Silent Saboteurs: Decoding Mycotoxins—From Chemistry and Prevalence to Health Risks, Detection, Management and Emerging Frontiers
Abstract
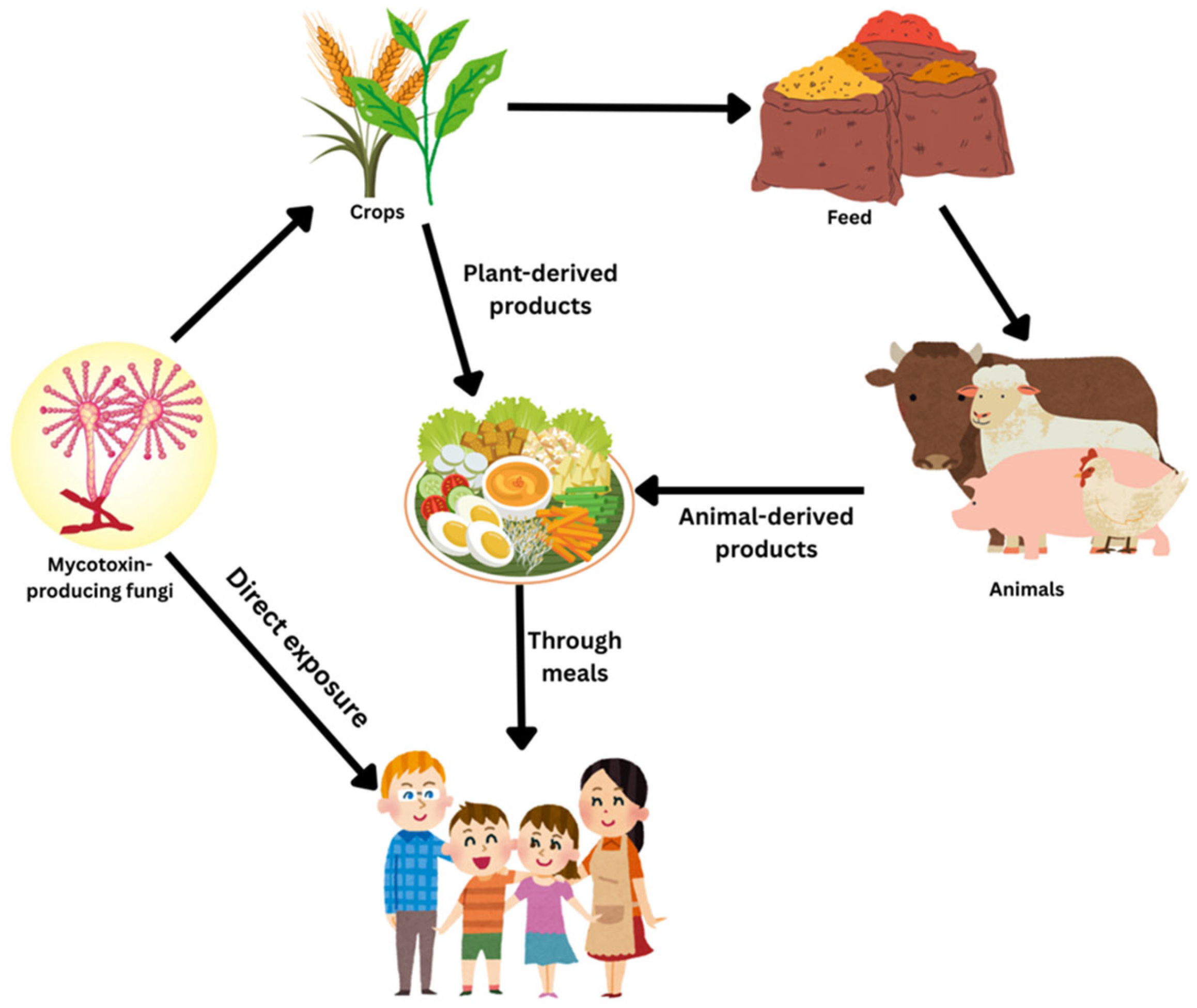
1. Introduction
2. Mycotoxin Types, Chemistry, Biosynthesis, and Mechanism of Action
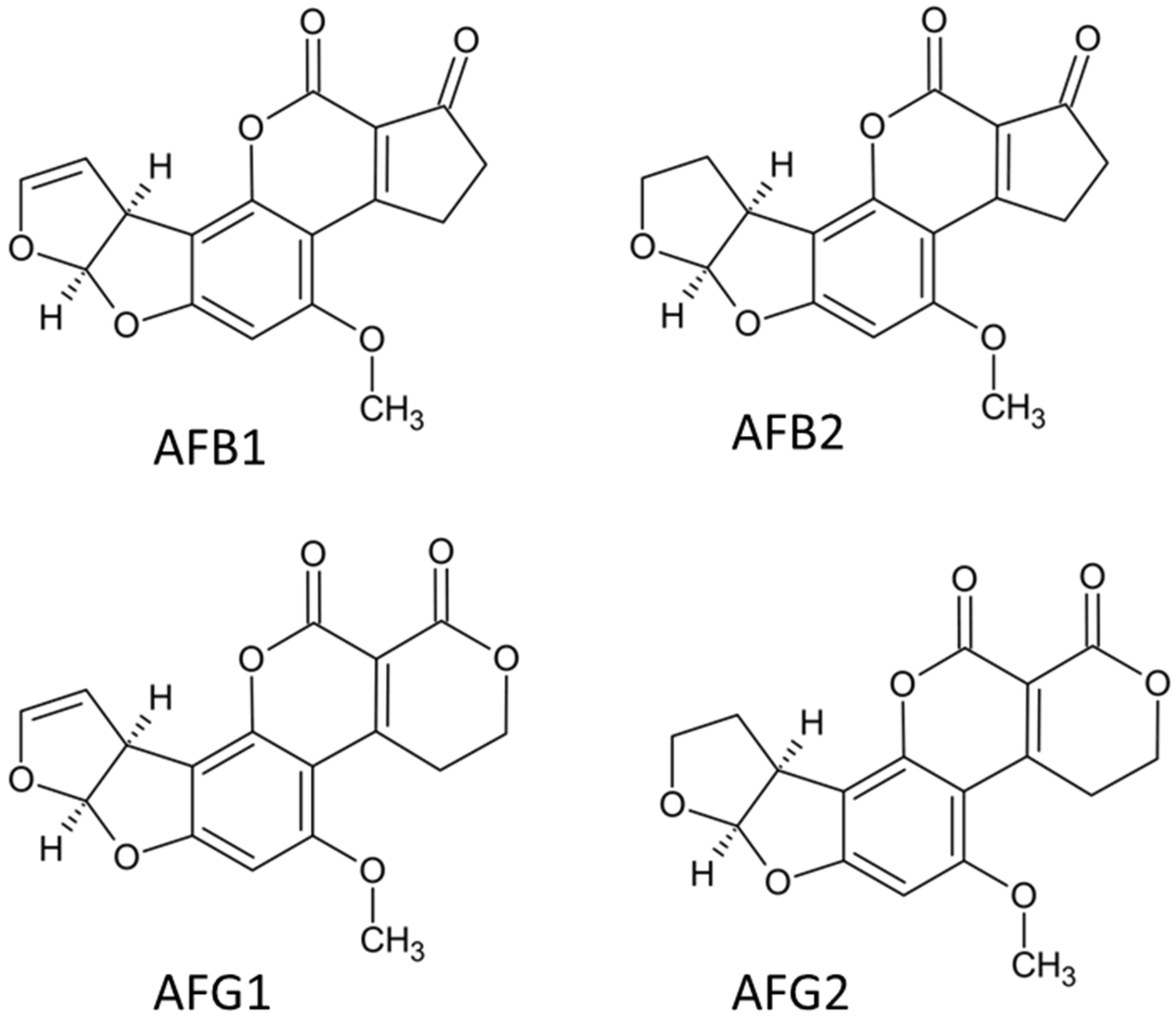
2.1. Aflatoxins
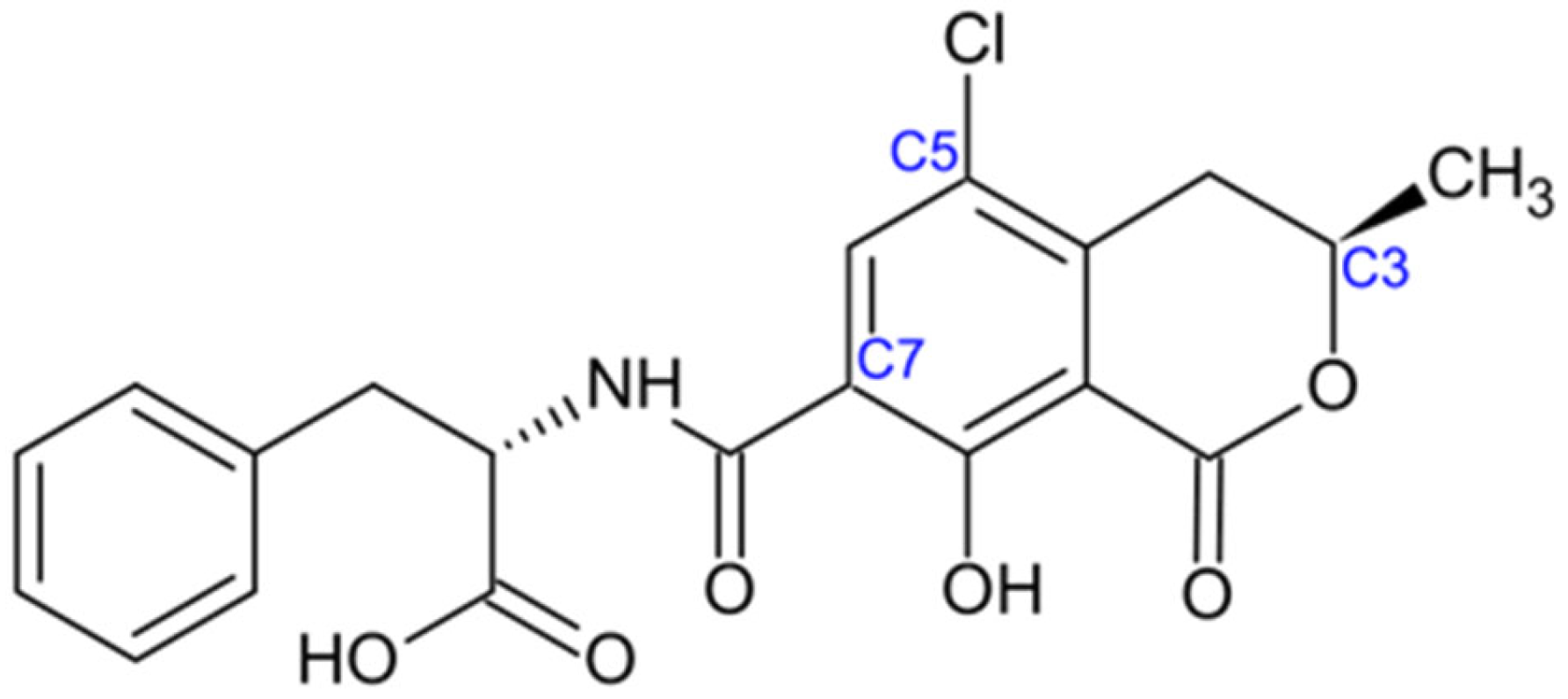
2.2. Ochratoxins
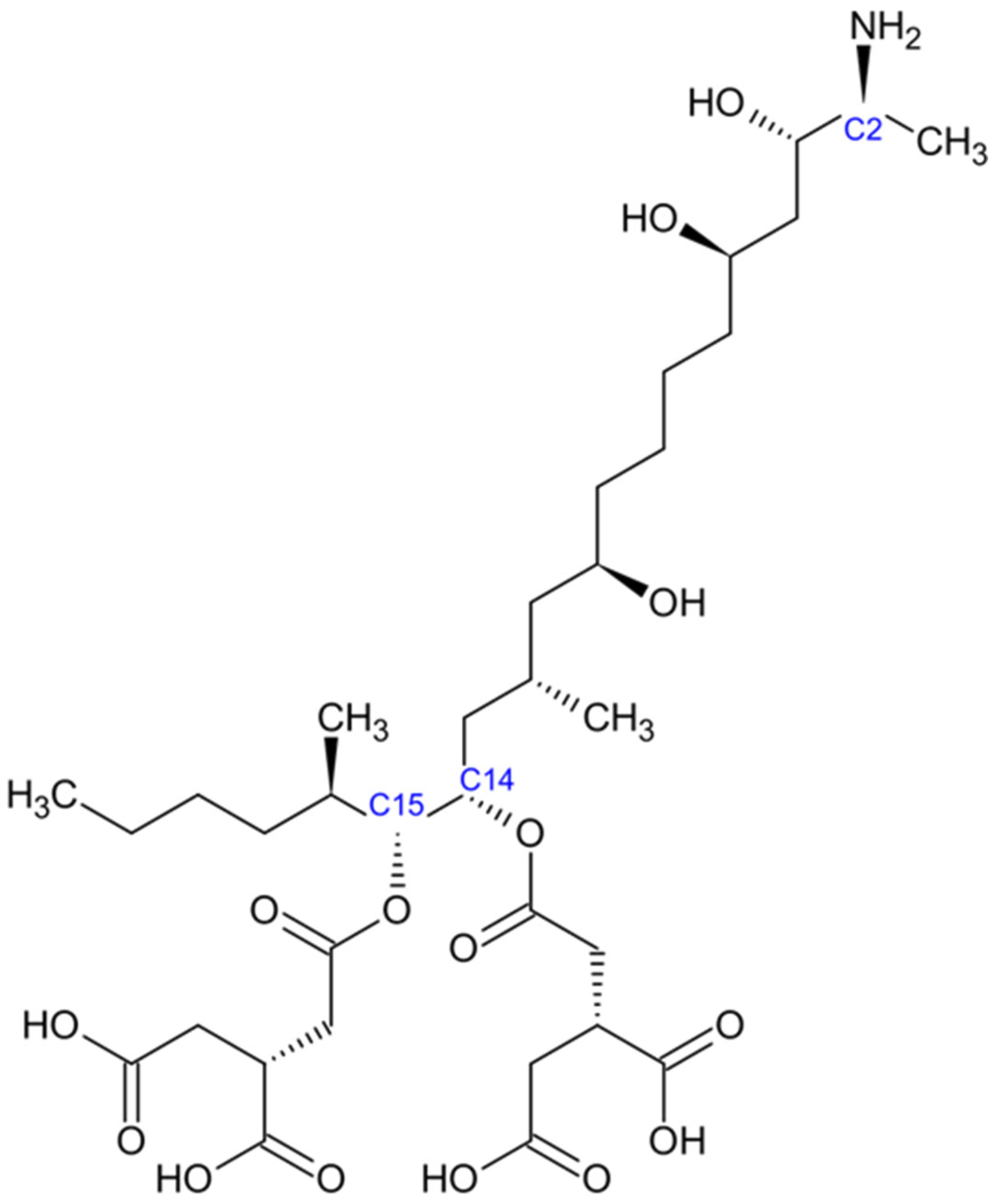
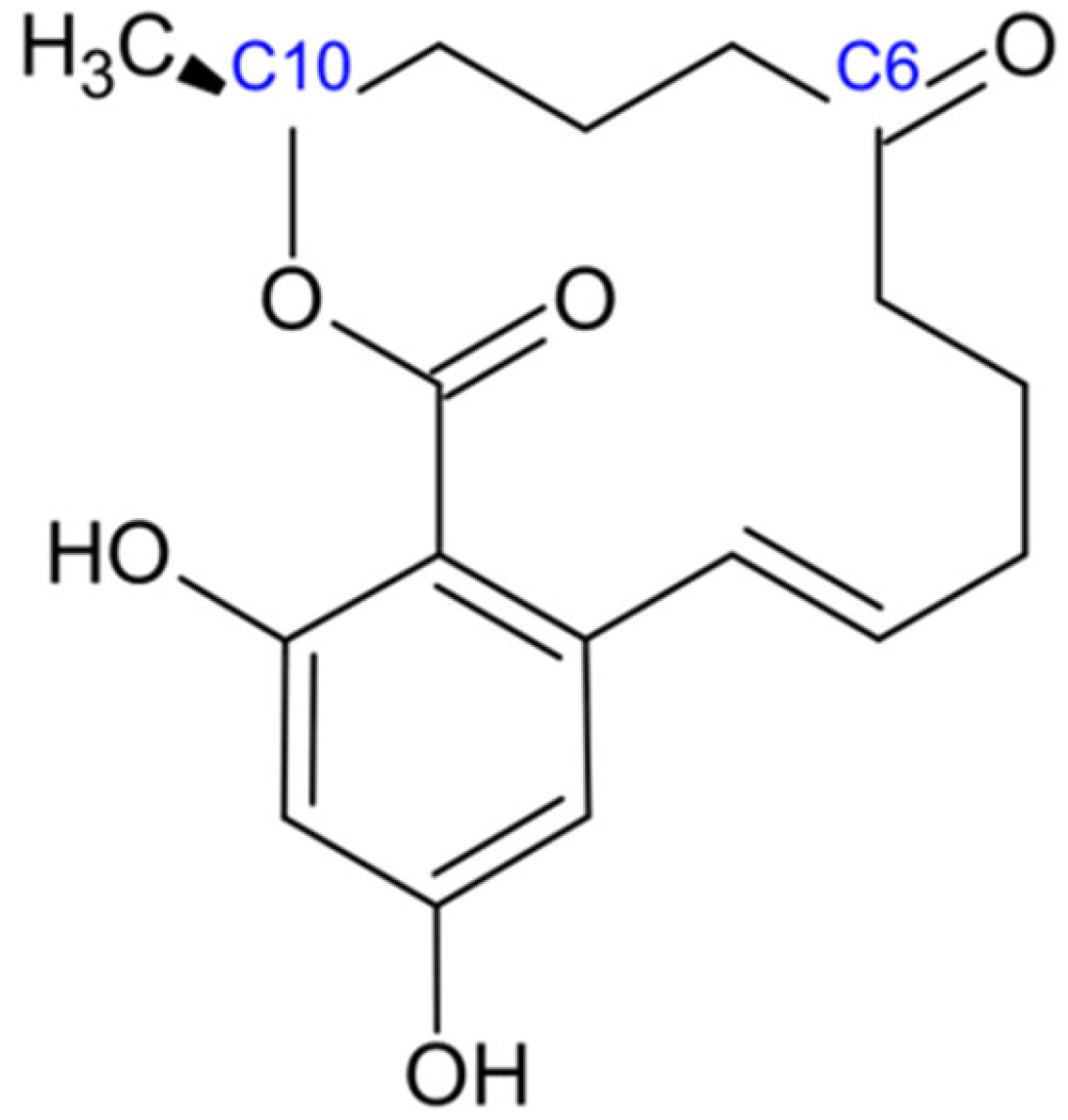
2.3. Fumonisins
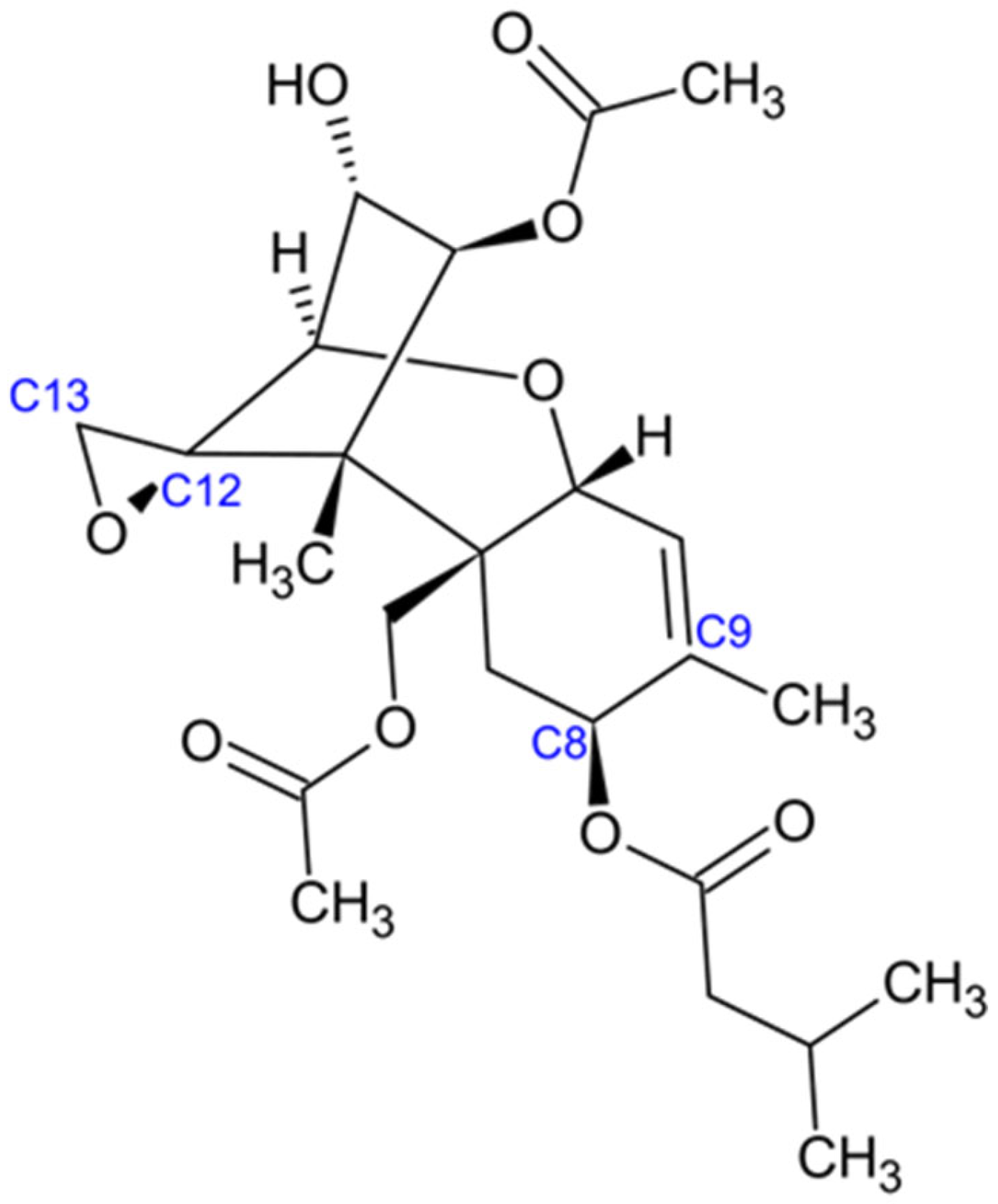
2.4. Trichothecenes
2.5. Zearalenone
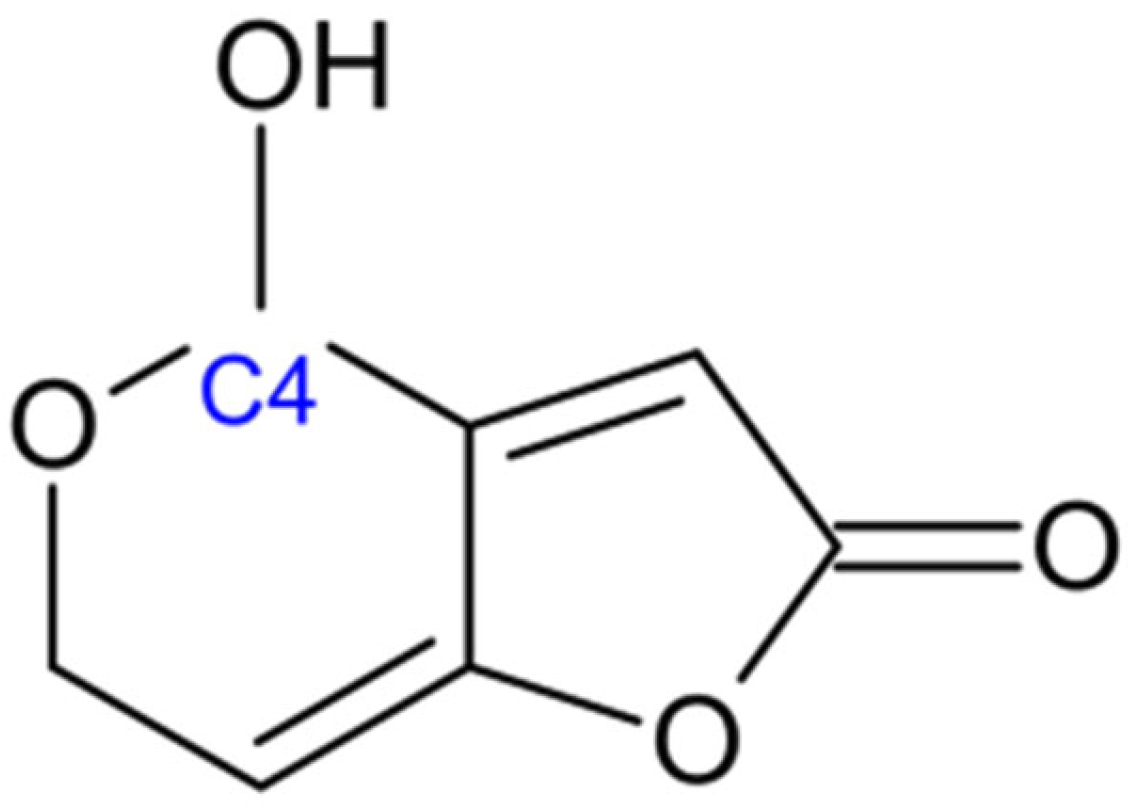
2.6. Patulin
3. Prevalence of Mycotoxins
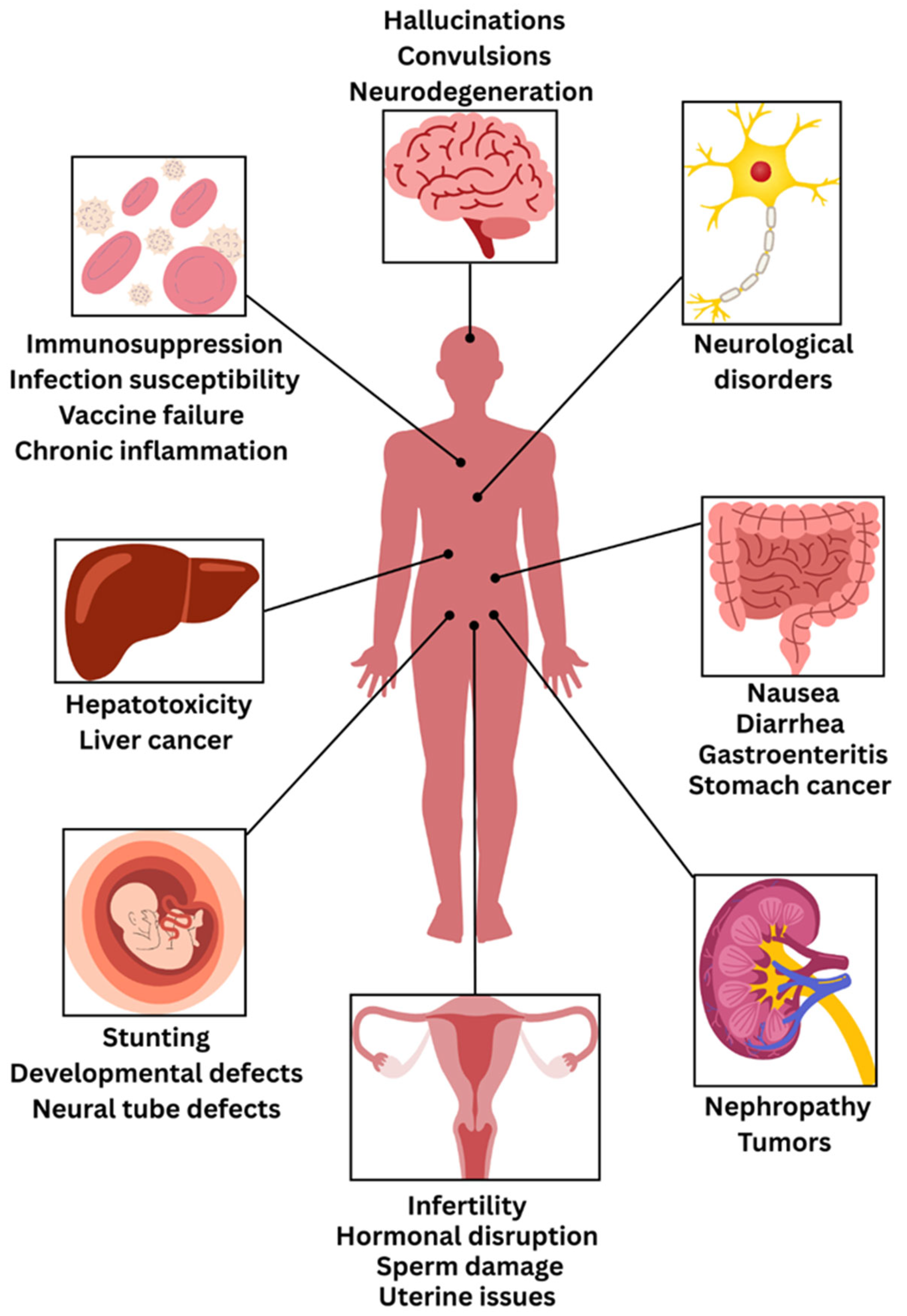
4. Consequences on Human and Animal Health
| Mycotoxin | Food Commodity | Impact on Humans | Impact on Animals | References |
|---|---|---|---|---|
| Aflatoxins | Wheat, walnut, maize, peanuts, eggs, milk, meat | Hepatotoxicity, teratogenicity, carcinogenicity, immunotoxicity | Immunosuppression, productivity reduction, appetite loss, organ damage (Liver, Kidney) | [38,156,157] |
| Ochratoxins | Coffee beans, oats, wheat, maize, wine, dried fruits, spices | Nephrotoxicity, hepatotoxicity, genotoxicity, neurotoxicity | Nephrotoxicity, reduction in growth rate, Immunosuppression, poor performance | [158,159,160] |
| Fumonisins | Corn flour, peanut, grapes, rice, wheat, barley, | Esophageal cancer, liver cancer, neural tube defects, child growth defects | Porcine pulmonary edema, Equine leucoencephalomalacia, skeletal abnormalities | [19,161,162] |
| Trichothecenes | Barley, oats, wheat, maize | Skin irritation, gastrointestinal distress, alimentary toxic aleukia, respiratory issues | Feed refusal, weight loss, immunosuppression, dermatitis | [163,164] |
| Zearalenone | Wheat, barley, sorghum, rye, rice | Reproductive system disorders, hepatotoxicity | Immunotoxicity, reproductive system defects, hormonal defects | [165,166,167] |
| Patulin | Apple, fig, tomatoes, grapes, | Gastrointestinal issues, nausea, vomiting | Teratogenicity, organ damage (kidney, liver), immune system toxicity, brain edema | [168,169,170] |
5. Mycotoxin Control and Prevention
5.1. Pre-Harvest Control Measures
5.2. Post-Harvest Control Measures
5.3. Mycotoxin Detoxification Strategies
5.3.1. Physical Detoxification Approaches
5.3.2. Chemical Detoxification Approaches
5.3.3. Adsorbent-Based Detoxification
5.4. Regulatory Frameworks and Risk Assessment
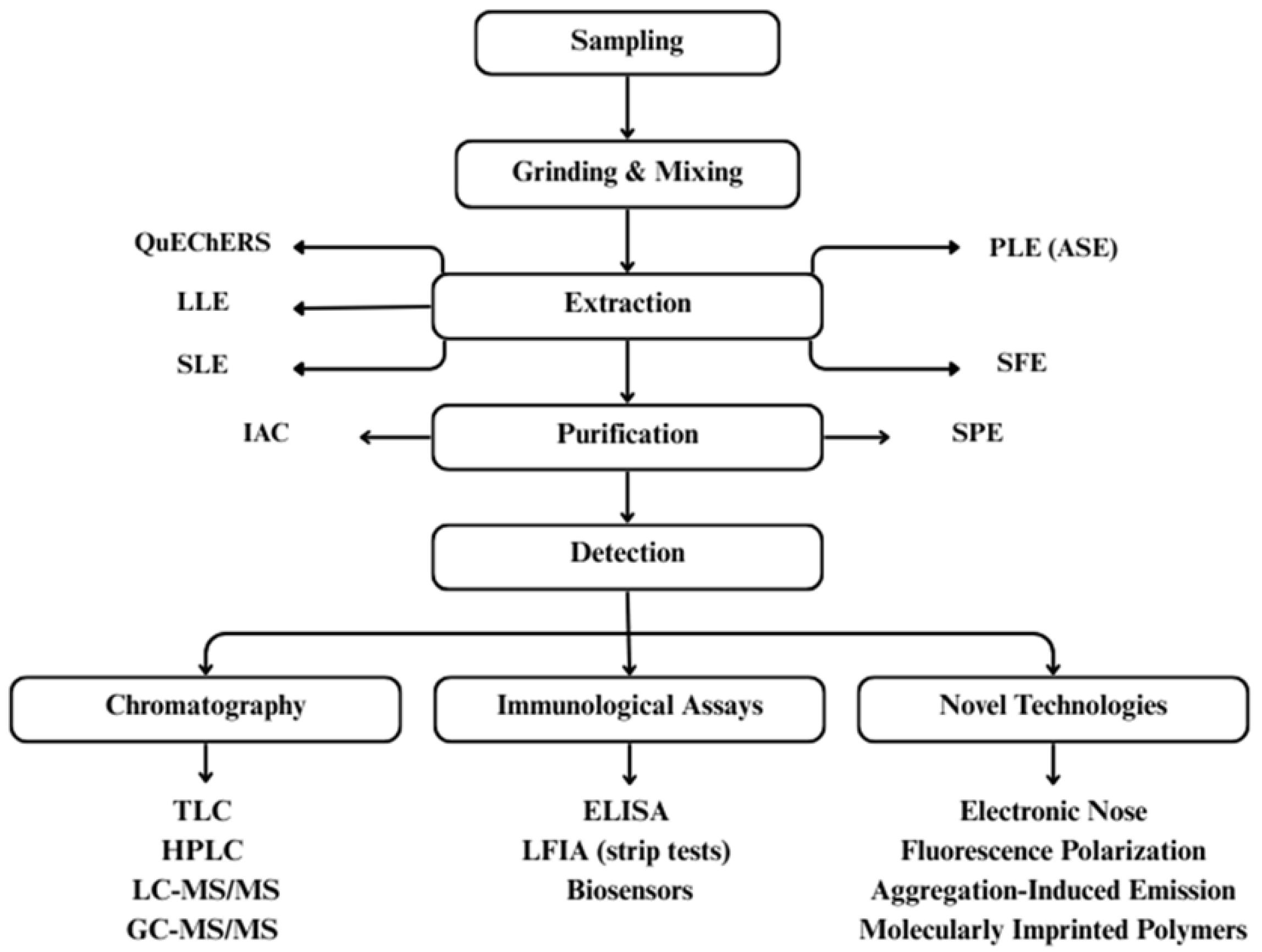
6. Emerging Detection Methods of Mycotoxins in Food
6.1. Artificial Intelligence and Machine Learning
6.2. Integration with Advanced Sensing Technologies
6.2.1. Hyperspectral Imaging
6.2.2. Electronic Nose Technology
6.2.3. Biosensor Technologies
7. Emerging Detection Methods of Mycotoxins in Food
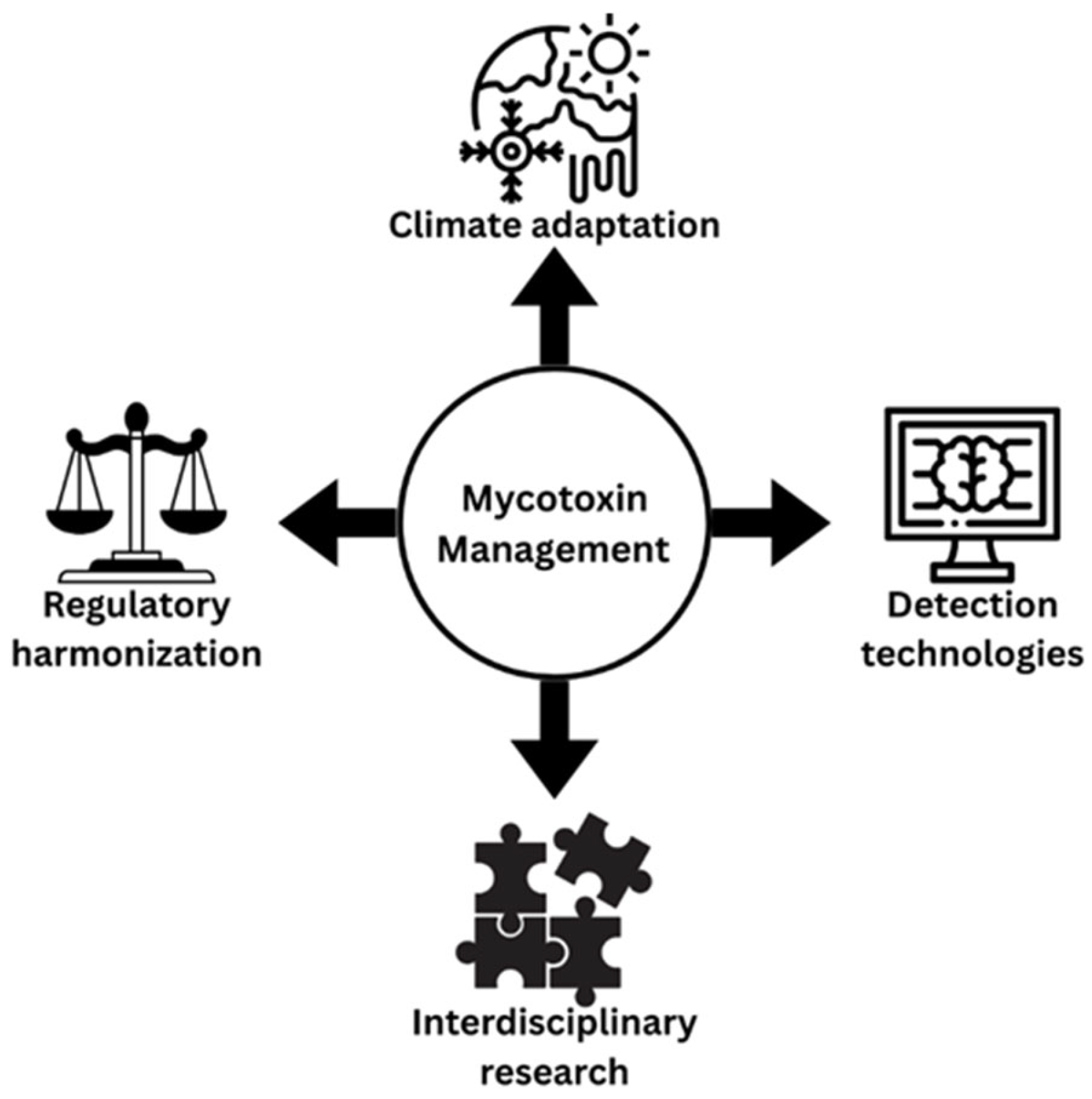
7.1. Climate Change and Mycotoxin Contamination Patterns
7.2. AI and ML in Mycotoxin Detection
7.3. Challenges in AI-Based Detection Implementation
7.4. Global Trade and Economic Impact
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, X.; Fu, R.; Yan, H.; Han, S.; Gerelt, K.; Cui, P.; Chen, J.; Qi, K.; Zhou, Y. Determination of six groups of mycotoxins in Chinese dark tea and the associated risk assessment. Environ. Pollut. 2020, 261, 114180. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Müller, A.; Rosen, R. Multi-Mycotoxin Contamination of Aquaculture Feed: A Global Survey. Toxins 2025, 17, 116. [Google Scholar] [CrossRef]
- Maguey-Gonzalez, J.A.; Latorre, J.D.; Laverty, L.; Castellanos-Huerta, I.; Shehata, A.A.; Eisenreich, W.; Tellez-Isaias, G. Advances in Anti-Mycotoxins. In Alternatives to Antibiotics Against Pathogens in Poultry; Springer: Cham, Switzerland, 2024; pp. 235–255. [Google Scholar]
- Joshi, P.; Chauysrinule, C.; Mahakarnchanakul, W.; Maneeboon, T. Multi-mycotoxin contamination, mold incidence and risk assessment of aflatoxin in maize kernels originating from Nepal. Microbiol. Res. 2022, 13, 258–277. [Google Scholar] [CrossRef]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twarużek, M. Multiannual mycotoxin survey in feed materials and feeding stuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Akinmoladun, O.F.; Fon, F.N.; Nji, Q.; Adeniji, O.O.; Tangni, E.K.; Njobeh, P.B. Multiple mycotoxin contamination in livestock feed: Implications for animal Health, productivity, and food safety. Toxins 2025, 17, 365. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z. Mycotoxins in food, recent development in food analysis and future challenges; a review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Dhakal, A.; Hashmi, M.F.; Sbar, E. Aflatoxin Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Samuel, N.; Ezri, Y.; Farah, R.; Igor, V.; Hussein, A.; Rubinshtein, O.; Assy, N. Acute aflatoxicosis resulting in fulminant hepatic failure and rhabdomyolysis. Gastroenterol. Res. 2009, 2, 48. [Google Scholar] [CrossRef]
- Mariappan, A.K.; Munusamy, P.; Latheef, S.K.; Singh, S.D.; Dhama, K. Hepato nephropathology associated with inclusion body hepatitis complicated with citrinin mycotoxicosis in a broiler farm. Vet. World 2018, 11, 112. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. Mycotoxins in food: Cancer risks and strategies for control. Foods 2024, 13, 3502. [Google Scholar] [CrossRef]
- Kuć-Szymanek, A.; Kubik-Machura, D.; Kościelecka, K.; Męcik-Kronenberg, T.; Radko, L. Neurotoxicological Effects of Some Mycotoxins on Humans Health and Methods of Neuroprotection. Toxins 2025, 17, 24. [Google Scholar] [CrossRef]
- Nie, T.; Li, J.; You, L.; Wu, Q. Environmental mycotoxins: A potential etiological factor for neurodegenerative diseases? Toxicology 2025, 511, 154056. [Google Scholar] [CrossRef]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Villeda, B.; Lobos, O.; Aguilar-Zuniga, K.; Carrasco-Sánchez, V. Ochratoxins in wines: A review of their occurrence in the last decade, toxicity, and exposure risk in humans. Toxins 2021, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.G. The occurrence of ochratoxin A in mouldy bread and flour. Food Cosmet. Toxicol. 1980, 18, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in apples and apple-based food products: The burdens and the mitigation strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef]
- Li, H.; Xing, L.; Zhang, M.; Wang, J.; Zheng, N. The toxic effects of aflatoxin B1 and aflatoxin M1 on kidney through regulating L-proline and downstream apoptosis. BioMed Res. Int. 2018, 2018, 9074861. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Ratnaseelan, A.M.; Tsilioni, I.; Theoharides, T.C. Effects of mycotoxins on neuropsychiatric symptoms and immune processes. Clin. Ther. 2018, 40, 903–917. [Google Scholar] [CrossRef]
- McKeon, H.P.; Schepens, M.A.; van den Brand, A.D.; de Jong, M.H.; van Gelder, M.M.; Hesselink, M.L.; Sopel, M.M.; Mengelers, M.J. Assessment of Mycotoxin Exposure and Associated Risk in Pregnant Dutch Women: The Human Biomonitoring Approach. Toxins 2024, 16, 278. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.O.; Ramalho, L.N.; Oliveira, C.A.; Ramalho, F.S. Reproductive, gestational, and fetal alterations induced by dietary mycotoxins: A systematic review. Pesqui. Veterinária Bras. 2024, 44, e07481. [Google Scholar] [CrossRef]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Xu, W.; Yang, F.; Wei, W.; Chen, X.; Zhang, Z.; Liu, Y. Reproductive Toxicity of Zearalenone and Its Molecular Mechanisms: A Review. Molecules 2025, 30, 505. [Google Scholar] [CrossRef]
- Sajjad, Y.; Dib, J.; Soliman, N.; Alhmoudi, M.; Sajjad, S.G.; Kandil, H.; Fakih, M. The role of mycotoxins in reproductive health: Mechanisms, evidence, and clinical implications. J. IVF-Worldw. 2025, 3, 42–55. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Wu, F. Mycotoxin risk assessment for the purpose of setting international regulatory standards. Environ. Sci. Technol. 2004, 38, 4049–4055. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological detoxification of mycotoxins: Current status and future advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef]
- Sotnichenko, A.; Pantsov, E.; Shinkarev, D.; Okhanov, V. Hydrophobized reversed-phase adsorbent for protection of dairy cattle against lipophilic toxins from diet. Efficiency in vitro and in vivo. Toxins 2019, 11, 256. [Google Scholar] [CrossRef]
- Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic mechanism and biological detoxification of fumonisins. Toxins 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Merrill, A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A.; Brown, M.P. Genetics and physiology of aflatoxin biosynthesis. Annu. Rev. Phytopathol. 1998, 36, 329–362. [Google Scholar] [CrossRef] [PubMed]
- Filazi, A.; Sireli, U.T. Occurrence of aflatoxins in food. In Aflatoxins: Recent Advances and Future Prospects; InTech: New Delhi, India, 2013; pp. 143–170. [Google Scholar]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.G.; Rădulescu, A.L.; Georgescu, S.E.; Dinischiotu, A. Aflatoxins in feed: Types, metabolism, health consequences in swine and mitigation strategies. Toxins 2022, 14, 853. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef]
- Liao, X.; Jia, B.; Sun, C.; Shi, L.; Liu, X.; Zhou, L.; Kong, W. Reuse of regenerated immunoaffinity column for excellent clean-up and low-cost detection of trace aflatoxins in malt. Microchem. J. 2020, 157, 105007. [Google Scholar] [CrossRef]
- Okechukwu, V.O.; Adelusi, O.A.; Kappo, A.P.; Njobeh, P.B.; Mamo, M.A. Aflatoxins: Occurrence, biosynthesis, mechanism of action and effects, conventional/emerging detection techniques. Food Chem. 2024, 436, 137775. [Google Scholar] [CrossRef]
- Huang, G.; Ma, J.; Li, J.; Yan, L. Study on the interaction between aflatoxin M1 and DNA and its application in the removal of aflatoxin M1. J. Mol. Liq. 2022, 355, 118938. [Google Scholar] [CrossRef]
- Gourama, H.; Bullerman, L.B. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic fungi of concern in foods and feeds: A review. J. Food Prot. 1995, 58, 1395–1404. [Google Scholar] [CrossRef]
- Cova, T.F.; Ferreira, C.; Nunes, S.C.; Pais, A.A. Structural Similarity, Activity, and Toxicity of Mycotoxins: Combining Insights from Unsupervised and Supervised Machine Learning Algorithms. J. Agric. Food Chem. 2025, 73, 6173–6188. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The existing methods and novel approaches in mycotoxins’ detection. Molecules 2021, 26, 3981. [Google Scholar] [CrossRef]
- Galaverna, G.; Dall’Asta, C. Sampling techniques for the determination of mycotoxins in food matrices. In Comprehensive Sampling and Sample Preparationi; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2012; Volume 4, pp. 381–403. [Google Scholar]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Yu, J.; Ehrlich, K.C. Toxins of filamentous fungi. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Osweiler, M.D., Seitz, T.S., Rottinghaus, G.E., Battaglia, M.A., Eds.; CABI: Wallingford, UK, 2006; pp. 171–194. [Google Scholar]
- Pitt, J.I. Toxigenic fungi and mycotoxins. Br. Med. Bull. 2000, 56, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Smela, M.E.; Currier, S.S.; Bailey, E.A.; Essigmann, J.M. The chemistry and biology of aflatoxin B1: From mutational spectrometry to carcinogenesis. Carcinogenesis 2001, 22, 535–545. [Google Scholar] [CrossRef]
- Shen, H.M.; Shi, C.Y.; Shen, Y.; Ong, C.N. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free. Radic. Biol. Med. 1996, 21, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.W.; Guengerich, F.P. Reaction of aflatoxin B1 exo-8,9-epoxide with DNA: Kinetics and evidence for a common intermediate. Chem. Res. Toxicol. 1997, 10, 362–370. [Google Scholar]
- Ben Miri, Y.; Benabdallah, A.; Chentir, I.; Djenane, D.; Luvisi, A.; De Bellis, L. Comprehensive Insights into Ochratoxin A: Occurrence, Analysis, and Control Strategies. Foods 2024, 13, 1184. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Bacha, N.; Atoui, A.; Mathieu, F.; Liboz, T.; Lebrihi, A. Aspergillus westerdijkiae polyketide synthase gene “aoks1” is involved in the biosynthesis of ochratoxin A. Fungal Genet. Biol. 2009, 46, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Färber, P.; Geisen, R. Analysis of differentially-expressed ochratoxin A biosynthesis genes of Penicillium nordicum. Eur. J. Plant Pathol. 2004, 110, 661–669. [Google Scholar] [CrossRef]
- Boudra, H.; Le Bars, P.; Le Bars, J. Thermostability of ochratoxin A in wheat under two moisture conditions. Appl. Environ. Microbiol. 1995, 61, 1156–1158. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.B.; Doi, E.; Kitabatake, N. Detoxification of ochratoxin A on heating under acidic and alkaline conditions. Biosci. Biotechnol. Biochem. 1992, 56, 741–745. [Google Scholar] [CrossRef]
- Więckowska, M.; Szelenberger, R.; Niemcewicz, M.; Harmata, P.; Poplawski, T.; Bijak, M. Ochratoxin A—The current knowledge concerning hepatotoxicity, mode of action and possible prevention. Molecules 2023, 28, 6617. [Google Scholar] [CrossRef]
- Gayathri, L.; Dhivya, R.; Dhanasekaran, D.; Periasamy, V.S.; Alshatwi, A.A.; Akbarsha, M.A. Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: In vitro study in HepG2 cell. Food Chem. Toxicol. 2015, 83, 151–163. [Google Scholar] [CrossRef]
- Qi, X.; Yang, X.; Chen, S.; He, X.; Dweep, H.; Guo, M.; Xu, W.; Luo, Y.; Gretz, N.; Dai, Q.; et al. Ochratoxin A induced early hepatotoxicity: New mechanistic insights from microRNA, mRNA and proteomic profiling studies. Sci. Rep. 2014, 4, 5163. [Google Scholar] [CrossRef]
- Więckowska, M.; Szelenberger, R.; Popławski, T.; Bijak, M.; Gorniak, L.; Stela, M.; Cichon, N. Gut as a Target of Ochratoxin A: Toxicological Insights and the Role of Microbiota. Int. J. Mol. Sci. 2025, 26, 9438. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Song, X.; Zhao, Y. Ochratoxin A alters liver and kidney functions via oxidative stress-mediated pathways in ducks. Toxins 2019, 11, 466. [Google Scholar]
- El Khoury, A.; Atoui, A. Ochratoxin A: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Liu, Y. A consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M.; Silbernagl, S.; Freudinger, R. Interaction of ochratoxin A with renal organic anion transport. J. Pharmacol. Exp. Ther. 1994, 268, 138–146. [Google Scholar]
- Creppy, E.E.; Schlegel, M.; Størmer, F.C.; Röschenthaler, R.; Dirheimer, G. Effect of ochratoxin A on liver and kidney protein synthesis in mice: An in vivo and in vitro study. Food Chem. Toxicol. 1983, 21, 587–593. [Google Scholar]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Müller, G.; Burkert, B.; Oetjen, J.; Köhler, H. Immunotoxic effects of ochratoxin A in rats. Mycoses 1999, 42, 495–502. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Z.; Wang, Y.; Huang, J.; Peng, D.; Li, Z.; Tang, J. Research progress on fumonisin B1 contamination and toxicity: A review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2017, 18, 33–50. [Google Scholar] [CrossRef]
- Smith, G.W. Chapter 71—Fumonisins. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1003–1018. [Google Scholar]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Fumonisins: Chemistry, biosynthesis, and biological effects. In Mycotoxins in Food, Feed and Bioweapons; Bhatnagar, P., Harris, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 75–104. [Google Scholar]
- Proctor, R.H.; Plattner, R.D.; Brown, D.W.; Seo, J.A.; Lee, Y.W. Discontinuous distribution of fumonisin biosynthetic genes in the Gibberella fujikuroi species complex. Fungal Genet. Biol. 2003, 39, 121–133. [Google Scholar] [CrossRef]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H. Inhibition of sphingolipid biosynthesis by fumonisins: Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [CrossRef]
- Merrill, A.H.; Wang, E.; Vales, T.R.; Smith, E.R.; Schroeder, J.J.; Menaldino, D.S.; Alexander, C.; Crane, H.M.; Xia, J.; Liotta, D.C.; et al. Fumonisin toxicity and sphingolipid metabolism. Environ. Health Perspect. 2001, 109 (Suppl. S2), 283–289. [Google Scholar]
- Gelderblom, W.C.A.; Kriek, N.P.J.; Marasas, W.F.O.; Thiel, P.G. Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 2001, 12, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Edwards, C.K.; Remick, D.G. Fumonisin-induced pulmonary edema: Inflammatory responses in a rat model. Toxicol. Appl. Pharmacol. 2005, 205, 198–204. [Google Scholar]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, D.; Nguyen, C.; McCormick, S.P.; Proctor, R.H.; Luo, S.; Zou, Y.; Hai, Y. Biosynthesis of the Central Tricyclic Skeleton of Trichothecene Mycotoxins. J. Am. Chem. Soc. 2025, 147, 10331–10338. [Google Scholar] [CrossRef]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef]
- Foroud, N.A.; Eudes, F. Trichothecenes in Cereal Grains. Int. J. Mol. Sci. 2009, 10, 147–173. [Google Scholar] [CrossRef]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of trichothecene biosynthesis in Fusarium fungi: Genes, regulatory mechanisms, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Zhou, H.R.; Islam, Z.; Pestka, J.J. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxin deoxynivalenol. Toxicol. Sci. 2003, 74, 297–309. [Google Scholar]
- Islam, Z.; Amuzie, C.J.; Harkema, J.R.; Pestka, J.J. Role of IL-1 in trichothecene-induced inflammation, apoptosis and cytokine gene expression. Toxicology 2006, 219, 56–66. [Google Scholar]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Sudakin, D.L. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Rao, P.V.L. Trichothecene mycotoxins: Role in pathogenesis. Curr. Neuropharmacol. 2010, 8, 174–180. [Google Scholar]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Pitt, J.I. Mycotoxins: Zearalenone. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 313–314. [Google Scholar]
- Urry, W.H.; Wehrmeister, W.H.; Hodge, E.B.; Hidy, P.H. The structure of zearalenone. Tetrahedron Lett. 1966, 7, 3109–3114. [Google Scholar] [CrossRef]
- Gromadzka, K.; Waskiewicz, A.; Chelkowski, J.; Golinski, P. Zearalenone and its metabolites: Occurrence, detection, toxicity and guidelines. World Mycotoxin J. 2008, 1, 209–220. [Google Scholar] [CrossRef]
- Hidy, P.H.; Baldwin, R.S.; Greasham, R.L.; Keith, C.L.; McMullen, J.R. Zearalenone and Some Derivatives: Production and Biological Activities. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1977; pp. 59–82. [Google Scholar]
- Kim, H.K.; Lee, S.; Yun, S.H. A putative polyketide synthase gene required for sexual reproduction in Gibberella zeae. Fungal Genet. Biol. 2005, 42, 44–54. [Google Scholar]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Domińska, K.; Piastowska-Ciesielska, A.W. Estrogen receptor α is crucial in zearalenone-induced invasion and migration of prostate cancer cells. Toxins 2018, 10, 98. [Google Scholar] [CrossRef]
- Ahamed, M.; Siddiqui, M.K.J.; Banu, N. Induction of chromosomal aberrations by Zearalenone in human lymphocytes in vitro. Toxicol. Vitr. 2001, 15, 535–540. [Google Scholar]
- Ding, L.; Kang, Y.; Cai, X.; He, Y.; Liu, D. Effects of zearalenone on steroid hormone profiles and expression of cytochrome P450 enzymes in mice. Environ. Toxicol. Pharmacol. 2006, 22, 234–239. [Google Scholar]
- Frizzell, C.; Meade, K.; Sheehan, D. Metabolic effects of the mycotoxin zearalenone: Implications for glucose and lipid homeostasis. Toxins 2011, 3, 1234–1248. [Google Scholar]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- Barad, S.; Sionov, E.; Prusky, D. Role of patulin in post-harvest diseases. Toxins 2014, 6, 2670–2683. [Google Scholar] [CrossRef]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- Pitt, J. Mycotoxins: Patulin. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 310–312. [Google Scholar]
- Guray, T.; Tuncel, M.; Uysal, U.D. A Rapid Determination of Patulin Using Capillary Zone Electrophoresis and its Application to Analysis of Apple Juices. J. Chromatogr. Sci. 2013, 51, 310–317. [Google Scholar] [CrossRef]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci. Technol. 2019, 83, 99–113. [Google Scholar] [CrossRef]
- White, T.C.; Simmonds, D.; Donaldson, S. Identification of genes associated with patulin biosynthesis in Penicillium griseofulvum. FEMS Microbiol. Lett. 2006, 255, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, A.S.; Franco, B.D.G.M.; Rosenthal, A. Influence of environmental conditions on the production of mycotoxins. Braz. J. Food Technol. 2008, 11, 89–97. [Google Scholar]
- Fliege, R.; Metzler, M. Electrophilic properties of patulin. N-acetylcysteine and glutathione adducts. Chem. Res. Toxicol. 2000, 13, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.D.; Clement, B.A.; Kubena, L.F. Inhibition of mitochondrial function and metabolism by patulin in vitro. Toxicol. Appl. Pharmacol. 1990, 104, 94–102. [Google Scholar]
- Liu, B.H.; Yu, F.Y.; Yu, H.Y.; Huang, X.L.; Chen, C.Y. Effects of mycotoxin patulin on expression of DNA damage-responsive genes in human lymphocytes. Toxicol. Lett. 2003, 143, 243–250. [Google Scholar]
- Assunção, R.; Martins, C.; Dupont, D.; Alvito, P. Patulin bioaccessibility in processed apple products using a simulated digestion model: Influence of the food matrix. Food Funct. 2016, 7, 2056–2065. [Google Scholar]
- Bourdiol, F.; Pinel, C.; Cravedi, J.P.; Cano, J.P. Effects of patulin on mouse spleen cell proliferation and protein synthesis. Food Chem. Toxicol. 1990, 28, 723–729. [Google Scholar]
- Gomes, A.L.; de Sousa, R.L.M.; das Neves, L.A.V.; da Gloria, E.M.; Burbarelli, M.F.C.; Seno, L.d.O.; Petrus, R.R.; Fernandes, A.M. Occurrence and Co-exposure of aflatoxins and fumonisins in conventional and organic corn. Food Control. 2024, 165, 110628. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Smith, J.R.; Lee, C.H. Global distribution and prevalence of mycotoxins in food and feed: A comprehensive review. Food Control 2023, 132, 108503. [Google Scholar]
- Nji, Q.N.; Meyer, M.; Mokoena, M.P. Three-year multi-mycotoxin analysis of South African commercial maize. Front. Fungal Biol. 2024, 5, 1426782. [Google Scholar] [CrossRef]
- Borràs-Vallverdú, B.; Ramos, A.J.; Cantero-Martínez, C.; Marín, S.; Sanchis, V.; Fernández-Ortega, J. Influence of agronomic factors on mycotoxin contamination in maize. Toxins 2022, 14, 620. [Google Scholar] [CrossRef]
- Nji, Q.N.; Babalola, O.O.; Mwanza, M. Climatic effects on aflatoxin contamination of maize. Toxicol. Rep. 2024, 13, 101711. [Google Scholar] [CrossRef]
- Cheli, F.; Campagnoli, A.; Dell’Orto, V.; Moro, A. Occurrence of ochratoxin A in cereals and cereal products: A review. Food Control 2013, 34, 429–436. [Google Scholar]
- Foroud, N.A.; Eudes, F. Trichothecenes in cereal grains—An update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Atnafu, B.; Garbaba, C.A.; Lemessa, F.; Migheli, Q.; Sulyok, M.; Chala, A. Multiple mycotoxins associated with maize (Zea mays L.) grains harvested from subsistence farmers’ fields in southwestern Ethiopia. Mycotoxin Res. 2024, 40, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Seyoum, C.; Yousuf, J.; Mweetwa, A.; Odera, J.A.; Okello, D.K.; Sulyok, M. Multi-mycotoxins analysis in post-harvest maize (Zea mays L.) grain from major producing areas of Ethiopia. World Mycotoxin J. 2023, 16, 261–272. [Google Scholar] [CrossRef]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Orina, A.; Gavrilova, O.P.; Gagkaeva, T.; Burkin, A.; Kononenko, G. The contamination of Fabaceae plants with fungi and mycotoxins. Agric. Food Sci. 2020, 29, 265–275. [Google Scholar] [CrossRef]
- Tseng, T.C.; Tu, J.C.; Soo, L.C. Natural occurrence of mycotoxins in Fusarium infected beans. Microbios 1995, 84, 21–28. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of historical data and a new outlook. Crit. Rev. Food Sci. Nutr. 2020, 60, 2779–2809. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-mycotoxin analysis in feed and feed ingredients: A review. Food Addit. Contam. Part A 2013, 30, 2079–2094. [Google Scholar]
- Omara, T.; Kiprop, A.K.; Wangila, P.; Wacoo, A.P.; Kagoya, S.; Nteziyaremye, P.; Peter Odero, M.; Kiwanuka Nakiguli, C.; Baker Obakiro, S. The Scourge of Aflatoxins in Kenya: A 60-Year Review (1960 to 2020). J. Food Qual. 2021, 2021, 8899839. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin contamination in food crops: Causes, detection, and management: A review. Food Prod. Process Nutr. 2021, 3, 17. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Alameri, M.M.; Kong, A.S.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.H.; Abushelaibi, A.; Lim, S.E.; Loh, J.Y.; et al. Aflatoxin Contamination: An Overview on Health Issues, Detection and Management Strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef]
- Söylemez, T.; Berger, R.G.; Krings, U.; Yamaç, M. Aflatoxin B1 (AFB1) biodegradation by a lignolytic phenoloxidase of Trametes hirsuta. Sci. Rep. 2025, 15, 6330. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, R.; Raghavendra, V.B.; Rachitha, P. A comprehensive review of mycotoxins, their toxicity, and innovative detoxification methods. Toxicol. Rep. 2025, 14, 101952. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.-G.; Elkaliny, N.E.; Darwish, O.A.; Ashraf, Y.; Ebrahim, R.A.; Das, S.P.; Yahya, G. Comprehensive review for aflatoxin detoxification with special attention to cold plasma treatment. Mycotoxin Res. 2025, 41, 277–300. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Jelaković, B.; Dika, Ž.; Arlt, V.M.; Stiborova, M.; Pavlović, N.M.; Nikolić, J.; Colet, J.M.; Vanherweghem, J.L.; Nortier, J.L. Balkan Endemic Nephropathy and the Causative Role of Aristolochic Acid. Semin. Nephrol. 2019, 39, 284–296. [Google Scholar] [CrossRef]
- Taroncher, M.; Fuentes, C.; Rodríguez-Carrasco, Y.; Ruiz, M.J. Assessment of the genotoxic and mutagenic effects induced by T-2 mycotoxin in HepG2 cells. Toxicology 2024, 501, 153712. [Google Scholar] [CrossRef]
- Hua, Z.; Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Li, W.; Lu, C.; et al. Contamination of aflatoxins induces severe hepatotoxicity through multiple mechanisms. Front. Pharmacol. 2021, 11, 605823. [Google Scholar] [CrossRef]
- Mak, D.; Kramvis, A. Epidemiology and aetiology of hepatocellular carcinoma in Sub-Saharan Africa. Hepatoma Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Ekwueme, C.T.; Uhegwu, C.C.; Ejileugha, C.; Augustine, J.; Okolo, C.A.; Onyeaka, H. A Review of the Mycotoxin Family of Fumonisins, Their Biosynthesis, Metabolism, Methods of Detection and Effects on Humans and Animals. Int. J. Mol. Sci. 2024, 26, 184. [Google Scholar] [CrossRef]
- Polak-Sliwińska, M.; Paszczyk, B. Trichothecenes in food and feed: Occurrence, toxicokinetics, and impact on human and animal health. Toxins 2021, 13, 876. [Google Scholar]
- Khan, R.; Anwar, F.; Ghazali, F.M. A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, T.; Chuturgoon, A.A. Mycotoxins exacerbate HIV infection: The potential of N6-methyladenosine RNA methylation. Epigenomics 2021, 13, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Alvito, P.; Pereira-da-Silva, L. Mycotoxin Exposure during the First 1000 Days of Life and Its Impact on Children’s Health: A Clinical Overview. Toxins 2022, 14, 189. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A.; et al. Fumonisins Disrupt Sphingolipid Metabolism, Folate Transport, and Neural Tube Development in Embryo Culture and In Vivo: A Potential Risk Factor for Human Neural Tube Defects among Populations Consuming Fumonisin-Contaminated Maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Humphrey, J.H.; Stoltzfus, R.J. Aflatoxin Exposure During Pregnancy, Maternal Anemia, and Adverse Birth Outcomes. Am. J. Trop. Med. Hyg. 2017, 96, 770–776. [Google Scholar] [CrossRef]
- Mielech, A.; Puścion-Jakubik, A.; Socha, K. Assessment of the Risk of Contamination of Food for Infants and Toddlers. Nutrients 2021, 13, 2358. [Google Scholar] [CrossRef]
- De Santis, B.; Brera, C.; Mezzelani, A.; Soricelli, S.; Ciceri, F.; Moretti, G.; Debegnach, F.; Bonaglia, M.C.; Villa, L.; Molteni, M.; et al. Role of mycotoxins in the pathobiology of autism: A first evidence. Nutr. Neurosci. 2017, 22, 132–144. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Owuamanam, I.C.; Ogueke, C.C.; Hannington, T. The impacts of mycotoxins on the proximate composition and functional properties of grains. Eur. Acad. Res. 2020, 8, 1024–1071. [Google Scholar]
- Jurišić, N.; Schwartz-Zimmermann, H.E.; Kunz-Vekiru, E.; Moll, W.D.; Schweiger, W.; Fowler, J.; Berthiller, F. Determination of aflatoxin biomarkers in excreta and ileal content of chickens. Poult. Sci. 2019, 98, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ogunade, I.M.; Vyas, D.; Adesogan, A.T. Aflatoxin in Dairy Cows: Toxicity, Occurrence in Feedstuffs and Milk and Dietary Mitigation Strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Battacone, G.; Nudda, A.; Pulina, G. Effects of Ochratoxin A on Livestock Production. Toxins 2010, 2, 1796–1824. [Google Scholar] [CrossRef]
- Chen, C.; Wu, F. The need to revisit ochratoxin A risk in light of diabetes, obesity, and chronic kidney disease prevalence. Food Chem. Toxicol. 2017, 103, 79–85. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Ma, Z.; Zhang, Q.; Li, H. The Occurrence and Contamination Level of Ochratoxin A in Plant and Animal-Derived Food Commodities. Molecules 2021, 26, 6928. [Google Scholar] [CrossRef]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H., Jr.; Rothman, K.J.; Hendricks, K.A. Exposure to Fumonisins and the Occurrence of Neural Tube Defects along the Texas–Mexico Border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B2 Production by Aspergillus niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Yagen, B.; Joffe, A.Z. Screeing of toxic isolates of Fusarium poae and Fusarium sporotrichiodes involved in causing alimentary toxic aleukia. Appl. Environ. Microbiol. 1976, 32, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004, 114, 205–239. [Google Scholar] [CrossRef]
- Yang, J.; Wang, G.X.; Liu, J.L.; Fan, J.J.; Cui, S. Toxic effects of zearalenone and its derivatives α-zearalenol on male reproductive system in mice. Reprod. Toxicol. 2007, 24, 381–387. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, L.; Chen, Y.; Sun, T.; Wang, N.; Chen, X.; Yang, Z.; Ge, J.; Jiang, S. Comparative study of stress response, growth and development of uteri in post-weaning gilts challenged with zearalenone and estradiol benzoate. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1885–1894. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Llewellyn, G.C.; McCay, J.A.; Brown, R.D.; Musgrove, D.L.; Butterworth, L.F.; Munson, A.E.; White, K.L., Jr. Immunological evaluation of the mycotoxin patulin in female b6C3F1 mice. Food Chem. Toxicol. 1998, 36, 1107–1115. [Google Scholar] [CrossRef]
- Drusch, S.; Ragab, W. Mycotoxins in Fruits, Fruit Juices, and Dried Fruits. J. Food Prot. 2003, 66, 1514–1527. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.; Marcone, M.F. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef]
- Rose, L.J.; Okoth, S.; Flett, B.C.; Van Rensburg, B.J.; Viljoen, A. Preharvest Management Strategies and Their Impact on Mycotoxigenic Fungi and Associated Mycotoxins. In Mycotoxins—Impact and Management Strategies; IntechOpen: London, UK, 2018; pp. 41–57. [Google Scholar]
- Milani, J.M. Ecological conditions affecting mycotoxin production in cereals: A review. Vet. Med. 2013, 2013, 405–411. [Google Scholar] [CrossRef]
- Kaiser, N.; Douches, D.; Dhingra, A.; Glenn, K.C.; Herzig, P.R.; Stowe, E.C.; Swarup, S. The role of conventional plant breeding in ensuring safe levels of naturally occurring toxins in food crops. Trends Food Sci. Technol. 2020, 100, 51–66. [Google Scholar] [CrossRef]
- Figlan, S.; Mwadzingeni, L. Breeding Tools for Assessing and Improving Resistance and Limiting Mycotoxin Production by Fusarium graminearum in Wheat. Plants 2022, 11, 1933. [Google Scholar] [CrossRef]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef]
- Senghor, A.L.; Ortega-Beltran, A.; Atehnkeng, J.; Jarju, P.; Cotty, P.J.; Bandyopadhyay, R.J.P.D. Aflasafe SN01 is the first biocontrol product approved for aflatoxin mitigation in two nations, Senegal and The Gambia. Plant Dis. 2021, 105, 1461–1473. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Szieberth, D.; Toldine, E.T.; Nagy, Z.; Szabó, B.; Herczig, B.; Tóth, B. Updating the methodology of identifying maize hybrids resistant to ear rot pathogens and their toxins—Artificial inoculation tests for kernel resistance to Fusarium graminearum, F. verticillioides, and Aspergillus flavus. J. Fungi 2022, 8, 293. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A. Biocontrol of Aflatoxins Using Non-Aflatoxigenic Aspergillus flavus: A Literature Review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef]
- Cotty, P.J.; Mellon, J.E. Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination Adaptations of aflatoxin-producing fungi Aflatoxins in the Sonoran desert. Mycotoxin Res. 2006, 22, 110–117. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Atehnkeng, J.; Ortega-Beltran, A.; Akande, A.; Falade, T.D.; Cotty, P.J. “Ground-truthing” efficacy of biological control for aflatoxin mitigation in farmers’ fields in Nigeria: From field trials to commercial usage, a 10-year study. Front. Microbiol. 2019, 10, 2528. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of Fungicide Resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health Rev. 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Q.; Webber, H.; Johnen, A.; Rossi, V.; Junior, A.F.N. Integrating machine learning and change detection for enhanced crop disease forecasting in rice farming: A multi-regional study. Eur. J. Agron. 2024, 160, 127317. [Google Scholar] [CrossRef]
- Focker, M.; van Eupen, M.; Verweij, P.; Liu, C.; van Haren, C.; Van der Fels-Klerx, H.J. Effects of climate change on areas suitable for maize cultivation and aflatoxin contamination in Europe. Toxins 2023, 15, 599. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Effects of planting date and environmental factors on Fusarium ear rot symptoms and fumonisin B1 accumulation in maize grown in six North American locations. Plant Pathol. 2012, 61, 1130–1142. [Google Scholar] [CrossRef]
- García-Lara, S.; García-Jaimes, E.; Ortíz-Islas, S. Field effectiveness of improved hermetic storage technologies on maize grain quality in Central Mexico. J. Stored Prod. Res. 2020, 87, 101585. [Google Scholar] [CrossRef]
- Obeng-Akrofi, G.; Maier, D.E.; White, W.S.; Akowuah, J.O.; Bartosik, R.; Cardoso, L. Effectiveness of hermetic bag storage technology to preserve physical quality attributes of shea nuts. J. Stored Prod. Res. 2023, 101, 102086. [Google Scholar] [CrossRef]
- Yewle, N.R.; Stroshine, R.L.; Ambrose, R.K.; Baributsa, D. Short-Term Hermetic Storage of Wet Maize and Its Effect on Quality. Foods 2023, 12, 891. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, T.D. Postcollection enhancement of mycotoxins and postharvest handling of samples. J. Environ. Pathol. Toxicol. Oncol. 1990, 10, 106–109. [Google Scholar] [PubMed]
- Dembedza, M.P.; Chidewe, C.; Benhura, M.A.; Mvumi, B.M.; Manema, L.R.; Nyanga, L.K. Effectiveness of hermetic maize grain storage technology in limiting aflatoxin exposure in women and children from smallholder farming areas. World Mycotoxin J. 2019, 12, 233–244. [Google Scholar] [CrossRef]
- Walker, S.; Jaime, R.; Kagot, V.; Probst, C. Comparative effects of hermetic and traditional storage devices on maize grain: Mycotoxin development, insect infestation and grain quality. J. Stored Prod. Res. 2018, 77, 34–44. [Google Scholar] [CrossRef]
- Murashiki, T.C.; Chidewe, C.; Benhura, M.A.; Manema, L.R.; Mvumi, B.M.; Nyanga, L.K. Effectiveness of hermetic technologies in limiting aflatoxin B1 and fumonisin B1 contamination of stored maize grain under smallholder conditions in Zimbabwe. World Mycotoxin J. 2018, 11, 459–470. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Colović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, A.; Yu, B.; Sun, X. Recent Advances in Non-Contact Food Decontamination Technologies for Removing Mycotoxins and Fungal Contaminants. Foods 2024, 13, 2244. [Google Scholar] [CrossRef]
- Abraham, N.; Chan, E.T.S.; Zhou, T.; Seah, S.Y. Microbial detoxification of mycotoxins in food. Front. Microbiol. 2022, 13, 957148. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Savvaidis, I.; Chokr, A.; Louka, N.; Atoui, A. Detoxification approaches of mycotoxins: By microorganisms, biofilms and enzymes. Int. J. Food Contam. 2022, 9, 3. [Google Scholar] [CrossRef]
- Sun, H.; He, Z.; Xiong, D.; Long, M. Mechanisms by which microbial enzymes degrade four mycotoxins and application in animal production: A review. Anim. Nutr. 2023, 15, 256–274. [Google Scholar] [CrossRef]
- Geofrey, N.; Kigozi, A.R.; Turyagyenda, L.; Mugerwa, S. The Role of Bentonite Clays in Aflatoxin-Decontamination, Assimilation and Metabolism in Commercial Poultry. Biomed. J. Sci. Tech. Res. 2022, 43, 34649–34658. [Google Scholar]
- Xie, S.; Huang, L.; Su, C.; Yan, J.; Chen, Z.; Li, M.; Du, M.; Zhang, H. Application of clay minerals as adsorbents for removing heavy metals from the environment. Green Smart Min. Eng. 2024, 1, 249–261. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Saari, N. Lactic Acid Bacteria in Antifungal Biopreservation. Molecules 2020, 25, 2655. [Google Scholar] [CrossRef]
- Nguyen, T.; Chen, X.; Ma, L.; Feng, Y. Mycotoxin Biodegradation by Bacillus Bacteria—A Review. Toxins 2024, 16, 478. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Mahbub, N.U.; Islam, M.A. Gut Microorganism-Mediated Neutralization of Mycotoxins: A Promising Approach to Combat Fungal Toxicity. Adv. Gut Microbiome Res. 2024, 2024, 8448547. [Google Scholar] [CrossRef]
- Pallarés, N.; Sebastià, A.; Martínez-Lucas, V.; Queirós, R.; Barba, F.J.; Berrada, H.; Ferrer, E. High Pressure Processing Impact on Emerging Mycotoxins (ENNA, ENNA1, ENNB, ENNB1) Mitigation in Different Juice and Juice-Milk Matrices. Foods 2022, 11, 190. [Google Scholar] [CrossRef]
- Urugo, M.M.; Teka, T.A.; Berihune, R.A.; Teferi, S.L.; Garbaba, C.A.; Adebo, J.A. Novel non-thermal food processing techniques and their mechanism of action in mycotoxins decontamination of foods. Innov. Food Sci. Emerg. Technol. 2023, 85, 103312. [Google Scholar] [CrossRef]
- Jakovac-Strajn, B.; Babič, J.; Pezo, L.; Banjac, V.; Čolović, R.; Kos, J.; Miljanić, J.; Janić Hajnal, E. Mitigation of Mycotoxin Content by a Single-Screw Extruder in Triticale (×Triticosecale Wittmack). Foods 2025, 14, 263. [Google Scholar] [CrossRef]
- Sorbo, A.; Pucci, E.; Nobili, C.; Taglieri, I.; Passeri, D.; Zoani, C. Food Safety Assessment: Overview of Metrological Issues and Regulatory Aspects in the European Union. Separations 2022, 9, 53. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef]
- Ibrahim, O.O.; Menkovska, M. The Nature, Sources, Detections and Regulations of Mycotoxins That Contaminate Foods and Feeds Causing Health Hazards for Both Human and Animals. J. Agric. Chem. Environ. 2019, 08, 33–57. [Google Scholar] [CrossRef]
- Anukul, N.; Vangnai, K.; Mahakarnchandkul, W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 2013, 21, 227–241. [Google Scholar] [CrossRef]
- Munasinghe, J.; De Silva, A.; Weerasinghe, G.; Gunaratne, A.; Corke, H. Food safety in Sri Lanka: Problems and solutions. Qual. Assur. Saf. Crops Foods 2014, 7, 37–44. [Google Scholar] [CrossRef]
- Miklós, G.; Angeli, C.; Ambrus, Á.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jóźwiak, Á.; Bartók, T. Detection of Aflatoxins in Different Matrices and Food-Chain Positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef] [PubMed]
- Kizis, D.; Vichou, A.E.; Natskoulis, P.I. Recent advances in mycotoxin analysis and detection of mycotoxigenic fungi in grapes and derived products. Sustainability 2021, 13, 2537. [Google Scholar] [CrossRef]
- Maphaisa, T.C.; Akinmoladun, O.F.; Adelusi, O.A.; Mwanza, M.; Fon, F.; Tangni, E.; Njobeh, P.B. Advances in mycotoxin detection techniques and the crucial role of reference material in ensuring food safety. A review. Food Chem. Toxicol. 2025, 200, 115387. [Google Scholar] [CrossRef]
- Logrieco, A.F.; Miller, J.D.; Eskola, M.; Krska, R.; Ayalew, A.; Bandyopadhyay, R.; Battilani, P.; Bhatnagar, D.; Chulze, S.; De Saeger, S.; et al. The mycotox charter: Increasing awareness of, and concerted action for, minimizing mycotoxin exposure worldwide. Toxins 2018, 10, 149. [Google Scholar] [CrossRef]
- Muhenga, A.S.; Alphonce, R. Consumer’s Awareness and Willingness to Pay for Aflatoxin-Free Sunflower Oil from Four Selected Regions in Tanzania. Sustainability 2023, 15, 12309. [Google Scholar] [CrossRef]
- Liew, W.-P.-P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Amuzie, C.; Bandyopadhyay, R.; Bhat, R.V.; Black, R.; Burger, H.; Cardwell, K.F.; Gelderblom, W.; Gong, Y.Y.; Groopman, J.D.; Kimanya, M.; et al. Mycotoxin Control in Low-and Middle-Income Countries; HAL: Lyon, France, 2016. [Google Scholar]
- Aggarwal, A.; Mishra, A.; Tabassum, N.; Kim, Y.M.; Khan, F. Detection of Mycotoxin Contamination in Foods Using Artificial Intelligence: A Review. Foods 2024, 13, 3339. [Google Scholar] [CrossRef]
- Faeli, L. Mycotoxins Diagnostic Methods from Past to Present. In Research on Mycotoxins—From Mycotoxigenic Fungi to Innovative Strategies of Diagnosis, Control and Detoxification; IntechOpen: London, UK, 2025. [Google Scholar]
- Inglis, M.B.; Gomes, A.A.; Silva, L.M. Machine learning applied to the detection of mycotoxin in food: A systematic review. Toxins 2024, 16, 268. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Dong, H.; Sun, J.; Huang, J.; Li, S.; Ma, C.; Guo, Y.; Sun, X. Spatio-temporal distribution patterns and quantitative detection of aflatoxin B1 and total aflatoxin in peanut kernels explored by short-wave infrared hyperspectral imaging. Food Chem. 2023, 424, 136441. [Google Scholar] [CrossRef]
- Tao, F.; Yao, H.; Hruska, Z.; Kincaid, R.; Rajasekaran, K. Near-infrared hyperspectral imaging for evaluation of aflatoxin contamination in corn kernels. Biosyst. Eng. 2022, 221, 181–194. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Wang, H.; Li, S.; Shao, X.; Xia, L.; Darwish, I.A.; Guo, Y.; Sun, X. Advancing detection of fungal and mycotoxins contamination in grains and oilseeds: Hyperspectral imaging for enhanced food safety. Food Chem. 2025, 470, 142689. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Shi, J.; Zhang, N.; Qin, Z.; Du, L.; Zhai, X.; Shen, T.; Zhang, R.; Zou, X.; et al. Advances in Hyperspectral Imaging Technology for Grain Quality and Safety Detection: A Review. Foods 2025, 14, 2977. [Google Scholar] [CrossRef]
- Wang, B.; Shen, F.; He, X.M.; Jiang, X.S.; Yuan, J.; Fang, Y.; Hu, Q.H.; Qiu, W.F.; Mamo, F.T. Simultaneous detection of harmful fungi and mycotoxin contamination in peanuts by electronic nose. Shipin Kexue/Food Sci. 2022, 43, 310–316. [Google Scholar]
- Cheli, F.; Ottoboni, M.; Fumagalli, F.; Mazzoleni, S.; Ferrari, L.; Pinotti, L. E-nose technology for mycotoxin detection in feed: Ready for a real context in field application or still an emerging technology? Toxins 2023, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Camardo Leggieri, M.; Mazzoni, M.; Bertuzzi, T.; Moschini, M.; Prandini, A.; Battilani, P. Electronic nose for the rapid detection of deoxynivalenol in wheat using classification and regression trees. Toxins 2022, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Leggieri, M.C.; Mazzoni, M.; Fodil, S.; Moschini, M.; Bertuzzi, T.; Prandini, A.; Battilani, P. An electronic nose supported by an artificial neural network for the rapid detection of aflatoxin B1 and fumonisins in maize. Food Control 2021, 123, 107722. [Google Scholar] [CrossRef]
- Ingle, A.P.; Gupta, I.; Jogee, P.; Rai, M. Role of nanotechnology in the detection of mycotoxins: A smart approach. In Nanomycotoxicology: Treating Mycotoxins in the Nano Way; Elsevier: Amsterdam, The Netherlands, 2019; pp. 11–33. [Google Scholar]
- Zhang, M.; Guo, X.; Wang, J. Advanced biosensors for mycotoxin detection incorporating miniaturized meters. Biosens. Bioelectron. 2023, 224, 115077. [Google Scholar] [CrossRef]
- Horky, P.; Skalickova, S.; Baholet, D.; Skladanka, J. Nanoparticles as a solution for eliminating the risk of mycotoxins. Nanomaterials 2018, 8, 727. [Google Scholar] [CrossRef]
- Adelere, I.A.; Lateef, A. A novel approach to the green synthesis of metallic nanoparticles: The use of agro-wastes, enzymes, and pigments. Nanotechnol. Rev. 2016, 5, 567–587. [Google Scholar] [CrossRef]
- Casu, A.; Leggieri, M.C.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, 13323. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.d.A.; Lee, I.; Singh, C.B.; Mishra, G.; Panda, B.K.; Lee, S.-H. Detection of Mycotoxins in Cereal Grains and Nuts Using Machine Learning Integrated Hyperspectral Imaging: A Review. Toxins 2025, 17, 219. [Google Scholar] [CrossRef] [PubMed]
- Focker, M.; Liu, C.; Wang, X.; van der Fels-Klerx, H.J. The use of artificial intelligence to improve mycotoxin management: A review. Mycotoxin Res. 2025, 41, 529–540. [Google Scholar] [CrossRef]
- Goda, A.A.; Shi, J.; Xu, J.; Liu, X.; Zhou, Y.; Xiao, L.; Abdel-Galil, M.; Salem, S.H.; Ayad, E.G.; Deabes, M.; et al. Global health and economic impacts of mycotoxins: A comprehensive review. Environ. Sci. Eur. 2025, 37, 1166. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Kabak, B. Occurrence of mycotoxins in dried fruits worldwide, with a focus on aflatoxins and ochratoxin A: A review. Toxins 2023, 15, 576. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2024/1022 of 7 May 2024 amending Regulation (EU) 2023/915 as regards maximum levels of deoxynivalenol in certain foodstuffs. Off. J. Eur. Union 2024, L, 1–7. [Google Scholar]
- Lakshman, P.L.N.; Dilrukshi, D.M.N.; Bulathgama, B.E.A.U. The aflatoxin occurrence of food commodities in Sri Lanka: An overview of prevalence, detection and decontamination techniques. Trop. Agric. Res. Ext. 2022, 25, 200. [Google Scholar] [CrossRef]
- Nanayakkara, T.M.; Rajapakse, N.P.; Mendis, E.P. Total Aflatoxin Levels in Coconut Oil Produced in Sri Lanka and Compliance to Specifications. J. Agric. Sci. Sri Lanka 2025, 20, 11305. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. IAEA-Supported Lab in Sri Lanka Helps Keep Contaminated Food Off the Market. 2021. Available online: https://www.iaea.org/newscenter/news/iaea-supported-lab-in-sri-lanka-helps-keep-contaminated-food-off-the-market (accessed on 24 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thambugala, K.M.; Dayananda, D.; Tennakoon, S.; Harischandra, H.; Jayatunga, P.; de Silva, N.; Dhanusha, A.; Madusanka, S.; Daranagama, D.A.; Gonapaladeniya, M.; et al. Silent Saboteurs: Decoding Mycotoxins—From Chemistry and Prevalence to Health Risks, Detection, Management and Emerging Frontiers. J. Fungi 2025, 11, 840. https://doi.org/10.3390/jof11120840
Thambugala KM, Dayananda D, Tennakoon S, Harischandra H, Jayatunga P, de Silva N, Dhanusha A, Madusanka S, Daranagama DA, Gonapaladeniya M, et al. Silent Saboteurs: Decoding Mycotoxins—From Chemistry and Prevalence to Health Risks, Detection, Management and Emerging Frontiers. Journal of Fungi. 2025; 11(12):840. https://doi.org/10.3390/jof11120840
Chicago/Turabian StyleThambugala, Kasun M., Dilakshini Dayananda, Samawansha Tennakoon, Hiruni Harischandra, Pamoda Jayatunga, Nissanka de Silva, Asanthi Dhanusha, Sahan Madusanka, Dinushani A. Daranagama, Madhusha Gonapaladeniya, and et al. 2025. "Silent Saboteurs: Decoding Mycotoxins—From Chemistry and Prevalence to Health Risks, Detection, Management and Emerging Frontiers" Journal of Fungi 11, no. 12: 840. https://doi.org/10.3390/jof11120840
APA StyleThambugala, K. M., Dayananda, D., Tennakoon, S., Harischandra, H., Jayatunga, P., de Silva, N., Dhanusha, A., Madusanka, S., Daranagama, D. A., Gonapaladeniya, M., Haituk, S., & Cheewangkoon, R. (2025). Silent Saboteurs: Decoding Mycotoxins—From Chemistry and Prevalence to Health Risks, Detection, Management and Emerging Frontiers. Journal of Fungi, 11(12), 840. https://doi.org/10.3390/jof11120840







