungi 11, no. 12: 880. https://doi.org/10.3390/jof11120880
APA StyleTangthirasunun, N., Gautier, V., Lalanne, C., Bonometti, L., Cros-Arteil, S., Hayes, R. D., Calhoun, S., Riley, R., Pangilinan, J., Lipzen, A., Ng, V., Grigoriev, I. V., Gladieux, P., Giraud, T., & Silar, P. (2025). Diversity of Sordariales Fungi: Identification of Seven New Species of Naviculisporaceae Through Morphological Analyses and Genome Sequencing. Journal of Fungi, 11(12), 880. https://doi.org/10.3390/jof11120880








