Trichoderma asperellum Ta1 Alleviates Root Rot Caused by Fusarium solani and Promotes the Growth of Panax notoginseng
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Fungal Materials
2.2. Isolation and Identification of Antagonistic Strains
2.3. Antifungal Analysis of T. asperellum Ta1 Fermentation Broth
2.4. Identification of Metabolites Secreted by T. asperellum Ta1
2.5. Root Rot Control and Growth Promotion Analysis of T. asperellum Ta1 on P. notoginseng
2.6. Determination of Growth and Agronomic Parameters of P. notoginseng
2.7. Transcriptome and Metabolome Analysis of P. notoginseng
3. Results
3.1. Trichoderma asperellum Ta1 Inhibits Root Rot Pathogens
3.2. T. asperellum Ta1 Fermentation Broth Inhibited Root Rot Pathogens
3.3. Identification of Metabolites in Ta1 Fermentation Broth via UPLC-MS/MS Analysis
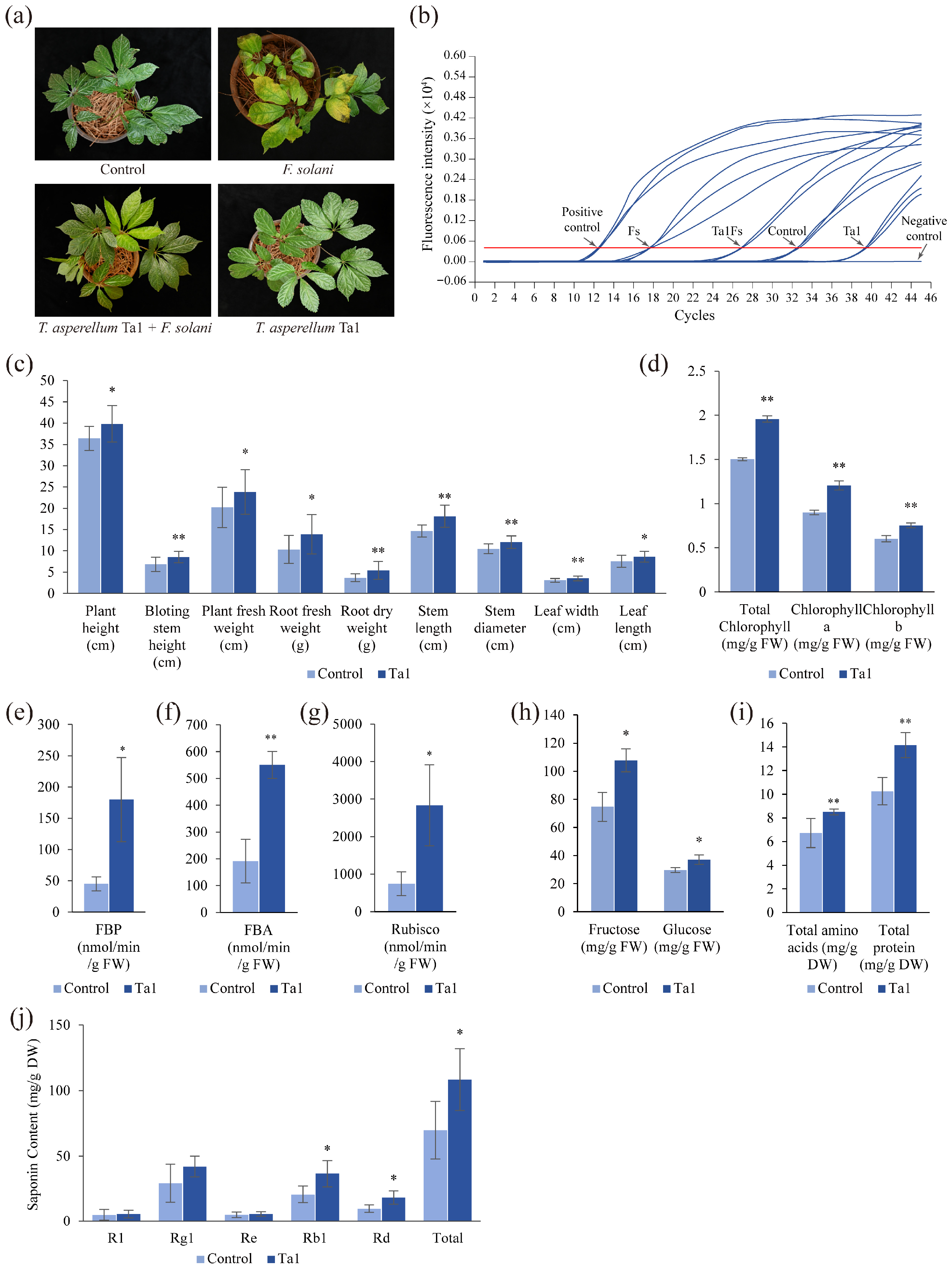
3.4. T. asperellum Ta1 Effectively Controlled Root Rot in P. notoginseng
3.5. T. asperellum Ta1 Promoted Growth and Agronomic Trait Development in P. notoginseng
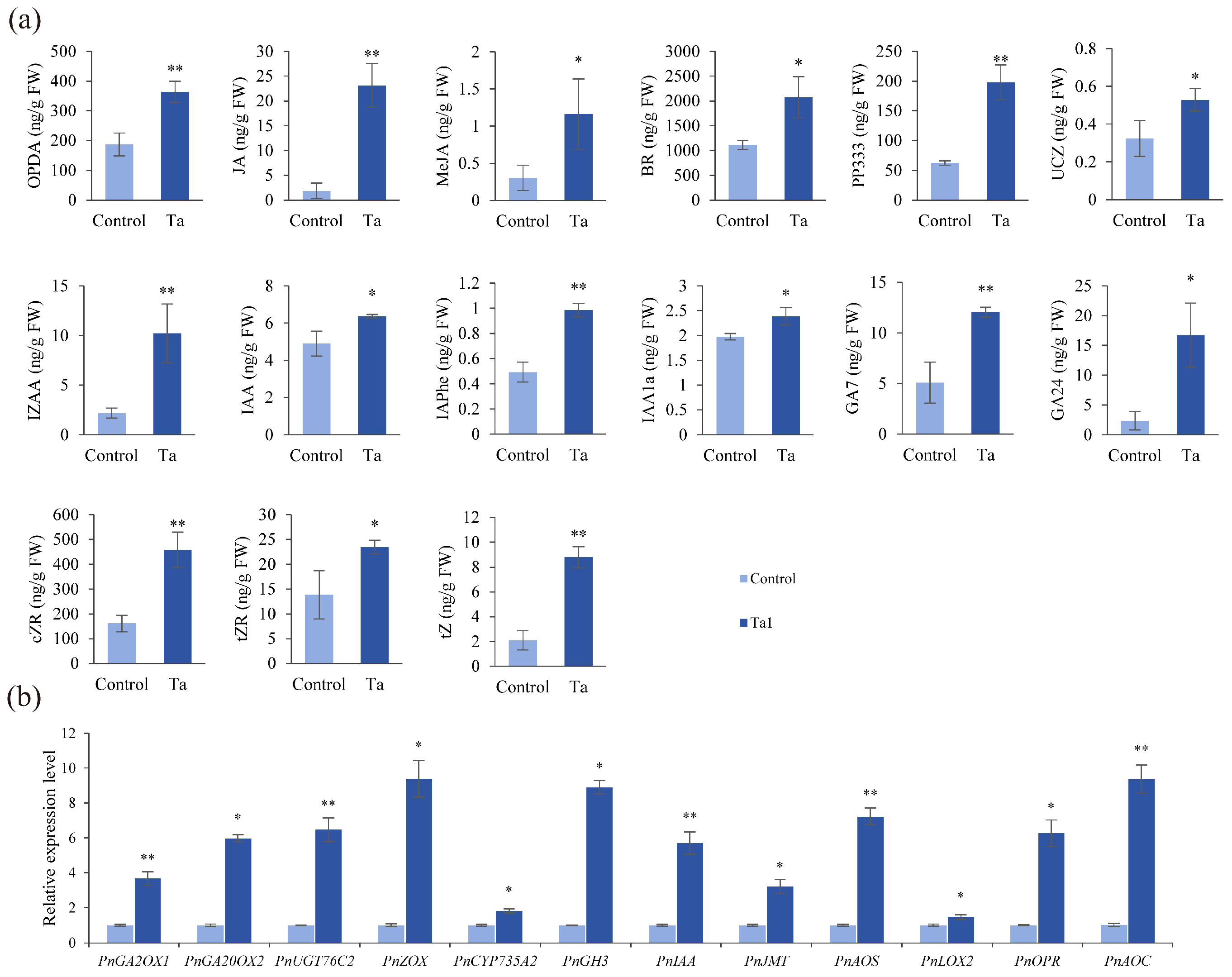
3.6. Ta1 Enhanced Biosynthesis of Growth-Promoting Hormones in P. notoginseng
3.7. Transcriptomics Profiling of P. notoginseng with Ta1 Treatment Against F. solani Infection
3.8. Metabolomics Profiling of P. notoginseng with Ta1 Treatment Against F. solani Infection
3.9. Combined Analysis of Transcriptome and Metabolome Reveals the Biocontrol and Growth-Promoting Mechanisms of Ta1 in P. notoginseng
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.L.; Feng, Y.Z.; Ma, J.C.; Zhang, Q.; Dong, Y.M.; Li, D.J.; Duan, X.M.; Zhou, L.Q.; Li, Z.H.; Yang, Y.; et al. Genomic and metabolomic insights into the antimicrobial compounds and plant growth-promoting potential of Bacillus velezensis B115. Sci. Rep. 2025, 15, 10666. [Google Scholar] [CrossRef]
- Xu, C.; Wang, W.; Wang, B.; Zhang, T.; Cui, X.; Pu, Y.; Li, N. Analytical methods and biological activities of Panax notoginseng saponins: Recent trends. J. Ethnopharmacol. 2019, 236, 443–465. [Google Scholar] [CrossRef]
- Su, L.L.; Li, W.Y.; Chen, X.H.; Wang, P.C.; Liu, D.Q. Proline-rich protein PRPL1 enhances Panax notoginseng defence against Fusarium solani by regulating reactive oxygen species balance and strengthening the cell wall barrier. Plant Cell Environ. 2024, 47, 2377–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Bao, Y.L.; Wang, Z.R.; Yang, Q.; Cui, X.M. Research progress in diseases of Panax notoginseng. Physiol. Mol. Plant Pathol. 2022, 121, 101878. [Google Scholar] [CrossRef]
- Chen, X.; Hu, L.F.; Huang, X.S.; Zhao, L.X.; Miao, C.P.; Chen, Y.W.; Xu, L.H.; Han, L.; Li, Y.Q. Isolation and characterization of new phenazine metabolites with antifungal activity against root-rot pathogens of Panax notoginseng from streptomyces. J. Agric. Food Chem. 2019, 67, 11403–11407. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Guo, H.L.; Zhang, K.X.; Zhao, M.Y.; Ruan, J.J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef]
- Lin, R.L.; Yang, Q.Y.; Xiao, J.W.; Solairaj, D.; Ngea, G.L.N.; Zhang, H.Y. Study on the biocontrol effect and physiological mechanism of Hannaella sinensis on the blue mold decay of apples. Int. J. Food Microbiol. 2022, 382, 109931. [Google Scholar] [CrossRef]
- Sun, D.G.; Li, F.Y.; Wang, L.L.; Chen, R.G.; Liu, F.; Guo, L.W.; Li, N.; Zhang, F.X.; Lei, L.C. Identification and application of an endophytic fungus Arcopilus aureus from Panax notoginseng against crop fungal disease. Front. Plant Sci. 2024, 15, 1305376. [Google Scholar] [CrossRef]
- Wang, C.X.; Liang, C.Y.; Wang, C.M.; Yin, F.; Zhang, W.D. Control of Panax notoginseng root rot through the combined application of biogas slurry and Bacillus and its mechanistic insights. Plant Soil 2024, 514, 987–1005. [Google Scholar] [CrossRef]
- Gan, K.F.; Chen, M.; Liang, T.T.; Li, X.M.; Li, G.; Liu, D.Q. Biocontrol effects and underlying mechanism of Bacillus subtilis Pn1 on Panax notoginseng root rot caused by Fusarium solani. Ind. Crops Prod. 2025, 229, 120963. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.J.; Diánez, F. Biological control of fungal diseases by Trichoderma aggressivum f. europaeum and its compatibility with fungicides. J. Fungi 2021, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.A.; Karuppiah, V.; Li, Y.Q.; Pandian, S.; Kumaran, S.; Chen, J. Role of cytochrome P450 genes of Trichoderma atroviride T23 on the resistance and degradation of dichlorvos. Chemosphere 2022, 290, 133173. [Google Scholar] [CrossRef]
- Kong, W.L.; Ni, H.; Wang, W.Y.; Wu, X.Q. Antifungal effects of volatile organic compounds produced by Trichoderma koningiopsis T2 against Verticillium dahliae. Front. Microbiol. 2022, 13, 1013468. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.M.; Balaraju, K.; Jeon, Y. Evaluation of Trichoderma atroviride and Trichoderma longibrachiatum as biocontrol agents in controlling red pepper anthracnose in Korea. Front. Plant Sci. 2023, 14, 1201875. [Google Scholar] [CrossRef] [PubMed]
- Boamah, S.; Zhang, S.W.; Xu, B.L.; Li, T.; Calderón-Urrea, A. Trichoderma longibrachiatum (TG1) enhances wheat seedlings tolerance to salt stress and resistance to Fusarium pseudograminearum. Front. Plant Sci. 2021, 12, 741231. [Google Scholar] [CrossRef]
- Chen, J.L.; Sun, S.Z.; Miao, C.P.; Wu, K.; Chen, Y.W.; Xu, L.H.; Guan, H.L.; Zhao, L.X. Endophytic Trichoderma gamsii YIM PH30019: A promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 2016, 40, 315–324. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, Y.X.; Zhang, Z.P.; Li, J.Q.; Zhu, S.S.; Yang, M.; Luo, L.X. Application of plant survival-promoting and pathogen-suppressing Trichoderma species for crop biofertilization and biocontrol of root rot in Panax notoginseng. J. Plant Pathol. 2022, 104, 1361–1369. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.Y.; Li, K.X.; Zhu, D.; Jiang, L.H.; Wu, L.X.; Li, Y.C.; He, X.H.; Du, Y.L. First report of Fusarium striatum causing root rot disease of Panax notoginseng in Yunnan, China. Phyton-Int. J. Exp. Bot. 2021, 91, 13–20. [Google Scholar] [CrossRef]
- Zhu, R.; Jin, L.; Sang, Y.; Hu, S.; Wang, B.T.; Jin, F.J. Characterization of potassium-solubilizing fungi, Mortierella spp., isolated from a poplar plantation rhizosphere soil. Arch. Microbiol. 2024, 206, 157. [Google Scholar] [CrossRef]
- Kunova, A.; Bonaldi, M.; Saracchi, M.; Pizzatti, C.; Chen, X.; Cortesi, P. Selection of Streptomyces against soil borne fungal pathogens by a standardized dual culture assay and evaluation of their effects on seed germination and plant growth. BMC Microbiol. 2016, 16, 272. [Google Scholar] [CrossRef]
- Serna-Domínguez, M.G.; Andrade-Michel, G.Y.; Arredondo-Bernal, H.C.; Gallou, A. Two efficient methods for isolation of high-quality genomic DNA from entomopathogenic fungi. J. Microbiol. Method 2018, 148, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Kinkel, L.L.; Samac, D.A. Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol. Control 2002, 23, 285–295. [Google Scholar] [CrossRef]
- Che, X.L.; Liang, T.T.; Qu, Y.; Liu, D.Q. Establishment and application of real-time fluorescent LAMP rapid detection of Fusarium solani of Panax notoginseng root rot. J. Agric. Biotechnol. 2024, 32, 939–948. (In Chinese) [Google Scholar]
- Chen, H.J.; Li, W.Y.; Chen, X.H.; Liu, G.Z.; Liu, X.Y.; Cui, X.M.; Liu, D.Q. Viral infections inhibit saponin biosynthesis and photosynthesis in Panax notoginseng. Plant Physiol. Biochem. 2023, 203, 108038. [Google Scholar] [CrossRef]
- Deng, J.; Che, X.L.; Gu, Y.; Qu, Y.; Liu, D.Q. Integrated multi-omics investigation revealed the importance of phenylpropanoid metabolism in the defense response of Lilium regale Wilson to fusarium wilt. Hortic. Res. 2024, 11, uhae140. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.R.; Ge, C.L.; Mao, W.K.; Chen, L.; Hu, H.P.; Chen, G.G.; Lang, Q.L.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. IMeta 2023, 2, e85. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Dong, T.; Xiao, S.; Lin, L.; He, Y.; Qu, F. Quantitative determination of thiabendazole in soil extracts by surface-enhanced raman spectroscopy. Molecules 2018, 23, 1949. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Alfano, G.; Ivey, M.L.L.; Cakir, C.; Bos, J.I.B.; Miller, S.A.; Madden, L.V.; Kamoun, S.; Hoitink, H.A.J. Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology 2007, 97, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Lodi, R.S.; Peng, C.E.; Dong, X.D.; Deng, P.; Peng, L.Z. Trichoderma hamatum and its benefits. J. Fungi 2023, 9, 994. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.Y.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Mironenka, J.; Rózalska, S.; Sobon, A.; Bernat, P. Trichoderma harzianum metabolites disturb Fusarium culmorum metabolism: Metabolomic and proteomic studies. Microbiol. Res. 2021, 249, 126770. [Google Scholar] [CrossRef]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 induces systemic resistance in tomato infected by Cucumber mosaic virus. Front. Plant Sci. 2016, 7, 1520. [Google Scholar] [CrossRef]
- Herrera-Tellez, V.I.; Cruz-Olmedo, A.K.; Plasencia, J.; Gavilanes-Ruiz, M.; Arce-Cervantes, O.; Hernandez-Leon, S.; Saucedo-Garcia, M. The protective effect of Trichoderma asperellum on tomato plants against Fusarium oxysporum and Botrytis cinerea diseases involves inhibition of reactive oxygen species production. Int. J. Mol. Sci. 2019, 20, 2007. [Google Scholar] [CrossRef]
- Kamble, M.V.; Joshi, S.M.; Hadimani, S.; Jogaiah, S. Biopriming with rhizosphere Trichoderma harzianum elicit protection against grapevine downy mildew disease by triggering histopathological and biochemical defense responses. Rhizosphere 2021, 19, 100398. [Google Scholar] [CrossRef]
- Intana, W.; Suwannarach, N.; Kumla, J.; Wonglom, P.; Sunpapao, A. Plant growth promotion and biological control against Rhizoctonia solani in thai local rice variety “Chor Khing” using Trichoderma breve Z2-03. J. Fungi 2024, 10, 417. [Google Scholar] [CrossRef]
- Ji, S.D.; Liu, Z.H.; Liu, B.; Wang, Y.C.; Wang, J.J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ.-Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]






| Group | Co-Differentially Expressed Pathways Name |
|---|---|
| Fs vs. Control | Linoleic acid metabolism |
| Phenylpropanoid biosynthesis | |
| Ta1Fs vs. Control | Diterpenoid biosynthesis |
| Limonene and pinene degradation | |
| Phenylpropanoid biosynthesis | |
| Monoterpenoid biosynthesis | |
| Linoleic acid metabolism | |
| Galactose metabolism | |
| alpha-Linolenic acid metabolism | |
| Starch and sucrose metabolism | |
| Ta1 vs. Control | Pentose and glucuronate interconversions |
| Galactose metabolism | |
| Starch and sucrose metabolism | |
| Amino sugar and nucleotide sugar metabolism | |
| Flavone and flavonol biosynthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Deng, J.; Li, Y.; Liu, D. Trichoderma asperellum Ta1 Alleviates Root Rot Caused by Fusarium solani and Promotes the Growth of Panax notoginseng. J. Fungi 2025, 11, 879. https://doi.org/10.3390/jof11120879
Gu Y, Deng J, Li Y, Liu D. Trichoderma asperellum Ta1 Alleviates Root Rot Caused by Fusarium solani and Promotes the Growth of Panax notoginseng. Journal of Fungi. 2025; 11(12):879. https://doi.org/10.3390/jof11120879
Chicago/Turabian StyleGu, Yue, Jie Deng, Youyu Li, and Diqiu Liu. 2025. "Trichoderma asperellum Ta1 Alleviates Root Rot Caused by Fusarium solani and Promotes the Growth of Panax notoginseng" Journal of Fungi 11, no. 12: 879. https://doi.org/10.3390/jof11120879
APA StyleGu, Y., Deng, J., Li, Y., & Liu, D. (2025). Trichoderma asperellum Ta1 Alleviates Root Rot Caused by Fusarium solani and Promotes the Growth of Panax notoginseng. Journal of Fungi, 11(12), 879. https://doi.org/10.3390/jof11120879






